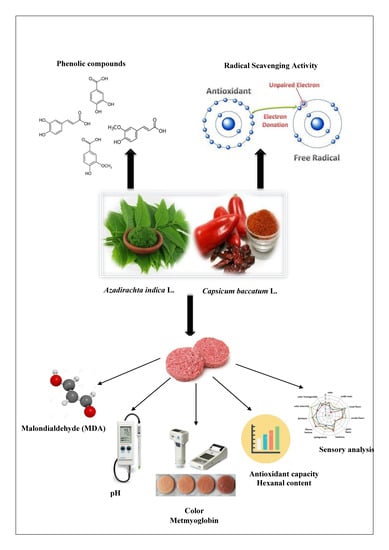
Effect of Neem (Azadirachta indica L.) on Lipid Oxidation in Raw Chilled Beef Patties
Abstract
1. Introduction
2. Materials and Methods
2.1. Natural Products
2.2. Microbial Strains
2.3. Meat
2.4. Chemicals and Products
2.5. Instrumentation
2.6. Total Polyphenol Content and Radical Scavenging Activity of A. indica and C. baccatum Extracts
2.6.1. Extracts Preparation
2.6.2. Total Polyphenols Content (TPC)
2.6.3. Radical Scavenging Activity (RSA): DPPH Assay
2.7. Antibacterial Activity of A. indica and C. baccatum Extracts, by Agar Disk-Diffusion Method (Inhibitory Zone Assay)
2.8. Antioxidant Effect of Powdered A. indica Dry Leaves on Beef Meat Quality
2.8.1. Preparation and Storage Conditions of Raw Beef Patties
2.8.2. Lipid Oxidation and pH Value Evolution
2.8.3. Antioxidant Capacity (AOC)
2.8.4. Color Fading Measurement
2.8.5. Metmyoglobin (MetMb) Reducing Activity
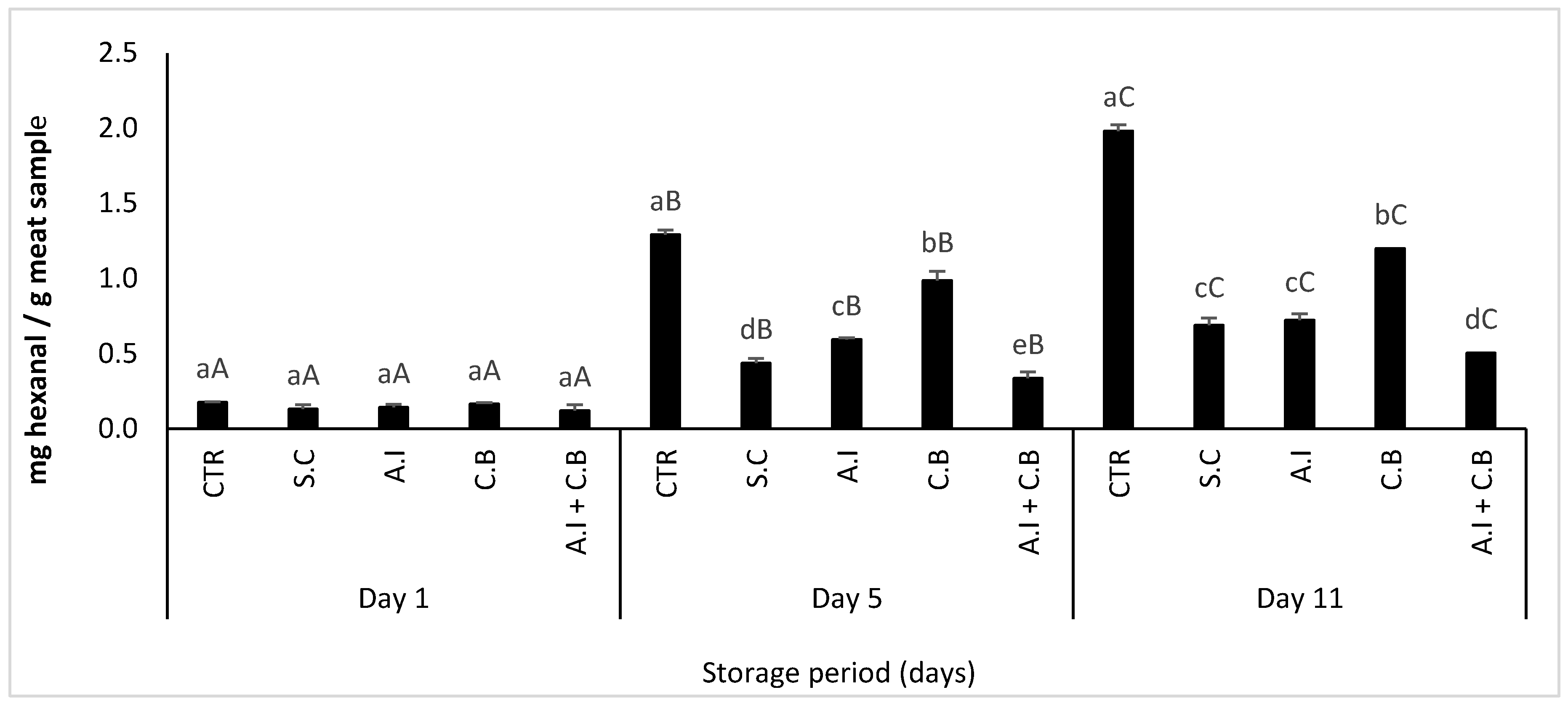
2.8.6. Hexanal Content Determination by HS-GC/MS
2.8.7. Microbial Analysis
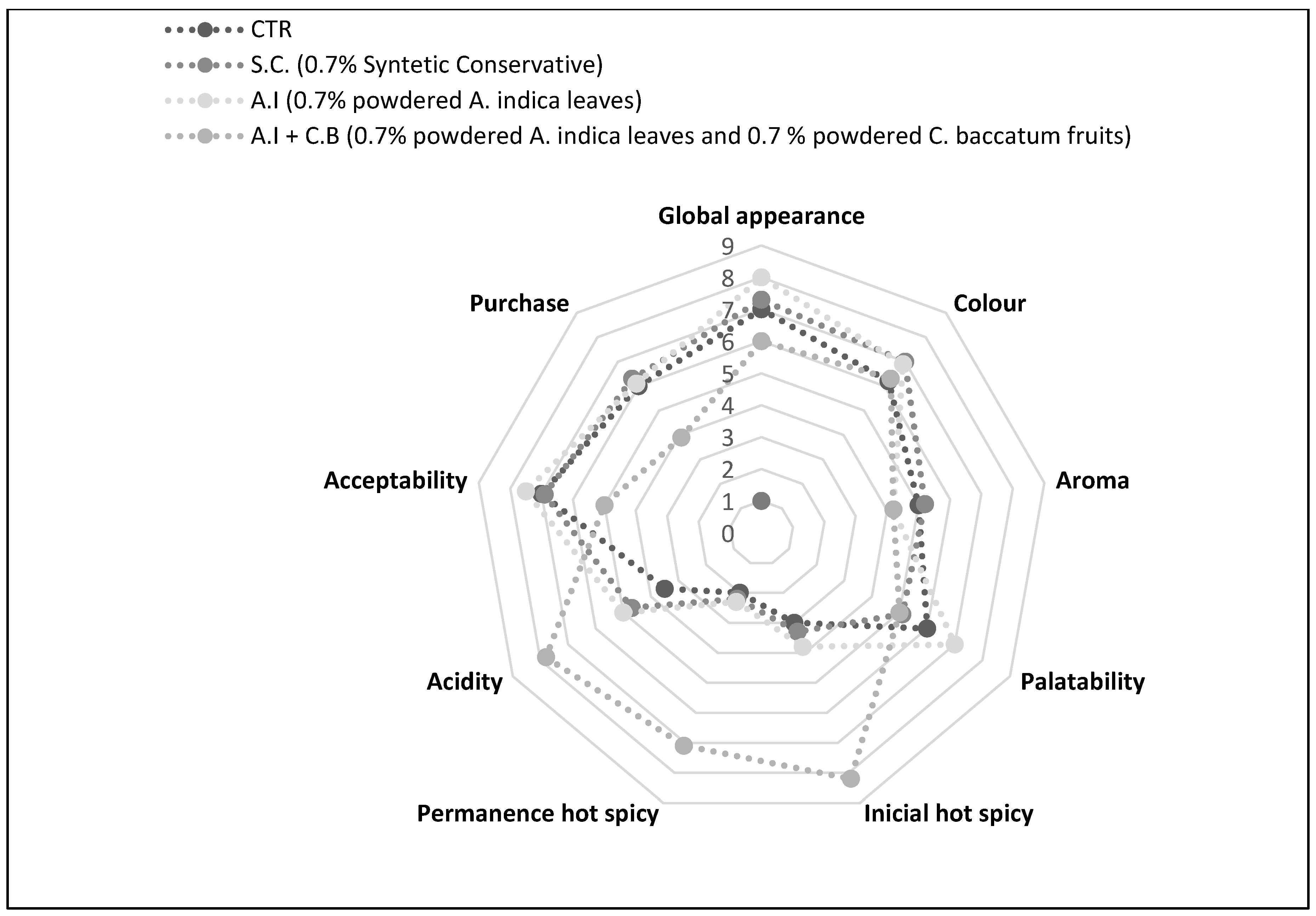
2.8.8. Sensory Analysis
2.8.9. Statistical Analysis
3. Results and Discussion
3.1. Total Polyphenol Content (TPC) and Radical Scavenging Activity (RSA)
3.2. Screening of Antibacterial Activity
3.3. Effect of Powdered A. indica Leaves on Beef Meat Quality During Refrigerated Storage
3.3.1. Lipid Oxidation and pH Variation
3.3.2. AOC Assay
3.3.3. Color Fading and MetMb Reducing Activity
3.3.4. Hexanal Content
3.3.5. Antimicrobial Analysis
3.3.6. Sensory Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, J.; Xiong, Y.L. Natural antioxidants as food and feed additives to promote health benefits and quality of meat products: A review. Meat Sci. 2016, 120, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gu, W.; Zhang, J.; Chu, Y.; Ye, X.; Hu, Y.; Chen, J. Effects of chitosan, aqueous extract of ginger, onion and garlic on quality and shelf life of stewed-pork during refrigerated storage. Food Chem. 2013, 141, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.A.; Bosco, S.J.D.; Mir, S.A. Plant extracts as natural antioxidants in meat and meat products. Meat Sci. 2014, 98, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Duman, M.; Özpolat, E. Effects of water extract of propolis on fresh shibuta (Barbus grypus) fillets during chilled storage. Food Chem. 2015, 189, 80–85. [Google Scholar] [CrossRef]
- Aziz, M.; Karboune, S. Natural antimicrobial/antioxidant agents in meat and poultry products as well as fruits and vegetables: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 486–511. [Google Scholar] [CrossRef]
- Lin, M.J.; Chang, S.C.; Jea, Y.S.; Liao, J.W.; Fan, Y.K.; Lee, T.T. In vitro antioxidant capability and performance assessment of White Roman goose supplemented with dried Toona sinensis. J. Appl. Anim.Res. 2016, 44, 395–402. [Google Scholar] [CrossRef]
- Gupta, S.; Abu-Ghannam, N. Recent developments in the application of seaweeds or seaweed extracts as a means for enhancing the safety and quality attributes of foods. Innov. Food Sci. Emerg. Technol. 2011, 12, 600–609. [Google Scholar] [CrossRef]
- Turgut, S.S.; Soyer, A.; Işıkçı, F. Effect of pomegranate peel extract on lipid and protein oxidation in beef meatballs during refrigerated storage. Meat Sci. 2016, 116, 126–132. [Google Scholar] [CrossRef]
- Mhalla, D.; Bouaziz, A.; Ennouri, K.; Chawech, R.; Smaoui, S.; Jarraya, R.; Tounsi, S.; Trigui, M. Antimicrobial activity and bioguided fractionation of Rumex tingitanus extracts for meat preservation. Meat Sci. 2017, 125, 22–29. [Google Scholar] [CrossRef]
- Baldi, G.; Chauhan, S.S.; Linden, N.; Dunshea, F.R.; Hopkins, D.L.; Sgoifo Rossi, C.A.; Dell’Orto, V.; Ponnampalam, E.N. Comparison of a grain-based diet supplemented with synthetic vitamin E versus a lucerne (alfalfa) hay-based diet fed to lambs in terms of carcass traits, muscle vitamin E, fatty acid content, lipid oxidation, and retail colour of meat. Meat Sci. 2019, 148, 105–112. [Google Scholar] [CrossRef]
- Seçil Karabacak, H.; Bozkurt, S. Effects of Urtica dioica and Hibiscus sabdariffa on the quality and safety of sucuk ( Turkish dry-fermented sausage ). Meat Sci. 2008, 78, 288–296. [Google Scholar] [CrossRef]
- Del Serrone, P.; Toniolo, C.; Nicoletti, M. Neem (Azadirachta indica A. Juss) Oil: A natural preservative to control meat spoilage. Foods 2015, 4, 3–14. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Kumar, V.S.; Navaratnam, V. Neem (Azadirachta indica): Prehistory to contemporary medicinal uses to humankind. Asian Pac. J. Trop. Biomed. 2013, 3, 505–514. [Google Scholar] [CrossRef]
- Fernandes, S.R.; Barreiros, L.; Oliveira, R.F.; Cruz, A.; Prudêncio, C.; Oliveira, A.I.; Pinho, C.; Santos, N.; Morgado, J. Chemistry, bioactivities, extraction and analysis of azadirachtin: State-of-the-art. Fitoterapia 2019, 134, 141–150. [Google Scholar] [CrossRef]
- Duangjai, A.; Nuengchamnong, N.; Lee, L.H.; Goh, B.H.; Saokaew, S.; Suphrom, N. Characterisation of an extract and fractions of Azadirachta indica flower on cholesterol lowering property and intestinal motility. Nat. Prod. Res. 2017, 6419, 1–4. [Google Scholar] [CrossRef]
- Al Akeel, R.; Mateen, A.; Janardhan, K.; Gupta, V.C. Analysis of anti-bacterial and anti oxidative activity of Azadirachta indica bark using various solvents extracts. Saudi J. Biol. Sci. 2017, 24, 11–14. [Google Scholar] [CrossRef]
- Slatnar, A.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Solar, A. Identification and quantification of phenolic compounds in kernels, oil and bagasse pellets of common walnut (Juglans regia L.). Food Res. Int. 2015, 67, 255–263. [Google Scholar] [CrossRef]
- Villasante, J.; Girbal, M.; Metón, I.; Almajano, M.P. Effects of pecan nut (Carya illinoiensis) and roselle flower (Hibiscus sabdariffa) as antioxidant and antimicrobial agents for sardines (Sardina pilchardus). Molecules 2019, 24, 85. [Google Scholar] [CrossRef]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Fan, X.J.; Liu, S.Z.; Li, H.H.; He, J.; Feng, J.T.; Zhang, X.; Yan, H. Effects of Portulaca oleracea L. extract on lipid oxidation and color of pork meat during refrigerated storage. Meat Sci. 2019, 147, 82–90. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.J.; Almajano, M.P. Caesalpinia decapetala extracts as inhibitors of lipid oxidation in beef patties. Molecules 2015, 20, 13913–13926. [Google Scholar] [CrossRef]
- Wrona, M.; Nerín, C.; Alfonso, M.J.; Caballero, M.Á. Antioxidant packaging with encapsulated green tea for fresh minced meat. Innov. Food Sci. Emerg. Technol. 2017, 41, 307–313. [Google Scholar] [CrossRef]
- Aini, N.; Azman, M.; Gallego, M.G.; Juliá, L.; Fajari, L.; Apilar Almajano, M.; Miguel, M.G.; Rocha, J.O.; Abourashed, E.A. The effect of Convolvulus arvensis dried extract as a potential antioxidant in food models. Antioxidants 2015, 4, 170–184. [Google Scholar] [CrossRef]
- Sirocchi, V.; Devlieghere, F.; Peelman, N.; Sagratini, G.; Maggi, F.; Vittori, S.; Ragaert, P. Effect of Rosmarinus officinalis L. essential oil combined with different packaging conditions to extend the shelf life of refrigerated beef meat. Food Chem. 2016. [Google Scholar] [CrossRef]
- Hawashin, M.D.; Al-Juhaimi, F.; Ahmed, I.A.M.; Ghafoor, K.; Babiker, E.E. Physicochemical, microbiological and sensory evaluation of beef patties incorporated with destoned olive cake powder. Meat Sci. 2016, 122, 32–39. [Google Scholar] [CrossRef]
- Saha, J.; Debnath, M.; Saha, A.; Ghosh, T.; Sarkar, P.K. Response surface optimisation of extraction of antioxidants from strawberry fruit, and lipid peroxidation inhibitory potential of the fruit extract in cooked chicken patties. J. Sci. Food Agric. 2011, 91, 1759–1765. [Google Scholar] [CrossRef]
- Christensen, Z.T.; Ogden, L.V.; Dunn, M.L.; Eggett, D.L. Multiple comparison procedures for analysis of ranked data. J. Food Sci. 2006, 71. [Google Scholar] [CrossRef]
- Ghimeray, A.K.; Jin, C.; Ghimire, B.K.; Cho, D.H. Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta Indica A. Juss grown in foothills of Nepal. J. Biotechnol. 2009, 8, 3084–3091. [Google Scholar]
- Datta, A.; Grün, I.U.; Kwasniewski, M.T.; Fernando, L.N. Comparison of two adsorbent based de-bittering procedures for neem (Azadirachta indica A. Juss) tea—Effect on polyphenols, anti-oxidant capacity, color and volatile profile. Plant Foods for Hum. Nutr. 2017, 72, 88–95. [Google Scholar] [CrossRef]
- Sora, G.T.S.; Haminiuk, C.W.I.; da Silva, M.V.; Zielinski, A.A.F.; Gonçalves, G.A.; Bracht, A.; Peralta, R.M. A comparative study of the capsaicinoid and phenolic contents and in vitro antioxidant activities of the peppers of the genus Capsicum: An application of chemometrics. J. Food Sci. Technol. 2015, 52, 8086–8094. [Google Scholar] [CrossRef]
- Dhanani, T.; Shah, S.; Gajbhiye, N.A.; Kumar, S. Effect of extraction methods on yield, phytochemical constituents and antioxidant activity of Withania somnifera. Arab. J. Chem. 2017, 10, S1193–S1199. [Google Scholar] [CrossRef]
- Lubomirova Christova-Bagdassarian, V.; Stefanova Atanassova, M.; Hristova, V.K.; Ayaz Ahmad, M. Soild-liquid extraction kinetics of total phenolic, total flavonoid, rutin and tannin contents in 50% ethanol extract of Cotinus coggygria. Int. J. Sci. Eng. Res. 2016, 7, 1466–1472. [Google Scholar]
- Gallego, M.; Skowyra, M.; Gordon, M.; Azman, N.; Almajano, M. Effect of leaves of Caesalpinia decapetala on oxidative stability of oil-in-water emulsions. Antioxidants 2017, 6, 19. [Google Scholar] [CrossRef]
- Kaviarasan, S.; Naik, G.H.; Gangabhagirathi, R.; Anuradha, C.V.; Priyadarsini, K.I. In vitro studies on antiradical and antioxidant activities of fenugreek (Trigonella foenum graecum) seeds. Food Chem. 2007, 103, 31–37. [Google Scholar] [CrossRef]
- Riazi, F.; Zeynali, F.; Hoseini, E.; Behmadi, H.; Savadkoohi, S. Oxidation phenomena and color properties of grape pomace on nitrite-reduced meat emulsion systems. Meat Sci. 2016, 121, 350–358. [Google Scholar] [CrossRef]
- Cui, Y.; Oh, Y.J.; Lim, J.; Youn, M.; Lee, I.; Pak, H.K.; Park, W.; Jo, W.; Park, S. AFM study of the differential inhibitory effects of the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG) against Gram-positive and Gram-negative bacteria. Food Microbiol. 2012, 29, 80–87. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant polyphenols as antioxidant and antibacterial agents for shelf-life extension of meat and meat products: Classification, structures, sources, and action mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Jones, R.N.; Messer, S.A.; Edmond, M.B.; Wenzel, R.P. National surveillance of nosocomial blood stream infection due to species of Candida other than Candida albicans: Frequency of occurrence and antifungal susceptibility in the SCOPE program. Diagn. Microbiol. Infect. Dis. 1998, 30, 121–129. [Google Scholar] [CrossRef]
- Chen, Y.-C. Stable susceptibility of Candida blood isolates to fluconazole despite increasing use during the past 10 years. J. Antimicrob. Chemother. 2003, 52, 71–77. [Google Scholar] [CrossRef][Green Version]
- Adejare, O.; Oduyebo, O.; Oladele, R.; Nwaokorie, F.; Ogunsola, F. In-vitro antifungal effect of Garcinia kola and garlic (Alliums sativum) on vaginal isolates of candida. Afr. J. Clin. Exp. Microbiol. 2013, 14, 140–145. [Google Scholar] [CrossRef][Green Version]
- Irene Cilla, L.M.; Beltrán, J.A.; Roncalés, P. Antioxidant effect of rosemary, borage, green tea, pu-erh tea and ascorbic acid on fresh pork sausages packaged in a modified atmosphere: Influence of the presence of sodium chloride. J. Sci. Food Agric. 2007, 86, 1298–1307. [Google Scholar] [CrossRef]
- Özer, C.O.; Seçen, S.M. Effects of quinoa flour on lipid and protein oxidation in raw and cooked beef burger during long term frozen storage. Food Sci. Technol. 2018, 2061, 1–7. [Google Scholar] [CrossRef]
- Abdelhakam, O.S.; Elsebaie, E.M.; Ghazi, A.K.; Gouda, M.S. Quality characteristics of beef hamburger enriched with red grape pomace powder during freezing storage. Slov. Vet. Res. 2019, 56, 333–340. [Google Scholar] [CrossRef]
- Hashimoto, T.A.; Caporaso, F.; Toto, C.; Were, L. Antioxidant capacity and sensory impact of coffee added to ground pork. Eur. Food Res. Technol. 2019, 245, 977–986. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.J.; Skowyra, M.; Almajano, M.P. Antioxidant properties of three aromatic herbs (rosemary, thyme and lavender) in oil-in-water emulsions. J. Am. Oil Chem. Soc. 2013, 90, 1559–1568. [Google Scholar] [CrossRef]
- Muela, E.; Sañudo, C.; Campo, M.M.; Medel, I.; Beltrán, J.A. Effect of freezing method and frozen storage duration on instrumental quality of lamb throughout display. Meat Sci. 2010, 84, 662–669. [Google Scholar] [CrossRef]
- Ouerfelli, M.; Bettaieb Ben Kâab, L.; Almajano, M.P. Radical scavenging and antioxidant activity of Anthyllis vulneraria leaves and flowers. Molecules 2018, 23, 1657. [Google Scholar] [CrossRef]
- De Oliveira Ferreira, N.S.; Rosset, M.; Lima, G.; Stuelp Campelo, P.M.; de Macedo, R.E.F. Effect of adding Brosimum gaudichaudii and Pyrostegia venusta hydroalcoholic extracts on the oxidative stability of beef burgers. LWT 2019, 108, 145–152. [Google Scholar] [CrossRef]
- Mancini, R.A.; Hunt, M.C. Current research in meat color. Meat Sci. 2005, 71, 100–121. [Google Scholar] [CrossRef]
- Domínguez, R.; Purriños, L.; Pérez-Santaescolástica, C.; Pateiro, M.; Barba, F.J.; Tomasevic, I.; Campagnol, P.C.B.; Lorenzo, J.M. Characterization of volatile compounds of dry-cured meat products using HS-SPME-GC/MS technique. Food Anal. Methods 2019. [Google Scholar] [CrossRef]
- Bosse Née Danz, R.; Wirth, M.; Konstanz, A.; Becker, T.; Weiss, J.; Gibis, M. Determination of volatile marker compounds in raw ham using headspace-trap gas chromatography. Food Chem. 2017, 219, 249–259. [Google Scholar] [CrossRef]
- Juntachote, T.; Berghofer, E.; Siebenhandl, S.; Bauer, F. The antioxidative properties of Holy basil and Galangal in cooked ground pork. Meat Sci. 2006, 72, 446–456. [Google Scholar] [CrossRef]
- Realini, C.E.; Guàrdia, M.D.; Díaz, I.; García-Regueiro, J.A.; Arnau, J. Effects of acerola fruit extract on sensory and shelf-life of salted beef patties from grinds differing in fatty acid composition. Meat Sci. 2014, 99, 18–24. [Google Scholar] [CrossRef]




| Samples | Extract Solvents | TPC (mg GAE/g DW) | RSA: DPPH Assay (mM TE/g DW) |
|---|---|---|---|
| A. indica | 80% EtOH | 47.47 ± 0.03 c | 0.37 ± 0.013 b |
| 80% MeOH | 107.41 ± 0.03 b | 0.72 ± 0.004 a | |
| Deionized water | 20.93 ± 0.08 a | 0.27 ± 0.002 c | |
| C. baccatum | 80% EtOH | 34.78 ± 0.03 a | 0.29 ± 0.002 b |
| 80% MeOH | 53.91 ± 0.02 b | 0.42 ± 0.001 a | |
| Deionized water | 20.23 ± 0.01 c | 0.17 ± 0.004 c |
| Inhibitory Zone (mm) | ||||||
|---|---|---|---|---|---|---|
| Microorganisms | Strains | A. indica | C. baccatum | penicillin | 80% MeOH | |
| Gram+ | S. aureus | ATCC 25423 | 19 | 22 | 24 | - |
| M. luteus | ATCC 4698 | 12 | 10 | 17 | - | |
| Listeria | ATCC 15313 | - | 10 | 15 | - | |
| B. cereus | ATCC 11778 | - | - | 10 | - | |
| Gram− | S. paratyphi | ATCC 9150 | 10 | - | 30 | - |
| E. coli | ATCC 25022 | 21 | - | 27 | - | |
| Trait | Day | CTR | S.C | A.I | C.B | A.I + C.B |
|---|---|---|---|---|---|---|
| Redness (a*) | 1 | 39.27 ± 2.37 aA | 50.12 ± 0.77 bA | 42.65 ± 2.16 cA | 48.36 ± 0.37 dA | 45.19 ± 1.26 cA |
| 2 | 33.32 ± 0.37 aB | 49.14 ± 1.32 bB | 39.43 ± 0.96 cB | 47.02 ± 0.05 bB | 38.77 ± 2.14 cB | |
| 3 | 30.15 ± 0.26 aC | 48.89 ± 1.52 bB | 38.73 ± 1.03 cC | 45.07 ± 0.33 dC | 38.23 ± 2.45 cB | |
| 4 | 27.65 ± 0.71 aD | 48.62 ± 0.51 bB | 38.31 ± 0.65 cC | 43.47 ± 0.23 dD | 37.45 ± 1.45 cC | |
| 5 | 27.12 ± 0.31 aD | 45.56 ± 0.21 bC | 38.04 ± 0.27 cC | 42.11 ± 0.15 bE | 37.01 ± 1.32 cC | |
| 7 | 25.88 ± 0.17 aE | 41.96 ± 0.23 bD | 36.01 ± 0.25 cD | 37.85 ± 1.22 cF | 36.04 ± 1.23 cD | |
| 8 | 25.24 ± 0.12 aE | 39.78 ± 1.02 bE | 34.29 ± 0.63 cE | 31.11 ± 0.47 dG | 35.77 ± 1.09 cE | |
| 9 | 23.99 ± 0,13 aF | 39.02 ± 1.01 bE | 31.12 ± 0.16 cF | 29.63 ± 0.23 dH | 35.19 ± 1.47 dE | |
| 10 | 23.06 ± 0.19 aF | 38.45 ± 0.98 bF | 28.29 ± 0.77 cG | 27.03 ± 0.11 cI | 30.44 ± 2.52 dF | |
| 11 | 22.89 ± 1.23 aG | 37.21 ± 1.00 bG | 26.64 ± 1.26 cH | 25.24 ± 1.14 cJ | 30.01 ± 0.99 dF | |
| Yellowness (b*) | 1 | 11.28 ± 0.11 aA | 15.25 ± 0.11 bA | 14.14 ± 0.67 bA | 10.55 ± 1.21 dA | 12.25 ± 1.01 cA |
| 2 | 10.56 ± 1.32 aB | 14.43 ± 1.14 bB | 13.49 ± 1.26 bB | 9.83 ± 2.01 dB | 12.04 ± 1.61 cA | |
| 3 | 9.89 ± 1.22 aC | 13.98 ± 1.22 bC | 13.32 ± 1.25 bB | 9.78 ± 0.55 aB | 11.78 ± 1.44 cB | |
| 4 | 9.54 ± 1.03 aC | 13.43 ± 0.11 bC | 12.87 ± 0.42 cC | 8.66 ± 1.06 dC | 11.59 ± 1.25 dB | |
| 5 | 9.43 ± 0.15 aC | 13.25 ± 0.25 bC | 12.44 ± 0.17 cC | 8.51 ± 0.09 dC | 11.23 ± 1.09 eB | |
| 7 | 7.45 ± 0.18 aD | 12.12 ± 0.13 bD | 11.01 ± 0.44 cD | 7.88 ± 0.15 aD | 10.76 ± 1.21 dC | |
| 8 | 5.33 ± 0.07 aE | 11.69 ± 1.09 bE | 10.82 ± 0.73 cE | 7.06 ± 0.45 dD | 10.66 ± 1.56 cC | |
| 9 | 5.24 ± 0.04 aE | 11.23 ± 0.33 bE | 10.12 ± 0.01 cE | 6.59 ± 0.16 dE | 10.09 ± 1.33 cC | |
| 10 | 5.17 ± 1.22 aE | 10.76 ± 0.26 bF | 9.72 ± 0.59 cF | 6.13 ± 0.19 dE | 9.44 ± 2.47 cD | |
| 11 | 5.02 ± 1.18 aE | 10.27 ± 0.11 bF | 9.42 ± 0.19 cF | 5.32 ± 1.69 aF | 9.21 ± 2.89 cD | |
| Lightness (L*) | 1 | 57.37 ± 0.74 aA | 70,79 ± 2.03 bA | 68,70 ± 2,24 cA | 65.16 ± 2.00 dA | 66.15 ± 2.20 dA |
| 2 | 56.32 ± 2.17 aB | 67.09 ± 3.02 bB | 66.46 ± 1,42 cB | 65.17 ± 2.02 dA | 66.01 ± 2.09 cA | |
| 3 | 56.01 ± 1.85 aB | 65.33 ± 1.23 bC | 64.09 ± 1.74 cC | 63.46 ± 1.46 dB | 65.19 ± 2.33 bB | |
| 4 | 55.84 ± 1.40 aC | 64.70 ± 3.53 bC | 62,61 ± 0,33 bD | 61.43 ± 1.52 cC | 61.23 ± 2.40 cC | |
| 5 | 54.98 ± 1.78 aD | 63.12 ± 2.10 bD | 58.12 ± 0.78 cE | 59.87 ± 1.96 cD | 54.54 ± 2.10 aD | |
| 7 | 53.13 ± 1.45 aE | 60.15 ± 1.64 bE | 56.45 ± 1.77 cF | 56.16 ± 1.69 cE | 52.33 ± 2.44 dE | |
| 8 | 52.41 ± 0.50 aF | 59.23 ± 0.23 bF | 54.15 ± 1.26 cG | 55.14 ± 1.62 cF | 51.46 ± 2.30 dF | |
| 9 | 51. 26 ± 1.45 aG | 56.54 ± 0.19 bG | 52. 49 ± 1.65 cH | 53.75 ± 1.36 dG | 50.73 ± 2.96 eG | |
| 10 | 47.46 ± 1.85 aH | 54.16 ± 0.15 bH | 50.46 ± 1.87 cI | 51.36 ± 1.64 dH | 49.16 ± 2.23 eH | |
| 11 | 43.23 ± 2.96 aI | 54.41± 2.65 bF | 49.22± 0.04 cJ | 48.04 ± 0.21 cI | 47.36 ± 2.08 dI |
| Aerobic Mesophilic Bacteria Presence | Refrigerated Storage Period (Days) | ||
|---|---|---|---|
| 0 | 5 | 11 | |
| CTR | − | + | + |
| S.C | − | − | − |
| A.I | − | − | − |
| C.B | − | − | + |
| A.I + C.B | − | − | − |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouerfelli, M.; Villasante, J.; Ben Kaâb, L.B.; Almajano, M. Effect of Neem (Azadirachta indica L.) on Lipid Oxidation in Raw Chilled Beef Patties. Antioxidants 2019, 8, 305. https://doi.org/10.3390/antiox8080305
Ouerfelli M, Villasante J, Ben Kaâb LB, Almajano M. Effect of Neem (Azadirachta indica L.) on Lipid Oxidation in Raw Chilled Beef Patties. Antioxidants. 2019; 8(8):305. https://doi.org/10.3390/antiox8080305
Chicago/Turabian StyleOuerfelli, Manel, Juliana Villasante, Leila Bettaieb Ben Kaâb, and MaríaPilar Almajano. 2019. "Effect of Neem (Azadirachta indica L.) on Lipid Oxidation in Raw Chilled Beef Patties" Antioxidants 8, no. 8: 305. https://doi.org/10.3390/antiox8080305
APA StyleOuerfelli, M., Villasante, J., Ben Kaâb, L. B., & Almajano, M. (2019). Effect of Neem (Azadirachta indica L.) on Lipid Oxidation in Raw Chilled Beef Patties. Antioxidants, 8(8), 305. https://doi.org/10.3390/antiox8080305