Reduction of Real-Time Imaging of M1 Macrophage Chemotaxis toward Damaged Muscle Cells is PI3K-Dependent
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
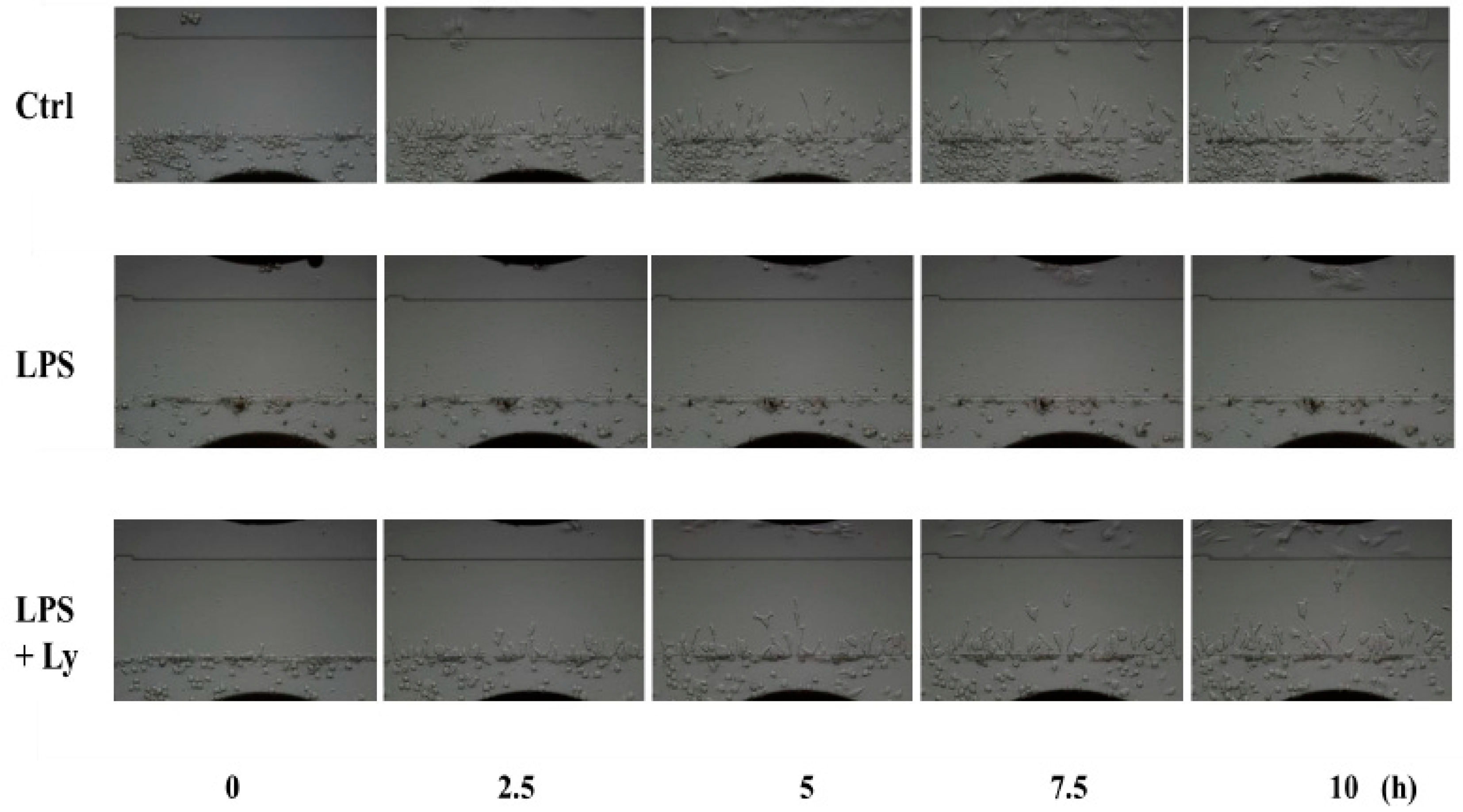
2.3. Real Time Horizontal Chemotaxis Assay Using TAXIScan
2.3.1. Flow Cytometric Analysis
2.3.2. Adherence to Plastic
2.3.3. Western Blot Analysis
2.3.4. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Statistical Analysis
3. Results
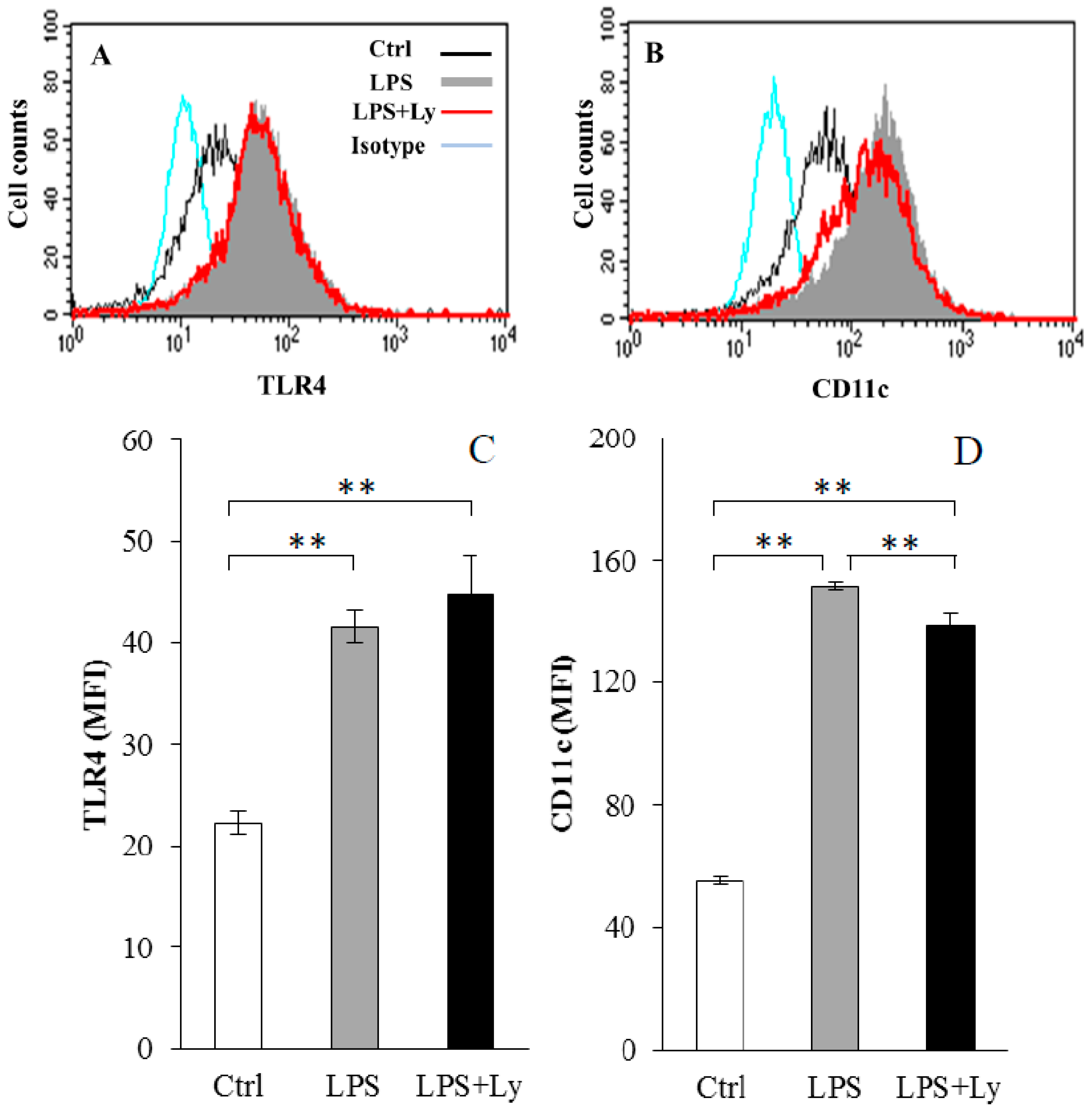
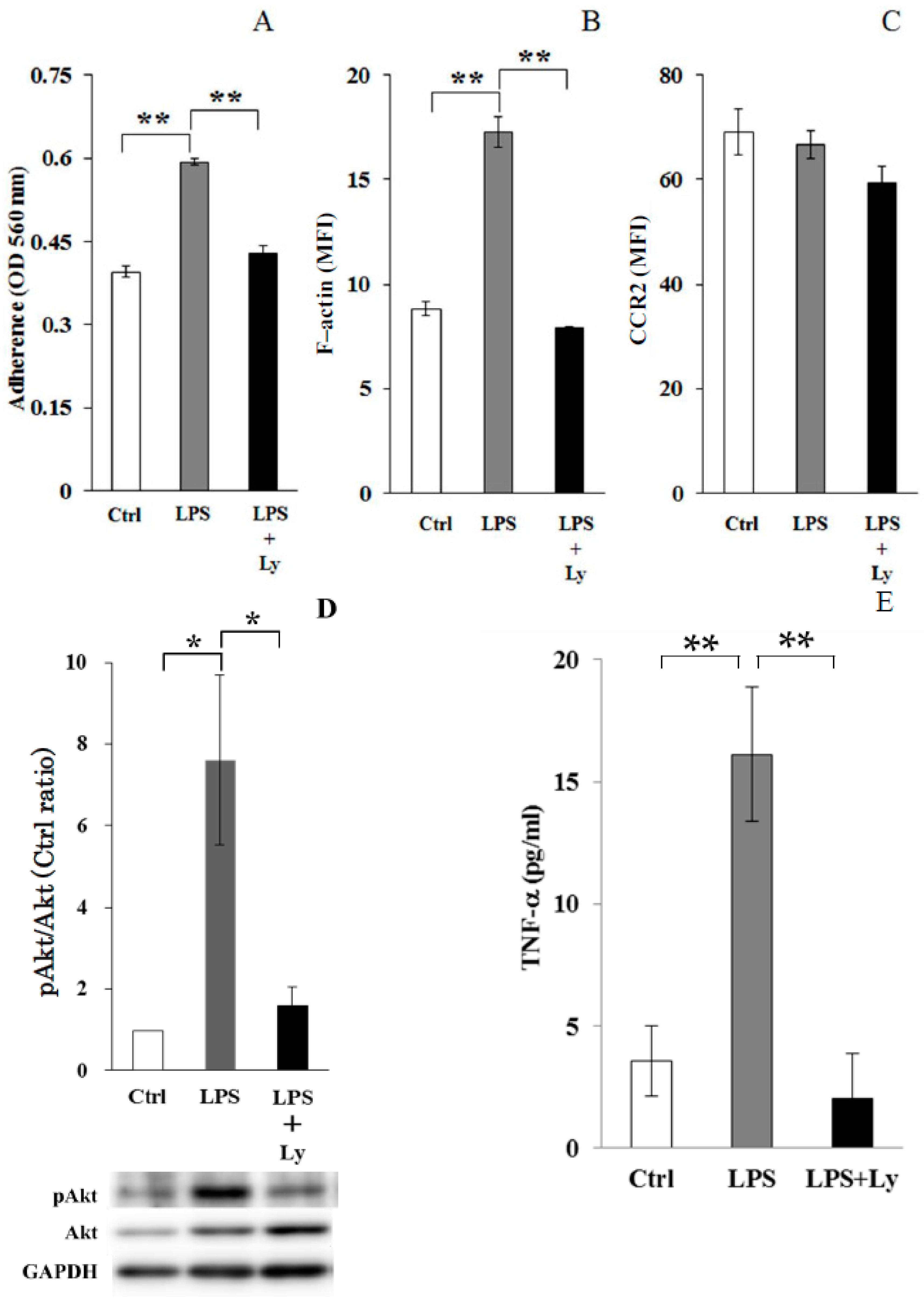
3.1. Effect of Ly Treatment on LPS-Induced Differentiation on the Macrophages
3.2. Effect of Ly Treatment on LPS-Induced Differentiation in the Macrophages
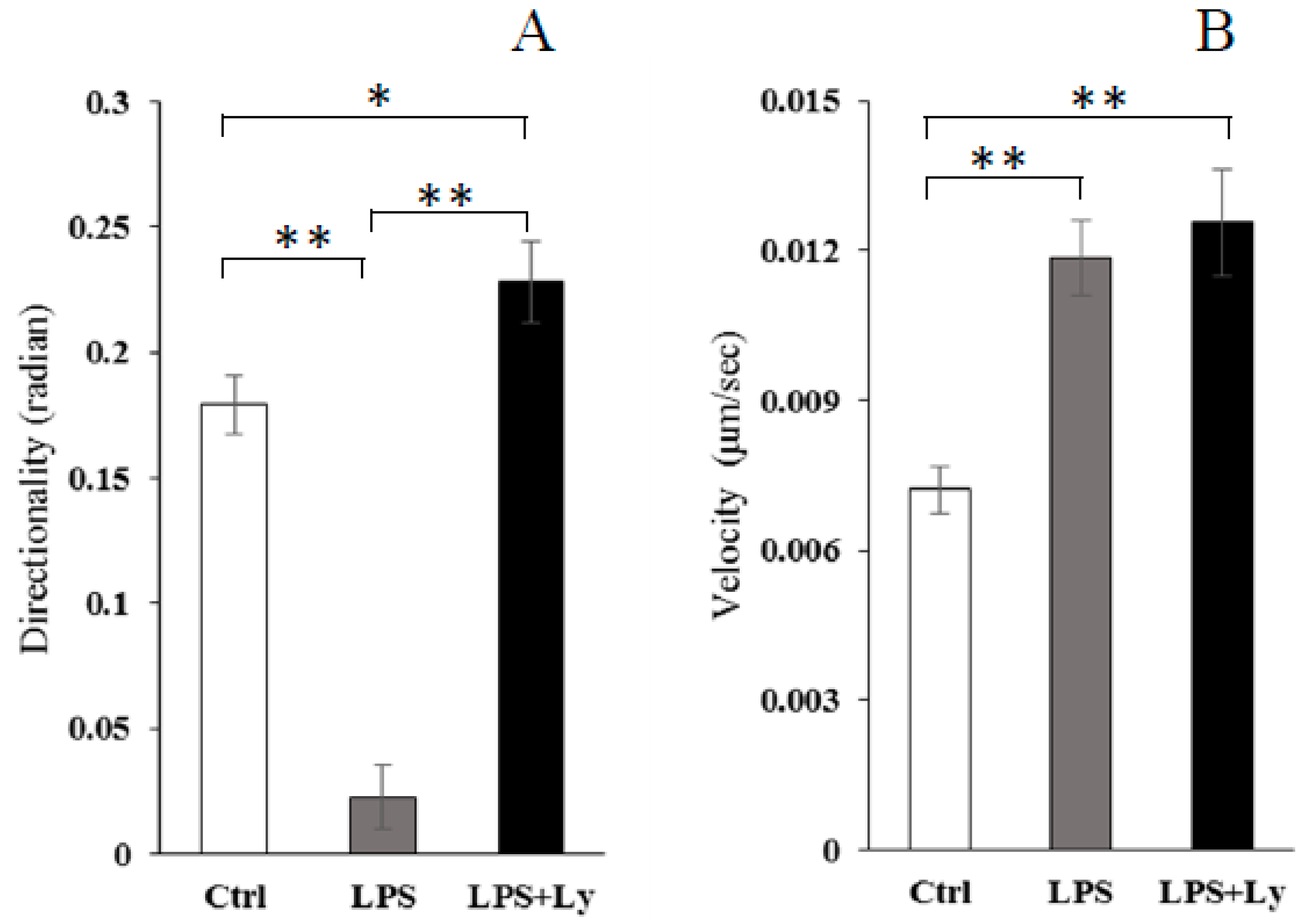
3.3. Effect of Ly Treatment on LPS-Induced Differentiation on the Cells Chemotactic Activity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tidball, J.G.; Berchenko, E.; Frenette, J. Macrophage Invasion does not Contribute to Muscle Membrane Injury during Inflammation. J. Leukoc. Biol. 1999, 65, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.X.; Tidball, J.G. Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro. J. Physiol. 2003, 547, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle Damage and Inflammation during Recovery from Exercise. J. Appl. Physiol. 2017, 122, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Tidball, J.G.; Villalta, S.A. Regulatory Interactions between Muscle and the Immune System during Muscle Regeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1173–R1187. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.; Della Gatta, P.; Cameron-Smith, D. Aging and Its Effects on Inflammation in Skeletal Muscle at Rest and Following Exercise-Induced Muscle Injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R1485–R1495. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, E.; Zordan, P.; Sciorati, C.; Rovere-Querini, P.; Brunelli, S. Macrophage Plasticity in Skeletal Muscle Repair. BioMed Res. Int. 2014. [Google Scholar] [CrossRef] [PubMed]
- Juban, G.; Chazaud, B. Metabolic Regulation of Macrophages during Tissue Repair: Insights from Skeletal Muscle Regeneration. FEBS Lett. 2017, 591, 3007–3021. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Melton, D.W.; Porter, L.; Sarwar, Z.U.; McManus, L.M.; Shireman, P.K. Altered Macrophage Phenotype Transition Impairs Skeletal Muscle Regeneration. Am. J. Pathol. 2014, 184, 1167–1184. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Qu, Q.; Zheng, B.; Xiong, S.; Fan, G.H. The Chemotaxis of M1 and M2 Macrophages is Regulated by Different Chemokines. J. Leukoc. Biol. 2015, 97, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Kleveta, G.; Borzęcka, K.; Zdioruk, M.; Czerkies, M.; Kuberczyk, H.; Sybirna, N.; Sobota, A.; Kwiatkowska, K. LPS Induces Phosphorylation of Actin-Regulatory Proteins Leading to Actin Reassembly and Macrophage Motility. J. Cell Biochem. 2012, 113, 80–92. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Oyanagi, E.; Miyachi, M.; Yamauchi, A.; Yano, H. Relationship between Macrophage Differentiation and the Chemotactic Activity toward Damaged Myoblast Cells. J. Immunol. Methods 2013, 393, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Huang, D.; Saederup, N.; Charo, I.F.; Ransohoff, R.M.; Zhou, L. Macrophages Recruited via CCR2 Produce Insulin-Like Growth Factor-1 to Repair Acute Skeletal Muscle Injury. FASEB J. 2011, 25, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Eräsalo, H.; Laavola, M.; Hämäläinen, M.; Leppänen, T.; Nieminen, R.; Moilanen, E. PI3K Inhibitors LY294002 and IC87114 Reduce Inflammation in Carrageenan-Induced Paw Oedema and Down-Regulate Inflammatory Gene Expression in Activated Macrophages. Basic Clin. Pharmacol. Toxicol. 2015, 116, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, B.; Lin, C.; Gong, Z.; An, X. TREM2 Inhibits Inflammatory Responses in Mouse Microglia by Suppressing the PI3K/NF-κB Signaling. Cell Biol. Int. 2018. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Cambronero, J. Rapamycin Inhibits GM-CSF-Induced Neutrophil Migration. FEBS Lett. 2003, 550, 94–100. [Google Scholar] [CrossRef]
- Fukata, M.; Nakagawa, M.; Kaibuchi, K. Roles of Rho-Family GTPases in Cell Polarisation and Directional Migration. Curr. Opin. Cell Biol. 2003, 15, 590–597. [Google Scholar] [CrossRef]
- Raftopoulou, M.; Hall, A. Cell Migration: Rho GTPases Lead the Way. Dev. Biol. 2004, 265, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, A.B.; Hall, A. Rho GTPases: Biochemistry and biology. Annu. Rev. Cell Dev. Biol. 2005, 21, 247–269. [Google Scholar] [CrossRef] [PubMed]
- Don, M.J.; Liao, J.F.; Lin, L.Y.; Chiou, W.F. Cryptotanshinone Inhibits Chemotactic Migration in Macrophages through Negative Regulation of the PI3K Signaling Pathway. Br. J. Pharmacol. 2007, 151, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Wall, A.A.; Yeo, J.C.; Condon, N.D.; Norwood, S.J.; Schoenwaelder, S.; Chen, K.W.; Jackson, S.; Jenkins, B.J.; Hartland, E.L.; et al. Rab8a Interacts Directly with PI3Kγ to Modulate TLR4-Driven PI3K and mTOR Signalling. Nat. Commun. 2014, 5, 4407. [Google Scholar] [CrossRef] [PubMed]
- Nitta, N.; Tsuchiya, T.; Yamauchi, A.; Tamatani, T.; Kanegasaki, S. Quantitative Analysis of Eosinophil Chemotaxis Tracked Using a Novel Optical Device—TAXIScan. J. Immunol. Methods 2007, 320, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Suganami, T.; Yamauchi, A.; Degawa-Yamauchi, M.; Tanaka, M.; Kouyama, R.; Kobayashi, Y.; Nitta, N.; Yasuda, K.; Hirata, Y.; et al. Role of CC Chemokine Receptor 2 in Bone Marrow Cells in the Recruitment of Macrophages into Obese Adipose Tissue. J. Biol. Chem. 2008, 283, 35715–35723. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.Y.; Heijnen, P.D.; Breur, M.; de Vries, H.E.; Tool, A.T.; Amor, S.; Dijkstra, C.D. Macrophages Migrate in an Activation-Dependent Manner to Chemokines Involved in Neuroinflammation. J. Neuroinflamm. 2014, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, A.; Yamamura, M.; Katase, N.; Itadani, M.; Okada, N.; Kobiki, K.; Nakamura, M.; Yamaguchi, Y.; Kuribayashi, F. Evaluation of Pancreatic Cancer Cell Migration with Multiple Parameters in Vitro by Using an Optical Real-Time Cell Mobility Assay Device. BMC Cancer 2017, 17, 234. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, N.; Yano, H.; Yokogawa, Y.; Suzuki, K. Exercise Training Inhibits Inflammation in Adipose Tissue via Both Suppression of Macrophage Infiltration and Acceleration of Phenotypic Switching from M1 to M2 Macrophages in High-Fat-Diet-Induced Obese Mice. Exerc. Immunol. Rev. 2010, 16, 105–118. [Google Scholar] [PubMed]
- Bugyi, B.; Carlier, M.F. Control of Actin Filament Treadmilling in Cell Motility. Annu. Rev. Biophys. 2010, 39, 449–470. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Rahimpour, R.; Ran, L.; Kong, C.; Biragyn, A.; Andrews, J.; Devries, M.; Wang, J.M.; Kelvin, D.J. Regulation of CCR2 Chemokine Receptor mRNA Stability. J. Leukoc. Biol. 1997, 62, 653–660. [Google Scholar] [CrossRef] [PubMed]
- Rowley, S.M.; Kuriakose, T.; Dockery, L.M.; Tran-Ngyuen, T.; Gingerich, A.D.; Wei, L.; Watford, W.T. Tumor Progression Locus 2 (Tpl2) Kinase Promotes Chemokine Receptor Expression and Macrophage Migration during Acute Inflammation. J. Biol. Chem. 2014, 289, 15788–15797. [Google Scholar] [CrossRef] [PubMed]
- Deci, M.B.; Ferguson, S.W.; Scatigno, S.L.; Nguyen, J. Modulating Macrophage Polarization through CCR2 Inhibition and Multivalent Engagement. Mol. Pharm. 2018, 15, 2721–2731. [Google Scholar] [CrossRef] [PubMed]
- Perdiguero, E.; Sousa-Victor, P.; Ruiz-Bonilla, V.; Jardí, M.; Caelles, C.; Serrano, A.L.; Muñoz-Cánoves, P. p38/MKP-1-Regulated AKT Coordinates Macrophage Transitions and Resolution of Inflammation during Tissue Repair. J. Cell Biol. 2011, 195, 307–322. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yano, H.; Uchida, M.; Saito, T.; Aoki, T.; Kremenik, M.J.; Oyanagi, E. Reduction of Real-Time Imaging of M1 Macrophage Chemotaxis toward Damaged Muscle Cells is PI3K-Dependent. Antioxidants 2018, 7, 138. https://doi.org/10.3390/antiox7100138
Yano H, Uchida M, Saito T, Aoki T, Kremenik MJ, Oyanagi E. Reduction of Real-Time Imaging of M1 Macrophage Chemotaxis toward Damaged Muscle Cells is PI3K-Dependent. Antioxidants. 2018; 7(10):138. https://doi.org/10.3390/antiox7100138
Chicago/Turabian StyleYano, Hiromi, Masataka Uchida, Tatsuya Saito, Takafumi Aoki, Michael J. Kremenik, and Eri Oyanagi. 2018. "Reduction of Real-Time Imaging of M1 Macrophage Chemotaxis toward Damaged Muscle Cells is PI3K-Dependent" Antioxidants 7, no. 10: 138. https://doi.org/10.3390/antiox7100138
APA StyleYano, H., Uchida, M., Saito, T., Aoki, T., Kremenik, M. J., & Oyanagi, E. (2018). Reduction of Real-Time Imaging of M1 Macrophage Chemotaxis toward Damaged Muscle Cells is PI3K-Dependent. Antioxidants, 7(10), 138. https://doi.org/10.3390/antiox7100138



