Protective Effects of Melon Extracts on Bone Strength, Mineralization, and Metabolism in Rats with Ovariectomy-Induced Osteoporosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Melon Extract and Animals
2.2. Surgical Procedures for Ovariectomy
2.3. Experimental Groups
2.4. Mechanical Test
2.5. Dual-Energy X-ray Absorptiometry
2.6. Blood Analysis
2.7. Statistical Analysis
3. Results
3.1. Measurement of Body Weight and Daily Food and Water Intake
3.2. Evaluation of Bone Strength
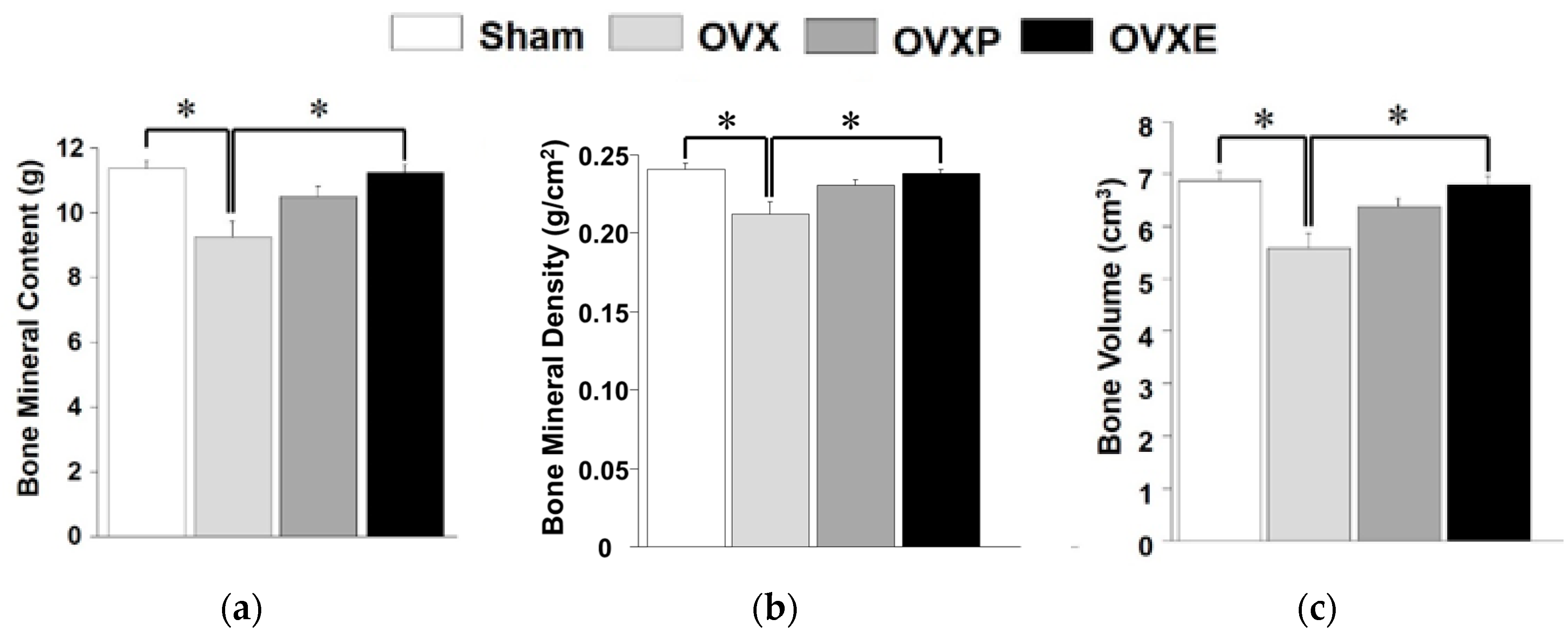
3.3. Bone Mineralization
3.4. Blood Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guglielmi, G.; Muscarella, S.; Bazzocchi, A. Integrated imaging approach to osteoporosis: State-of-the-art review and update. Radiographics 2011, 31, 1343–1364. [Google Scholar] [CrossRef] [PubMed]
- Sözen, T.; Özışık, L.; Başaran, L.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef]
- Anonymous. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, M.B.; Buckwalter, J.; Saltzman, C. Osteoporosis: The increasing role of the orthopaedist. Iowa Orthop. J. 1999, 19, 43–52. [Google Scholar] [PubMed]
- Golds, G.; Houdek, D.; Arnason, T. Male hypogonadism and osteoporosis: The effects, clinical consequences, and treatment of testosterone deficiency in bone health. Int. J. Endocrinol. 2017, 2017, 4602129. [Google Scholar] [CrossRef] [PubMed]
- Grodstein, F.; Stampfer, M.J.; Colditz, G.A.; Willett, W.C.; Manson, J.E.; Joffe, M.; Rosner, B.; Fuchs, C.; Hankinson, S.E.; Hunter, D.J.; et al. Postmenopausal hormone therapy and mortality. N. Engl. J. Med. 1997, 336, 1769–1775. [Google Scholar] [CrossRef] [PubMed]
- Maraka, S.; Kennel, K.A. Bisphosphonates for the prevention and treatment of osteoporosis. BMJ 2015, 351, h3783. [Google Scholar] [CrossRef] [Green Version]
- Watts, N.B.; Adler, R.A.; Bilezikian, J.P.; Drake, M.T.; Eastell, R.; Orwoll, E.S.; Finkelstein, J.S. Osteoporosis in men: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2012, 97, 1802–1822. [Google Scholar] [CrossRef] [PubMed]
- Austermann, K.; Baecker, N.; Stehle, P.; Heer, M. Putative effects of nutritive polyphenols on bone metabolism in vivo-evidence from human studies. Nutrients 2019, 11, 871. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Choi, B.R.; Kim, S.H.; Yi, H.Y.; Park, H.R.; Song, C.H.; Ku, S.K.; Lee, Y.J. Selection of the optimal herbal compositions of red clover and pomegranate according to their protective effect against climacteric symptoms in ovariectomized mice. Nutrients 2016, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, H.; Park, J.; Kim, H.J.; Kim, K.R.; Son, S.H.; Park, K.K.; Chung, W.Y. Artemisia annua extract prevents ovariectomy-induced bone loss by blocking receptor activator of nuclear factor kappa-B ligand-induced differentiation of osteoclasts. Sci. Rep. 2017, 7, 17332. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, Y.; Xiang, L.; Wang, Z.; Xiao, G.G.; Hu, J. Effect of curcumin on the diversity of gut microbiota in ovariectomized rats. Nutrients 2017, 9, 1146. [Google Scholar] [CrossRef] [PubMed]
- Jeon, I.S.; Kim, W.D.; Choi, J.K. Effect of melon extracts on the cellular signaling of C2C12 osteoblast cells. Chungbuk Med. J. 2016, 26, 71–78. [Google Scholar]
- Ghebretinsae, A.G.; Thulin, M.; Barber, J.C. Relationships of cucumbers and melons unraveled: Molecular phylogenetics of Cucumis and related genera (Benincaseae, Cucurbitaceae). Am. J. Bot. 2007, 94, 1256–1266. [Google Scholar] [CrossRef]
- Garcia-Mas, J.; Benjak, A.; Sanseverino, W.; Bourgeois, M.; Mir, G.; González, V.M.; Hénaff, E.; Câmara, F.; Cozzuto, L.; Lowy, E.; et al. The genome of melon (Cucumis melo L.). Proc. Natl. Acad. Sci. USA 2012, 109, 11872–11877. [Google Scholar] [CrossRef]
- Vouldoukis, I.; Lacan, D.; Kamate, C.; Coste, P.; Calenda, A.; Mazier, D.; Conti, M.; Dugas, B. Antioxidant and anti-inflammatory properties of a Cucumis melo LC. extract rich in superoxide dismutase activity. J. Ethnopharmacol. 2004, 94, 67–75. [Google Scholar] [CrossRef]
- Dhiman, K.; Gupta, A.; Sharma, D.K.; Gill, N.S.; Goyal, A. A review on the medicinally important plants of the family Cucurbitaceae. Asian J. Clin. Nutr. 2012, 4, 16–26. [Google Scholar] [CrossRef]
- Vella, F.M.; Cautela, D.; Laratta, B. Characterization of polyphenolic compounds in cantaloupe melon by-products. Foods 2019, 8, 196. [Google Scholar] [CrossRef]
- Zeb, A. Phenolic profile and antioxidant activity of melon (Cucumis melo L.) seeds from Pakistan. Foods 2016, 5, 67. [Google Scholar] [CrossRef]
- Heiss, C.; Kern, S.; Malhan, D.; Böcker, W.; Engelhardt, M.; Daghma, D.E.S.; Stoetzel, S.; Schmitt, J.; Ivo, M.; Kauschke, V.; et al. A new clinically relevant t-score standard to interpret bone status in a sheep model. Med. Sci. Monit. Basic Res. 2017, 23, 326–335. [Google Scholar] [CrossRef]
- Devareddy, L.; Hooshmand, S.; Collins, J.K.; Lucas, E.A.; Chai, S.C.; Arjmandi, B.H. Blueberry prevents bone loss in ovariectomized rat model of postmenopausal osteoporosis. J. Nutr. Biochem. 2008, 19, 694–699. [Google Scholar] [CrossRef]
- Kalu, D.N.; Salerno, E.; Liu, C.C.; Ferarro, F.; Arjmandi, B.N.; Salih, M.A. Ovariectomy-induced bone loss and the hematopoietic system. Bone Miner. 1993, 23, 145–161. [Google Scholar] [CrossRef]
- Sigrist, I.M.; Gerhardt, C.; Alini, M.; Schneider, E.; Egermann, M. The long-term effects of ovariectomy on bone metabolism in sheep. J. Bone Miner. Metab. 2007, 25, 28–35. [Google Scholar] [CrossRef]
- Qi, M.; Zhang, L.; Ma, Y.; Shuai, Y.; Li, L.; Luo, K.; Liu, W.; Jin, Y. Autophagy maintains the function of bone marrow mesenchymal stem cells to prevent estrogen deficiency-induced osteoporosis. Theranostics 2017, 7, 4498–4516. [Google Scholar] [CrossRef]
- Wang, F.S.; Wu, R.W.; Lain, W.S.; Tsai, T.C.; Chen, Y.S.; Sun, Y.C.; Ke, H.J.; Li, J.C.; Hwang, J.; Ko, J.Y. Sclerostin vaccination mitigates estrogen deficiency induction of bone mass loss and microstructure deterioration. Bone 2018, 112, 24–34. [Google Scholar] [CrossRef]
- Abe, T.; Chow, J.; Lean, J.M.; Chambers, T.J. Estrogen does not restore bone lost after ovariectomy in the rat. J. Bone Miner. Res. 1993, 8, 831–838. [Google Scholar] [CrossRef]
- Noh, D.; Lim, Y.; Lee, H.; Kim, H.; Kwon, O. Soybean-hop alleviates estrogen deficiency-related bone loss and metabolic dysfunction in ovariectomized rats fed a high-fat diet. Molecules 2018, 23, 1205. [Google Scholar] [CrossRef]
- Kim, G.H.; Baek, H.K.; Lee, J.S.; Kim, S.J.; Yi, S.S. Chronic oral administration of Tenebrio molitor extract exhibits inhibitory effect on glucocorticoid receptor overexpression in the hippocampus of ovariectomy-induced estrogen deficient mice. J. Food Sci. 2019, 84, 687–694. [Google Scholar] [CrossRef]
- Shahnazari, M.; Martin, B.R.; Legette, L.L.; Lachcik, P.J.; Welch, J.; Weaver, C.M. Diet calcium level but not calcium supplement particle size affects bone density and mechanical properties in ovariectomized rats. J. Nutr. 2009, 139, 1308–1314. [Google Scholar] [CrossRef]
- Van Pelt, R.E.; Gavin, K.M.; Kohrt, W.M. Regulation of body composition and bioenergetics by estrogens. Endocrinol. Metab. Clin. North Am. 2015, 44, 663–676. [Google Scholar] [CrossRef]
- Fan, J.Z.; Wang, Y.; Meng, Y.; Li, G.W.; Chang, S.X.; Nian, H.; Liang, Y.J. Panax notoginseng saponins mitigate ovariectomy-induced bone loss and inhibit marrow adiposity in rats. Menopause 2015, 22, 1343–1350. [Google Scholar] [CrossRef]
- Fonseca, H.; Moreira-Gonçalves, D.; Coriolano, H.J.; Duarte, J.A. Bone quality: The determinants of bone strength and fragility. Sports Med. 2014, 44, 37–53. [Google Scholar] [CrossRef]
- Tanaka, S.M.; Yorozuya, Y.; Takatsu, D. Random electromyostimulation promotes osteogenesis and the mechanical properties of rat bones. Ann. Biomed. Eng. 2017, 45, 2837–2846. [Google Scholar] [CrossRef]
- Shaban, N.Z.; Talaat, I.M.; Elrashidy, F.H.; Hegazy, A.Y.; Sultan, A.S. Therapeutic role of Punica granatum (pomegranate) seed oil extract on bone turnover and resorption induced in ovariectomized rats. J. Nutr. Health Aging 2017, 21, 1299–1306. [Google Scholar] [CrossRef]
- Levin, V.A.; Jiang, X.; Kagan, R. Estrogen therapy for osteoporosis in the modern era. Osteoporos. Int. 2018, 29, 1049–1055. [Google Scholar] [CrossRef]
- Fakkert, I.E.; Teixeira, N.; Abma, E.M.; Slart, R.; Mourits, M.; de Bock, G.H. Bone mineral density and fractures after surgical menopause: Systematic review and meta-analysis. BJOG 2017, 124, 1525–1535. [Google Scholar] [CrossRef]
- Keaveny, T.M.; Kopperdahl, D.L.; Melton, L.J., 3rd; Hoffmann, P.F.; Amin, S.; Riggs, B.L.; Khosla, S. Age-dependence of femoral strength in white women and men. J. Bone Miner. Res. 2010, 25, 994–1001. [Google Scholar] [CrossRef]
- Kanis, J.A.; McCloskey, E.V.; Harvey, N.C.; Johansson, H.; Leslie, W.D. Thresholds and the diagnosis of osteoporosis. J. Bone Miner. Res. 2015, 30, 1747–1753. [Google Scholar] [CrossRef]
- Bell, S.; Ajami, E.; Davies, J.E. An improved mechanical testing method to assess bone-implant anchorage. J. Vis. Exp. 2014, 84, e51221. [Google Scholar] [CrossRef]
- Kalaiselvi, V.S.; Prabhu, K.; Ramesh, M.; Venkatesan, V. The association of serum osteocalcin with the bone mineral density in post menopausal women. J. Clin. Diagn. Res. 2013, 7, 814–816. [Google Scholar]
- Zheng, H.; Qi, S.; Chen, C. Salidroside improves bone histomorphology and prevents bone loss in ovariectomized diabetic rats by upregulating the OPG/RANKL ratio. Molecules 2018, 23, 2398. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Kim, H.Y.; Woo, K.S.; Hwang, I.G.; Lee, Y.R.; Jeong, H.S. Effects of heat treatments on the antioxidant activities of fruits and vegetables. Korean J. Food Sci. Technol. 2008, 40, 166–170. [Google Scholar]
- Turkmen, N.; Sari, F.; Velioglu, Y.S. The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chem. 2005, 93, 713–718. [Google Scholar] [CrossRef]
- Li, H.; Huang, C.; Zhu, J.; Gao, K.; Fang, J.; Li, H. Lutein suppresses oxidative stress and inflammation by Nrf2 activation in an osteoporosis rat model. Med. Sci. Monit. 2018, 24, 5071–5075. [Google Scholar] [CrossRef]
- Abdollahi, M.; Larijani, B.; Rahimi, R.; Salari, P. Role of oxidative stress in osteoporosis. Therapy 2005, 2, 787–796. [Google Scholar] [CrossRef]
- Tan, S.P.; Stathopoulos, C.; Parks, S.; Roach, P. An Optimised aqueous extract of phenolic compounds from bitter melon with high antioxidant capacity. Antioxidants 2014, 3, 814–829. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Lee, S.-H.; Song, S.-J.; Kim, W.H.; Song, E.-S.; Lee, J.-C.; Lee, S.-J.; Han, D.-W.; Lee, J.-H. Protective Effects of Melon Extracts on Bone Strength, Mineralization, and Metabolism in Rats with Ovariectomy-Induced Osteoporosis. Antioxidants 2019, 8, 306. https://doi.org/10.3390/antiox8080306
Kim B, Lee S-H, Song S-J, Kim WH, Song E-S, Lee J-C, Lee S-J, Han D-W, Lee J-H. Protective Effects of Melon Extracts on Bone Strength, Mineralization, and Metabolism in Rats with Ovariectomy-Induced Osteoporosis. Antioxidants. 2019; 8(8):306. https://doi.org/10.3390/antiox8080306
Chicago/Turabian StyleKim, Bongju, Sung-Ho Lee, Su-Jin Song, Won Hyeon Kim, Eun-Sung Song, Jae-Chang Lee, Sung-Jae Lee, Dong-Wook Han, and Jong-Ho Lee. 2019. "Protective Effects of Melon Extracts on Bone Strength, Mineralization, and Metabolism in Rats with Ovariectomy-Induced Osteoporosis" Antioxidants 8, no. 8: 306. https://doi.org/10.3390/antiox8080306





