Abstract
β-hydroxy β-methylbutyrate (HMB) is a bioactive metabolite formed from the breakdown of the branched-chain amino acid, leucine. Given the popularity of HMB supplements among different athletes, specifically, those who participate in regular resistance training, this review was performed to summarize current literature on some aspects of HMB supplementation that have received less attention. Because of the small number of published studies, it has not been possible to conclude the exact effects of HMB on cardiovascular parameters, oxidative stress, and inflammatory markers. Thus, the interpretation of outcomes should be taken cautiously. However, the data presented here suggest that acute HMB supplementation may attenuate the pro-inflammatory response following an intense bout of resistance exercise in athletes. Also, the available findings collectively indicate that chronic HMB consumption with resistance training does not improve cardiovascular risk factors and oxidative stress markers greater than resistance training alone. Taken together, there is clearly a need for further well-designed, long-term studies to support these findings and determine whether HMB supplementation affects the adaptations induced by resistance training associated with the body’s inflammatory condition, antioxidative defense system, and cardiovascular risk factors in humans.
1. Introduction
Some athletes believe that most normal diets do not provide sufficient demands for an optimum performance during intensive training and competitions. Dietary supplements are a commonly used strategy to improve exercise performance and recovery, and many athletes use them as a part of their regular training or competition routine [1]. Over the last decades, numerous studies have been conducted to identify anabolic nutrients for skeletal muscles.
β-hydroxy β-methylbutyrate (HMB) is a type of amino acid supplement on the market. Due to its beneficial effects on muscle function and protein synthesis [2,3], HMB is fast becoming popular among different athletes as an ergogenic aid [4,5]. HMB is added to many training protocols, with the hopes of an enhanced lean body mass and sports performance [6]. Scientific research during the past 20 years demonstrate that HMB supplementation in conjunction with resistance training may improve body composition [7,8,9,10,11,12], muscle strength [2,7,9,10,11,12,13], and power [7,10,13]. It has also been reported that supplying HMB promotes favorable changes in aerobic [14,15,16] and anaerobic [11,14,17] capacity, and muscle recovery after exercise [12,18,19,20,21] in different athletes. To date, several systematic reviews and meta-analyses have investigated the aforementioned actions of HMB supplementation in a variety of populations [4,5,22,23,24,25,26]. Although recent studies have proposed some other actions of HMB with regard to effects on inflammatory, cardiovascular, and oxidative stress markers, these new aspects have received less attention. Therefore, we aimed to review recent findings in these contexts.
2. A Brief Overview of HMB Metabolism
HMB is a metabolic by-product of the essential branched-chain amino acid (BCAA), leucine, which has key roles in protein metabolism [27]. Figure 1 shows the different steps in the production of HMB [25,28]. Following the reversible transamination of leucine to α-ketoisocaproate (KIC) through the enzymatic action of BCAA transferase [26], KIC in the liver can either produce isovaleryl-CoA by the enzyme, branched-chain ketoacid dehydrogenase [28], or generate HMB by the cytosolic enzyme, KIC dioxygenase [29]. Most amounts of produced KIC are metabolized into isovaleryl-CoA, and it has been estimated that only approximately 2–10% of leucine is oxidized into HMB [25,30]. The normal plasma range of HMB concentrations is 1 to 4 µmol/L, but can increase 5-to 10-fold following leucine administration [31]. Although some foods, including citrus fruits, some fish, and breast milk, have some HMB [28], it is impractical to provide the typical 3 g daily dosage of HMB used in most previous human studies that demonstrate an improvement of body composition [7,8,10,11,12] and muscle strength [2,7,10,11,12,13]. Therefore, HMB supplementation is a reasonable way for different athletes, specifically, those who participate in resistance training programs.
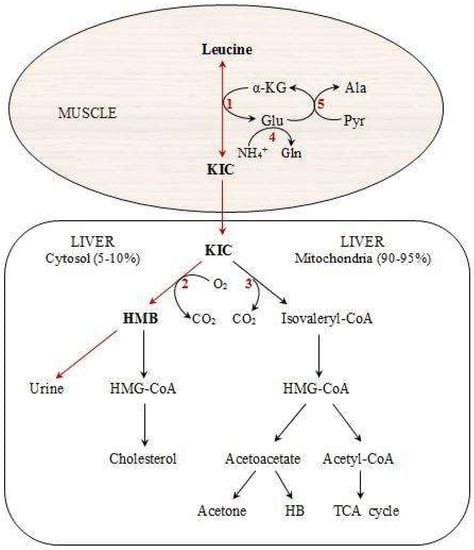
Figure 1.
Pathways of HMB metabolism from the amino acid, leucine. Modified from Nissen and Abumrad [31]. HMB: Beta-hydroxy-beta-methylbutyrate, KIC: Alpha-ketoisocaproic acid, HB: Beta-hydroxybutyrate, HMG-CoA: 3-hydroxy-3-methyl-glutaryl-CoA, Ala: Alanine, Pyr: Pyruvate, 1: branched-chain amino acid (BCAA) aminotransferase, 2: KIC dioxygenase, 3: Branched-chain alpha-keto acid dehydrogenase (BCKAD), 4: Glutamine synthetase, 5: Alanine aminotransferase.
There are two commercially available forms of HMB supplement, including (i) calcium HMB (HMB-Ca), a mono-hydrated calcium salt; and (ii) a free acid form of HMB (HMB-FA), beta-hydroxy-beta-methylbutyric acid [23,32]. Depending upon the dose and its ingestion with other additional nutrients, the magnitude and rate of appearance in blood circulation, and the clearance rate of HMB following consumption are different. In this context, Vukovich et al. [33] compared two doses of HMB-Ca and found that a 3-g dose can cause a peak in plasma concentrations of HMB 1 h after consumption, while a peak HMB level occurred 2 h after the ingestion of a 1-g dose. Plasma concentrations of HMB and urinary losses with the 3-g dose were also significantly higher than the 1-g dose (300% and 14%, respectively). The authors also reported that adding 75 g of glucose to the HMB-Ca dosage may delay peak HMB concentrations by 1 h and decrease its magnitude because of slow gastric emptying or an improvement in HMB clearance [33]. However, compared to 1 g of HMB-Ca, the absorption rate of 0.8 g of HMB-FA was higher and it only took 36 min to reach peak plasma concentrations following ingestion [32]. Furthermore, these increases in peak plasma levels were accompanied by a higher plasma clearance rate (25%) compared to HMB-Ca, indicating greater tissue uptake and utilization [23,25,34]. Although these benefits provided more effective and practical effects on muscle recovery after exercise through the greater intra-muscular HMB bioavailability [23,32,35], another study reported higher bioavailability of HMB after HMB-Ca intake when compared with an equivalent dosage of HMB-FA [36]. Taken together, most published studies have administered HMB-Ca, and further research is needed in this area.
3. An Overview of Different HMB Effects and Its Potential Mechanisms
We have summarized a number of the most important and beneficial effects of HMB supplementation and its suggested mechanisms of action (Table 1).

Table 1.
A summary of potential mechanisms proposed for the different beneficial effects of HMB supplementation on skeletal muscle.
4. Effects of HMB on Inflammation
4.1. Effects on Inflammation without Exercise
HMB improves immune function, especially under stressful conditions. In vitro, HMB has been shown to increase lymphocyte blastogenesis in a dose-dependent fashion [31]. In an animal study, Peterson et al. [48] found that HMB enhanced nitrite production in macrophages and also antibody production. The favorable effects of HMB supplementation on the number of CD3 and CD8 cells and human immunodeficiency virus (HIV) load have also been reported [49]. These data were supported by Hsieh et al. [50], who concluded that supplementation of HMB at 3 g/day for seven days may have an anti-inflammatory effect in a group of elderly patients with chronic obstructive pulmonary disease (COPD).
4.2. Effects on Inflammation Following Exercise
It has been well documented that acute exercise, specifically high-intensity exercise, results in increased inflammatory markers [51]. However, regular exercise training exerts anti-inflammatory effects on the blood [52]. The investigation of new strategies for decreasing the body’s inflammatory reactions after exercise may provide further insight into improved recovery and subsequent performance. It has been suggested that some pro-inflammatory factors, such as interleukin (IL) 1 beta (IL-1β) and tumor necrosis factor alpha (TNF-α), increase proteolysis and may modulate protein turnover [53]. Because HMB is associated with less proteolysis [39,41], to date, several studies have focused on unraveling whether HMB supplementation affects the inflammatory responses following exercise training (Table 2). Beneficial effects of acute HMB supplementation on attenuating pro-inflammatory mediators have been reported in resistance-trained athletes [54,55]. Townsend et al. [54] showed that circulating pro-inflammatory markers, TNF-α and monocyte TNF-α receptor 1 (TNFR1), expression was elevated during an acute bout of heavy resistance exercise and subsequent recovery in healthy, resistance-trained men. However, HMB-FA supplementation (acute ingestion before and/or after exercise) decreased these mediators immediately after resistance exercise. However, another study did not support these findings. Additionally, in another well-designed study, acute HMB ingestion (30 min before, and 2 and 6 h after exercise) had no effect on the responses of macrophage inflammatory protein (MIP)-1β, but attenuated the significant peak expression of complement receptor type 3 (CR3) at 30 min post-exercise (four sets of up to 10 repetitions of three resistance exercises at 70–80% 1-repetition maximum). No increases in CR3 expression after exercise or during recovery was also observed by HMB. However, these changes did not contribute to a more rapid recovery or improve subsequent performance [55]. Longer duration of HMB supplementation may also have some beneficial effects on the inflammatory response after exercise. Hoffman et al. [56] revealed that 23 days of HMB supplementation in combat soldiers can attenuate the inflammatory response (TNF-α, IL-8, IL-10, granulocyte colony-stimulating factor, interferon-γ, and fractalkine) to intense military training, and maintain muscle quality.

Table 2.
Main characteristics of studies examining the effects of HMB supplementation on inflammatory, cardiovascular, and oxidative stress markers.
Although these data provide evidence for a potential blunted or delayed inflammatory response following intense exercise protocols with acute or three weeks of HMB supplementation, anti-inflammatory effects of HMB were not confirmed by others that examined acute or long-term effects of HMB. For instance, Vulcan [57] investigated the effect of acute supplementation of HMB (before, or before and after exercise) on inflammatory responses following three sets of 50 eccentric leg extensions on each leg. It was observed that a decrease in serum concentration of IL-1 receptor antagonist (IL-1ra) and TNF-α for the placebo group was attenuated by HMB at 48-h and 72-h post exercise. In another study, it was investigated whether a longer period of HMB administration can influence inflammatory mediators in elite, national team level adolescent volleyball players. In this study, Portal et al. [17] found that seven-week consumption of HMB (3 g/day) did not change serum concentrations of IL-6 and IL-1ra during the early phase of the volleyball season. It should be considered that the training stimulus in this research was different from that performed by the four aforementioned studies [54,55,56,57]. Therefore, additional studies with long-term supplementation with HMB (at least 10 to 12 weeks) are needed to determine the impact of this dietary supplement on inflammatory responses to resistance exercise.
Because of limited scientific data regarding HMB effects on oxidative stress and cardiovascular parameters following resistance exercise, we propose new approaches for future research.
5. An Approach to Oxidative Stress
To the best of our knowledge, the available scientific literature on the effect of HMB supplementation on oxidative stress in humans is still preliminary in nature and it should be taken into more accurate consideration. Although HMB may improve immune function in humans [54,55,57], unfortunately, there is only one study [58] that has directly examined the effects of HMB supplementation on oxidative stress responses to exercise training (Table 2). In a randomized, double-blind, placebo-controlled trial, we investigated the effects of six-week HMB-FA supplementation on oxidative stress markers in 16 healthy young males. In this study, 8-hydroxy-2-deoxyguanosine (8-OHdG), malondialdehyde (MDA), and protein carbonyl (PC) were measured 48 h before and after resistance training. A significant decrease in MDA and PC was observed in both placebo and HMB groups. However, 8-OHdG did not change after resistance training in any of the groups. Thus, it seems that adding HMB supplementation to resistance training had no further improvements related to oxidative stress markers [58]. A transient increase in oxidative stress following acute exercise may be linked to the health benefits of regular exercise. Therefore, it is unknown what suppression of this oxidative response may do long-term with HMB supplementation. Given the limited available data about HMB effects on oxidative stress mediators, more research examining its effects is warranted. Only after more well-designed trials of HMB have been performed and its effects on the oxidative stress profile have been better defined will it be possible to comment on the effectiveness of HMB as a dietary supplement.
6. An Approach to Cardiovascular Risk Factors
6.1. Cardiovascular Effects of HMB without Exercise
As shown in Figure 1, cytosolic β-hydroxy-β-methylglutarate-Co-A (HMG-CoA), which is produced from HMB in the cytosol of the liver, can be used for cholesterol synthesis [59]. Thus, HMB action as a precursor for cellular cholesterol synthesis can be important for membrane production during periods of high muscular stress, especially during exercise training and the subsequent recovery period. This is known as the cholesterol synthesis hypothesis (CSH) [60]. There are controversial findings about the effect of HMB on some cardiovascular risk factors. For example, different authors have found no change [61], an increase [62], or a decrease [60] in low-density lipoprotein (LDL) or total cholesterol (TC). In a comprehensive study, Nissen et al. [60] summarized data from nine studies in which humans were fed 3 g HMB/day for three to eight weeks and reported that HMB supplementation results in a net decrease in TC (5.8%), a decrease in LDL cholesterol (7.3%), and a decrease in systolic blood pressure (4.4 mmHg). However, Hsieh et al. [61] concluded that HMB supplementation (2 g/day) for two or four weeks has no significant effect on serum lipids in bed-ridden elderly men and women. More recently, an animal study examined the effectiveness of a 12-week HMB administration on insulin resistance induced by a high fructose diet in rats. Compared to the control group, HMB significantly enhanced insulin tolerance and decreased fasting insulin, insulin resistance index (HOMA-IR), glycosylated hemoglobin (HbA1c), hepatic glycogen content, and serum triglycerides (TG), LDL, and very low-density lipoprotein (VLDL) [63]. It has also been demonstrated that two-week supplementation with HMB reduces arrhythmias during ischemia induced in rats [64]. Generally, these data suggest that HMB supplementation may result in a decrease in the risk of heart attack and stroke.
6.2. Cardiovascular Effects of HMB Following Exercise
Only one study investigated the cardiovascular effects of HMB supplementation in conjunction with exercise training (Table 2). Arazi et al. [65] examined the effect of HMB-Ca (3 g/day) on cardiovascular risk factors after four weeks of resistance training (three sessions per week) in athletes. After the training period, TC, LDL, and TG were significantly decreased in both groups and diastolic blood pressure was reduced only in the HMB group. However, no significant differences were found between HMB and placebo groups. Because of limited data regarding this topic, it is difficult to declare the certain and exact cardiovascular effects of HMB supplementation when combined with exercise training. Thus, further prolonged investigations are needed to determine these effects.
7. Conclusions
The available data collectively indicate that acute ingestion of HMB before and after resistance exercise can attenuate some circulating pro-inflammatory mediators, which improves the subsequent recovery process. However, more research is needed to support these effects and verify if chronic HMB consumption in conjunction with resistance training has more favorable effects on pro- and anti-inflammatory markers. Although the number of studies examining the interaction effects of HMB and exercise training on inflammation, oxidative stress, and cardiovascular parameters are limited, it seems that adding HMB supplements to a resistance exercise protocol did not produce further benefits. Generally, future research should be performed to specify the effectiveness of HMB supplementation on the inflammatory profile, the body’s antioxidative defense system and oxidative stress markers, and cardiovascular risk factors, when combined with exercise training.
Author Contributions
The authors’ contributions were as follows: All authors designed and wrote the review. Also, all of them read and approved the final manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maughan, R.J.; Depiesse, F.; Geyer, H. The use of dietary supplements by athletes. J. Sports Sci. 2007, 25, S103–S113. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.S.; Watson, P.E.; Rowlands, D.S. Effects of nine weeks of β-Hydroxy-β-Methylbutyrate supplementation on strength and body composition in resistance trained men. J. Strength Cond. Res. 2009, 23, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Cheng, X.W.; Inoue, A.; Hu, L.; Koike, T.; Kuzuya, M. β-hydroxy-β-methylbutyrate facilitates pi3k/akt-dependent mammalian target of rapamycin and foxo1/3a phosphorylations and alleviates tumor necrosis factor α/interferon γ–induced murf-1 expression in c2c12 cells. Nutr. Res. 2014, 34, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.H.; Mohammadi, H.; Eshaghi, H.; Askari, G.; Miraghajani, M. The effects of beta-hydroxy-beta-methylbutyrate supplementation on recovery following exercise-induced muscle damage: A systematic review and meta-analysis. J. Am. Coll. Nutr. 2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Martinez, J.; Santos-Lozano, A.; Garcia-Hermoso, A.; Sadarangani, K.; Cristi-Montero, C. Effects of beta-hydroxy-beta-methylbutyrate supplementation on strength and body composition in trained and competitive athletes: A meta-analysis of randomized controlled trials. J. Sci. Med. Sport 2017, 21, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Palisin, T.; Stacy, J.J. β-Hydroxy-β-methylbutyrate and its use in athletics. Curr. Sports. Med. Rep. 2005, 4, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Lowery, R.P.; Joy, J.M.; Rathmacher, J.A.; Baier, S.M.; Fuller, J.C.; Shelley, M.C.; Jäger, R.; Purpura, M.; Wilson, S.; Wilson, J.M. Interaction of beta-hydroxy-beta-methylbutyrate free acid and adenosine triphosphate on muscle mass, strength, and power in resistance trained individuals. J. Strength Cond. Res. 2016, 30, 1843–1854. [Google Scholar] [CrossRef] [PubMed]
- Stout, J.R.; Fukuda, D.H.; Kendall, K.L.; Smith-Ryan, A.E.; Moon, J.R.; Hoffman, J.R. β-Hydroxy-β-methylbutyrate (HMB) supplementation and resistance exercise significantly reduce abdominal adiposity in healthy elderly men. Exp. Gerontol. 2015, 64, 33–34. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, H.; Gill, P.; Fernandes Filho, J.; Fernandes, L.C. Effects of 12-weeks of supplementation with β-hydroxy-β-methylbutyrate-Ca (HMB-Ca) on athletic performance. J. Exerc. Physiol. Online 2015, 18, 84–94. [Google Scholar]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Andersen, J.; Wilson, S.M.; Stout, J.R.; Duncan, N.; Fuller, J.C.; Baier, S.M.; Naimo, M.A. The effects of 12 weeks of beta-hydroxy-beta-methylbutyrate free acid supplementation on muscle mass, strength, and power in resistance-trained individuals: A randomized, double-blind, placebo-controlled study. Eur. J. Appl. Physiol. 2014, 114, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Dunsmore, K.; Lowery, R.P.; Duncan, N.; Davis, G.; Rathmacher, J.; Baier, S.; Sikorski, E.; Morrison, T.; Naimo, M.; Walters, J. Effects of 12 weeks of beta-hydroxy-beta-methylbutyrate free acid Gel supplementation on muscle mass, strength, and power in resistance trained individuals (Abstract). J. Int. Soc. Sports Nutr. 2012, 9. [Google Scholar] [CrossRef]
- Panton, L.B.; Rathmacher, J.A.; Baier, S.; Nissen, S. Nutritional supplementation of the leucine metabolite β-hydroxy-β-methylbutyrate (hmb) during resistance training. Nutrition 2000, 16, 734–739. [Google Scholar] [CrossRef]
- Asadi, A.; Arazi, H.; Suzuki, K. Effects of β-hydroxy-β-methylbutyrate-free acid supplementation on strength, power and hormonal adaptations following resistance training. Nutrients 2017, 9, 1316. [Google Scholar] [CrossRef] [PubMed]
- Durkalec-Michalski, K.; Jeszka, J.; Podgórski, T. The effect of a 12-week beta-hydroxy-beta-methylbutyrate (HMB) supplementation on highly-trained combat sports athletes: A randomised, double-blind, placebo-controlled crossover study. Nutrients 2017, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Durkalec-Michalski, K.; Jeszka, J. The effect of β-hydroxy-β-methylbutyrate on aerobic capacity and body composition in trained athletes. J. Strength Cond. Res. 2016, 30, 2617–2626. [Google Scholar] [CrossRef] [PubMed]
- Durkalec-Michalski, K.; Jeszka, J. The efficacy of a β-hydroxy-β-methylbutyrate supplementation on physical capacity, body composition and biochemical markers in elite rowers: A randomised, double-blind, placebo-controlled crossover study. J. Int. Soc. Sports Nutr. 2015, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Portal, S.; Zadik, Z.; Rabinowitz, J.; Pilz-Burstein, R.; Adler-Portal, D.; Meckel, Y.; Cooper, D.M.; Eliakim, A.; Nemet, D. The effect of HMB supplementation on body composition, fitness, hormonal and inflammatory mediators in elite adolescent volleyball players: A prospective randomized, double-blind, placebo-controlled study. Eur. J. Appl. Physiol. 2011, 111, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.L.M.; de Lima, F.D.; Bottaro, M.; Vieira, A.; da Fonseca, A.C.; Lima, R.M. Pre-exercise β-hydroxy-β-methylbutyrate free-acid supplementation improves work capacity recovery: A randomized, double-blinded, placebo-controlled study. Appl. Physiol. Nutr. Metab. 2018, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Stout, J.R.; Jajtner, A.R.; Townsend, J.R.; Wells, A.J.; Beyer, K.S.; Boone, C.H.; Pruna, G.J.; Mangine, G.T.; Scanlon, T.M. Effects of β-hydroxy-β-methylbutyrate free acid and cold water immersion on post-exercise markers of muscle damage. Amino Acids 2014, 46, 1501–1511. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Walters, J.A.; Baier, S.M.; Fuller, J.C.; Stout, J.R.; Norton, L.E.; Sikorski, E.M.; Wilson, S.M. β-Hydroxy-β-methylbutyrate free acid reduces markers of exercise-induced muscle damage and improves recovery in resistance-trained men. Br. J. Nutr. 2013, 110, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Knitter, A.; Panton, L.; Rathmacher, J.; Petersen, A.; Sharp, R. Effects of β-hydroxy-β-methylbutyrate on muscle damage after a prolonged run. J. Appl. Physiol. 2000, 89, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Engelen, M.P.; Deutz, N.E. Is β-hydroxy β-methylbutyrate an effective anabolic agent to improve outcome in older diseased populations? Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Silva, V.R.; Belozo, F.L.; Micheletti, T.O.; Conrado, M.; Stout, J.R.; Pimentel, G.D.; Gonzalez, A.M. β-Hydroxy-β-methylbutyrate free acid supplementation may improve recovery and muscle adaptations after resistance training: A systematic review. Nutr. Res. 2017, 45, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Albert, F.J.; Morente-Sánchez, J.; Ortega, F.B.; Castillo, M.J.; Gutiérrez, Á. Usefulness of β-hydroxy-β-methylbutyrate (HMB) supplementation in different sports: An update and practical implications. Nutr. Hosp. 2015, 32, 20–33. [Google Scholar] [PubMed]
- Wilson, J.M.; Fitschen, P.J.; Campbell, B.; Wilson, G.J.; Zanchi, N.; Taylor, L.; Wilborn, C.; Kalman, D.S.; Stout, J.R.; Hoffman, J.R. International Society of Sports Nutrition Position Stand: Beta-hydroxy-beta-methylbutyrate (HMB). J. Int. Soc. Sports Nutr. 2013, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Zanchi, N.E.; Gerlinger-Romero, F.; Guimaraes-Ferreira, L.; de Siqueira Filho, M.A.; Felitti, V.; Lira, F.S.; Seelaender, M.; Lancha, A.H. HMB supplementation: Clinical and athletic performance-related effects and mechanisms of action. Amino Acids 2011, 40, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J. The role of leucine in the regulation of protein metabolism. J. Nutr. 2005, 135, 1553S–1556S. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.H.J.; Guimaraes-Ferreira, L.; Gerlinger-Romero, F.; Curi, R. An overview on beta-hydroxy-beta-methylbutyrate (HMB) supplementation in skeletal muscle function and sports performance. In Nutrition and Enhanced Sports Performance; Elsevier: Amsterdam, The Netherlands, 2014; pp. 455–463. [Google Scholar]
- Sabourin, P.J.; Bieber, L.L. Formation of beta-hydroxyisovalerate by an alpha-ketoisocaproate oxygenase in human liver. Metabolism 1983, 32, 160–164. [Google Scholar] [CrossRef]
- Van Koevering, M.; Nissen, S. Oxidation of leucine and alpha-ketoisocaproate to beta-hydroxy-beta-methylbutyrate in vivo. Am. J. Physiol. Endocrinol. Metab. 1992, 262, E27–E31. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.L.; Abumrad, N.N. Nutritional role of the leucine metabolite β-hydroxy β-methylbutyrate (HMB). J. Nutr. Biochem. 1997, 8, 300–311. [Google Scholar] [CrossRef]
- Fuller, J.C.; Sharp, R.L.; Angus, H.F.; Baier, S.M.; Rathmacher, J.A. Free acid gel form of β-hydroxy-β-methylbutyrate (HMB) improves HMB clearance from plasma in human subjects compared with the calcium HMB salt. Br. J. Nutr. 2011, 105, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Vukovich, M.D.; Slater, G.; Macchi, M.B.; Turner, M.J.; Fallon, K.; Boston, T.; Rathmacher, J. β-hydroxy-β-methylbutyrate (HMB) kinetics and the influence of glucose ingestion in humans. J. Nutr. Biochem. 2001, 12, 631–639. [Google Scholar] [CrossRef]
- Fuller, J.C.; Sharp, R.L.; Angus, H.F.; Khoo, P.Y.; Rathmacher, J.A. Comparison of availability and plasma clearance rates of β-hydroxy-β-methylbutyrate delivery in the free acid and calcium salt forms. Br. J. Nutr. 2015, 114, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.; Hossain, T.; Hill, D.; Phillips, B.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.; Breen, L.; Phillips, S. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Shreeram, S.; Johns, P.W.; Subramaniam, S.; Ramesh, S.; Vaidyanathan, V.; Puthan, J.K.; Mandal, S.; Mamidi, V.K.; Gelling, R.W. The relative bioavailability of the calcium salt of β-hydroxy-β-methylbutyrate is greater than that of the free fatty acid form in rats–3. J. Nutr. 2014, 144, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Kornasio, R.; Riederer, I.; Butler-Browne, G.; Mouly, V.; Uni, Z.; Halevy, O. β-hydroxy-β-methylbutyrate (HMB) stimulates myogenic cell proliferation, differentiation and survival via the MAPK/ERK and PI3K/Akt pathways. Biochim. Biophys. Acta Mol. Cell Res. 2009, 1793, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Gerlinger-Romero, F.; Guimarães-Ferreira, L.; Giannocco, G.; Nunes, M. Chronic supplementation of beta-hydroxy-beta methylbutyrate (HMβ) increases the activity of the GH/IGF-I axis and induces hyperinsulinemia in rats. Growth Horm. IGF Res. 2011, 21, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.J.; Mukerji, P.; Tisdale, M.J. Attenuation of proteasome-induced proteolysis in skeletal muscle by β-hydroxy-β-methylbutyrate in cancer-induced muscle loss. Cancer Res. 2005, 65, 277–283. [Google Scholar] [PubMed]
- Kovarik, M.; Muthny, T.; Sispera, L.; Holecek, M. Effects of β-hydroxy-β-methylbutyrate treatment in different types of skeletal muscle of intact and septic rats. J. Physiol. Biochem. 2010, 66, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Eley, H.L.; Russell, S.T.; Tisdale, M.J. Attenuation of depression of muscle protein synthesis induced by lipopolysaccharide, tumor necrosis factor, and angiotensin II by β-hydroxy-β-methylbutyrate. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1409–E1416. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Jackson, J.R.; Wang, Y.; Edens, N.; Pereira, S.L.; Alway, S.E. β-Hydroxy-β-methylbutyrate reduces myonuclear apoptosis during recovery from hind limb suspension-induced muscle fiber atrophy in aged rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R701–R715. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Beta-hydroxy-beta-methylbutyrate supplementation and skeletal muscle in healthy and muscle-wasting conditions. J. Cachexia Sarcopenia Muscle 2017, 8, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.; Sharp, R.; Ray, M.; Rathmacher, J.; Rice, D.; Fuller, J., Jr.; Connelly, A.; Abumrad, N. Effect of leucine metabolite β-hydroxy-β-methylbutyrate on muscle metabolism during resistance-exercise training. J. Appl. Physiol. 1996, 81, 2095–2104. [Google Scholar] [CrossRef] [PubMed]
- Vallejo, J.; Spence, M.; Cheng, A.-L.; Brotto, L.; Edens, N.K.; Garvey, S.M.; Brotto, M. Cellular and physiological effects of dietary supplementation with β-hydroxy-β-methylbutyrate (HMB) and β-alanine in late middle-aged mice. PLoS ONE 2016, 11, e0150066. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Duan, Y.; Yao, K.; Li, F.; Hou, Y.; Wu, G.; Yin, Y. β-Hydroxy-β-methylbutyrate, mitochondrial biogenesis, and skeletal muscle health. Amino Acids 2016, 48, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Da Justa Pinheiro, C.H.; Gerlinger-Romero, F.; Guimaraes-Ferreira, L.; de Souza, A.L., Jr.; Vitzel, K.F.; Nachbar, R.T.; Nunes, M.T.; Curi, R. Metabolic and functional effects of beta-hydroxy-beta-methylbutyrate (HMB) supplementation in skeletal muscle. Eur. J. Appl. Physiol. 2012, 112, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Peterson, A.; Qureshi, M.; Ferket, P.; Fuller, J., Jr. In vitro exposure with β-hydroxy-β-methylbutyrate enhances chicken macrophage growth and function. Vet. Immunol. Immunopathol. 1999, 67, 67–78. [Google Scholar] [CrossRef]
- Clark, R.H.; Feleke, G.; Din, M.; Yasmin, T.; Singh, G.; Khan, F.A.; Rathmacher, J.A. Nutritional treatment for acquired immunodeficiency virus-associated wasting using β-hydroxy β-methylbutyrate, glutamine, and arginine: A randomized, double-blind, placebo-controlled study. J. Parenter. Enter. Nutr. 2000, 24, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.; Chien, S.; Huang, M.; Tseng, H.; Chang, C. Anti-inflammatory and anticatabolic effects of short-term beta-hydroxy-beta-methylbutyrate supplementation on chronic obstructive pulmonary disease patients in intensive care unit. Asia Pac. J. Clin. Nutr. 2006, 15, 544–550. [Google Scholar] [PubMed]
- Suzuki, K. Cytokine response to exercise and its modulation. Antioxidants 2018, 7, 17. [Google Scholar] [CrossRef]
- Ma, S.; Suzuki, K. Toll-like receptor 4: Target of lipotoxicity and exercise-Induced anti-inflammatory effect? Ann. Nutr. Food Sci. 2018, 2, 1–3. [Google Scholar]
- Clowes, G.H., Jr.; George, B.C.; Villee, C.A., Jr.; Saravis, C.A. Muscle proteolysis induced by a circulating peptide in patients with sepsis or trauma. N. Engl. J. Med. 1983, 308, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Townsend, J.R.; Fragala, M.S.; Jajtner, A.R.; Gonzalez, A.M.; Wells, A.J.; Mangine, G.T.; Robinson, E.H., IV; McCormack, W.P.; Beyer, K.S.; Pruna, G.J. β-Hydroxy-β-methylbutyrate (HMB)-free acid attenuates circulating TNF-α and TNFR1 expression postresistance exercise. J. Appl. Physiol. 2013, 115, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.M.; Fragala, M.S.; Jajtner, A.R.; Townsend, J.R.; Wells, A.J.; Beyer, K.S.; Boone, C.H.; Pruna, G.J.; Mangine, G.T.; Bohner, J.D. Effects of β-hydroxy-β-methylbutyrate free acid and cold water immersion on expression of CR3 and MIP-1β following resistance exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 306, R483–R489. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Gepner, Y.; Stout, J.R.; Hoffman, M.W.; Ben-Dov, D.; Funk, S.; Daimont, I.; Jajtner, A.R.; Townsend, J.R.; Church, D.D. β-Hydroxy-β-methylbutyrate attenuates cytokine response during sustained military training. Nutr. Res. 2016, 36, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Vulcan, P.R. Role of β-Hydroxy-β-methylbutyrate (HMB) on Inflammation after Eccentric Exercise. Master’s Thesis, Iowa State University, Ames, IA, USA, October 2012. [Google Scholar]
- Arazi, H.; Asadi, A.; Suzuki, K. The effects of beta-hydroxy-beta-methylbutyrate-free acid supplementation and resistance training on oxidative stress markers: A randomized, double-blind, placebo-controlled study. Antioxidants 2018, 7, 76. [Google Scholar] [CrossRef] [PubMed]
- Rudney, H. The biosynthesis of β-hydroxy-β-methylglutaric acid. J. Biol. Chem. 1957, 227, 363–377. [Google Scholar] [PubMed]
- Nissen, S.; Sharp, R.; Panton, L.; Vukovich, M.; Trappe, S.; Fuller, J., Jr. β-hydroxy-β-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J. Nutr. 2000, 130, 1937–1945. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.-C.; Chow, C.-J.; Chang, W.-C.; Liu, T.-H.; Chang, C.-K. Effect of β-hydroxy-β-methylbutyrate on protein metabolism in bed-ridden elderly receiving tube feeding. Asia. Pac. J. Clin. Nutr. 2010, 19, 200–208. [Google Scholar] [PubMed]
- Holecek, M.; Muthny, T.; Kovarik, M.; Sispera, L. Effect of beta-hydroxy-beta-methylbutyrate (HMB) on protein metabolism in whole body and in selected tissues. Food Chem. Toxicol. 2009, 47, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Sharawy, M.H.; El-Awady, M.S.; Megahed, N.; Gameil, N.M. The ergogenic supplement β-hydroxy-β-methylbutyrate (HMB) attenuates insulin resistance through suppressing GLUT-2 in rat liver. Can. J. Physiol. Pharmacol. 2015, 94, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Ravanbakhsh, N.; Torabi, N.; Foadoddini, M. Preventive effects of beta-hydroxy-beta-methyl butyrate on cardiac arrhythmias in rats (Abstract). Ofogh-E-Danesh 2016, 22, 209–214. [Google Scholar]
- Arazi, H.; Rohani, H.; Ghiasi, A.; Davaran, M. The effect of HMB supplementation on cardiovascular risk factors after four weeks of resistance training in amateur athletes. Int. Cardiovasc. Res. J. 2015, 9, 89–93. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).