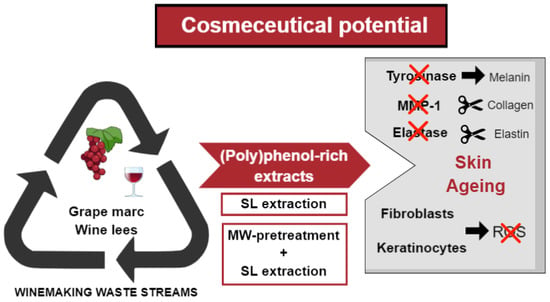
Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Samples
2.3. Methods
2.3.1. Phytochemical Characterization
Total Phenolic Content (TPC)
Total Anthocyanin Content (TAC)
High Performance Liquid Chromatography–Mass Spectrometry (HPLC-DAD-MS/MS)
2.3.2. Antioxidant Activity
2.3.3. Enzymatic Assays
Inhibition of Tyrosinase
Inhibition of Elastase
Inhibition of MMP-1
2.3.4. Cell-based Assays
Cell Culture
Cytotoxicity Evaluation
Cellular Antioxidant Activity
Protection against Oxidant-Induced Cytotoxicity
2.3.5. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical and Antioxidant Activity Characterization
3.1.1. Total Phenolic Content (TPC) and Total Anthocyanin Content (TAC)
3.1.2. Identification of Compounds by HPLC-MS/MS Analysis
3.1.3. Antioxidant Activity Characterization
3.2. Screening of the Cosmetic Potential of Wine Lees and Grape Marc Extracts
3.2.1. Anti-Hyperpigmentation Activity
Inhibition of Tyrosinase
3.2.2. Anti-ageing Activity
Inhibition of Elastase
Inhibition of MMP-1
3.2.3. Cellular Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bettini, O.; Sloop, C. Eu-27, Wine Annual: Wine Annual Report and Statistics 2015. USDA Foreign Agricultural Service—Global Agricultural Information Network (GAIN), 2015; 1–28, GAIN Report Number: IT1512. [Google Scholar]
- Ruggieri, L.; Cadena, E.; Martínez-Blanco, J.; Gasol, C.M.; Rieradevall, J.; Gabarrell, X.; Gea, T.; Sort, X.; Sánchez, A. Recovery of organic wastes in the Spanish wine industry. Technical, economic and environmental analyses of the composting process. J. Clean. Prod. 2009, 17, 830–838. [Google Scholar] [CrossRef] [Green Version]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, H. Skin aging and dry skin. J. Dermatol. 2004, 31, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Gilchrest, B.A.; Blog, F.B.; Szabo, G. Effects of Aging and Chronic Sun Exposure on Melanocytes in Human Skin. J. Investig. Dermatol. 1979, 73, 141–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakur, R.; Batheja, P.; Kaushik, D.; Michniak, B. Structural and Biochemical Changes in Aging Skin and Their Impact on Skin Permeability Barrier. In Skin Aging Handbook: An Integrated Approach to Biochemistry and Product Development; Dayan, N., Ed.; William Andrew: Norwich, NY, USA, 2008; pp. 55–90. [Google Scholar]
- Sárdy, M. Role of Matrix Metalloproteinases in Skin Ageing. Connect. Tissue Res. 2009, 50, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.S.; Hili, P.; Naughton, D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Wittenauer, J.; Mäckle, S.; Sußmann, D.; Schweiggert-Weisz, U.; Carle, R. Inhibitory effects of polyphenols from grape pomace extract on collagenase and elastase activity. Fitoterapia 2015, 101, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative Stress and Antioxidant Defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Yaar, M.; Gilchrest, B. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csörgő, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Chen, H.J.; Huang, J.P.; Lee, P.C.; Tsai, C.R.; Hsu, T.F.; Huang, W.Y. Kinetics of Tyrosinase Inhibitory Activity Using Vitis vinifera Leaf Extracts. BioMed Res. Int. 2017, 2017, 5232680-5. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díez, R.; Matos, M.; Rodrigues, L.; Bronze, M.R.; Rodríguez-Rojo, S.; Cocero, M.; Matias, A. Microwave and ultrasound pre-treatments to enhance anthocyanins extraction from different wine lees. Food Chem. 2019, 272, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Perestrelo, R.; Silva, C.; Pereira, J.; Câmara, J.S. Wines: Madeira, Port and Sherry Fortified Wines—The Sui Generis and Notable Peculiarities. Major Differences and Chemical Patterns. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2015; pp. 534–555. [Google Scholar]
- Álvarez, A.; Poejo, J.; Matias, A.A.; Duarte, C.M.; Cocero, M.J.; Mato, R.B. Microwave pretreatment to improve extraction efficiency and polyphenol extract richness from grape pomace. Effect on antioxidant bioactivity. Food Bioprod. Process. 2017, 106, 162–170. [Google Scholar] [CrossRef]
- Sólyom, K.; Solá, R.; Cocero, M.J.; Mato, R.B.; Alonso, M.J.C. Thermal degradation of grape marc polyphenols. Food Chem. 2014, 159, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1998, 299, 152–178. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the pH differential method: collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Moore, J.; Yin, J.J.; Yu, L. Novel Fluorometric Assay for Hydroxyl Radical Scavenging Capacity (HOSC) Estimation. J. Agric. Food Chem. 2006, 54, 617–626. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Flanagan, J.; Deemer, E.K.; Prior, R.L.; Huang, D. Novel Fluorometric Assay for Hydroxyl Radical Prevention Capacity Using Fluorescein as the Probe. J. Agric. Food Chem. 2002, 50, 2772–2777. [Google Scholar] [CrossRef] [PubMed]
- Bolling, B.W.; Chen, Y.Y.; Kamil, A.G.; Chen, C.Y.O. Assay Dilution Factors Confound Measures of Total Antioxidant Capacity in Polyphenol-Rich Juices. J. Food Sci. 2012, 77, H69–H75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, E.; Lim, Y.; Wong, L.; Lianto, F.; Wong, S.; Lim, K.; Joe, C.; Lim, T.; Chan, E.W.C. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Shimokawa, K.I.; Katayama, M.; Matsuda, Y.; Takahashi, H.; Hara, I.; Sato, H. Complexes of gelatinases and tissue inhibitor of metalloproteinases in human seminal plasma. J. Androl. 2003, 24, 73–77. [Google Scholar] [PubMed]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assessing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rojo, S.; Visentin, A.; Maestri, D.; Cocero, M. Assisted extraction of rosemary antioxidants with green solvents. J. Food Eng. 2012, 109, 98–103. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Weller, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Fleuriet, A.; Macheix, J. Phenolic Acids in Fruits and Vegetables. In Flavonoids in Health and Disease, 2nd ed.; Rice-Evans, C.A., Packer, L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2003; pp. 1–41. [Google Scholar]
- Peralbo-Molina, Á.; Luque deCastro, M.D. Potential of residues from the Mediterranean agriculture and agrifood industry. Trends Food Sci. Technol. 2013, 32, 16–24. [Google Scholar] [CrossRef]
- Databank PhytoHub. Available online: www.phytohub.eu (accessed on 31 May 2018).
- Databank Phenol-Explorer. Available online: www.phenol-explorer.eu (accessed on 31 May 2018).
- Donato, P.; Rigano, F.; Cacciola, F.; Schure, M.; Farnetti, S.; Russo, M.; Dugo, P.; Mondello, L. Comprehensive two-dimensional liquid chromatography–tandem mass spectrometry for the simultaneous determination of wine polyphenols and target contaminants. J. Chromatogr. A 2016, 1458, 54–62. [Google Scholar] [CrossRef]
- Blanco-Vega, D.; López-Bellido, F.J.; Alía-Robledo, J.M.; Hermosín-Gutiérrez, I. HPLC–DAD–ESI-MS/MS Characterization of Pyranoanthocyanins Pigments Formed in Model Wine. J. Agric. Food Chem. 2011, 59, 9523–9531. [Google Scholar] [CrossRef]
- De La Torre, M.P.D.; De Castro, M.D.L.; Priego-Capote, F. Characterization and Comparison of Wine Lees by Liquid Chromatography–Mass Spectrometry in High-Resolution Mode. J. Agric. Food Chem. 2015, 63, 1116–1125. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, M.; Quast, P.; Von Baer, D.; Winterhalter, P. Vitisin A Content in Chilean Wines from Vitis viniferaCv. Cabernet Sauvignon and Contribution to the Color of Aged Red Wines. J. Agric. Food Chem. 2003, 51, 6261–6267. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Prior, R.L. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef] [PubMed]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.; Matias, A.; Bronze, M. Phenolic characterization of aging wine lees: Correlation with antioxidant activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da Silva, M.A.; Salas, E.; Oliveira, J.; Teixeira, N.; De Freitas, V. Screening of Anthocyanins and Anthocyanin-Derived Pigments in Red Wine Grape Pomace Using LC-DAD/MS and MALDI-TOF Techniques. J. Agric. Food Chem. 2015, 63, 7636–7644. [Google Scholar]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Guo, M.; Perez, C.; Wei, Y.; Rapoza, E.; Su, G.; Bou-Abdallah, F.; Chasteen, N.D. Iron-binding properties of plant phenolics and cranberry’s bio-effects†. Dalton Trans. 2007, 43, 4951–4961. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Biswas, R.; Sharma, A.; Banerjee, S.; Biswas, S.; Katiyar, C. Validation of medicinal herbs for anti-tyrosinase potential. J. Herb. Med. 2018, 14, 1–16. [Google Scholar] [CrossRef]
- Chang, T.S. An Updated Review of Tyrosinase Inhibitors. Int. J. Mol. Sci. 2009, 10, 2440–2475. [Google Scholar] [CrossRef] [Green Version]
- Ya, W.; Chun-Meng, Z.; Tao, G.; Yi-Lin, Z.; Ping, Z. Preliminary screening of 44 plant extracts for anti-tyrosinase and antioxidant activities. Pak. J. Pharm. Sci. 2015, 28, 1737–1744. [Google Scholar]
- Brás, N.F.; Gonçalves, R.; Mateus, N.; Fernandes, P.A.; Ramos, M.J.; De Freitas, V. Inhibition of Pancreatic Elastase by Polyphenolic Compounds. J. Agric. Food Chem. 2010, 58, 10668–10676. [Google Scholar] [CrossRef] [PubMed]
- Sartor, L.; Pezzato, E.; Dell’Aica, I.; Caniato, R.; Biggin, S.; Garbisa, S. Inhibition of matrix-proteases by polyphenols: Chemical insights for anti-inflammatory and anti-invasion drug design. Biochem. Pharmacol. 2002, 64, 229–237. [Google Scholar] [CrossRef]
- Nar, H.; Werle, K.; Bauer, M.M.; Dollinger, H.; Jung, B. Crystal structure of human macrophage elastase (MMP-12) in complex with a hydroxamic acid inhibitor. J. Mol. Biol. 2001, 312, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Matias, A.; Nunes, S.L.; Poejo, J.; Mecha, E.; Serra, A.T.; Madeira, P.J.A.; Bronze, M.R.; Duarte, C.M.M.; Bronze, M. Antioxidant and anti-inflammatory activity of a flavonoid-rich concentrate recovered from Opuntia ficus-indica juice. Food Funct. 2014, 5, 3269–3280. [Google Scholar] [CrossRef] [PubMed]
- Serra, A.T.; Duarte, R.O.; Bronze, M.R.; Duarte, C.M. Identification of bioactive response in traditional cherries from Portugal. Food Chem. 2011, 125, 318–325. [Google Scholar] [CrossRef]
- Tammela, P.; Laitinen, L.; Galkin, A.; Wennberg, T.; Heczko, R.; Vuorela, H.; Slotte, J.; Vuorela, P. Permeability characteristics and membrane affinity of flavonoids and alkyl gallates in Caco-2 cells and in phospholipid vesicles. Arch. Biochem. Biophys. 2004, 425, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Svobodová, A.; Rambousková, J.; Walterová, D.; Vostalová, J. Bilberry extract reduces UVA-induced oxidative stress in HaCaT keratinocytes: A pilot study. BioFactors 2008, 33, 249–266. [Google Scholar] [CrossRef] [PubMed]
- Varasteh-kojourian, M.; Abrishamchi, P.; Matin, M.M.; Asili, J. Antioxidant, cytotoxic and DNA protective properties of Achillea eriophora DC. and Achillea biebersteinii Afan. extracts: A comparative study. Avicenna J. Phytomed. 2017, 7, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, V.; Cornelius, C.; Salinaro, A.T.; Cambria, M.T.; Locascio, M.; Rienzo, L.; Condorelli, D.F.; Mancuso, C.; De Lorenzo, A.; Calabrese, E. The Hormetic Role of Dietary Antioxidants in Free Radical-Related Diseases. Curr. Pharm. Des. 2010, 16, 877–883. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous Antioxidants—Double-Edged Swords in Cellular Redox State: Health Beneficial Effects at Physiologic Doses versus Deleterious Effects at High Doses. Oxid. Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]




| Phytochemical Composition | Antioxidant Activity | |||||
|---|---|---|---|---|---|---|
| Extract | TPC (mg GAE/g Extract) | TAC (mg malv-3-O-gl/g Extract) | ORAC (µmol TE/g Extract) | HOSC (µmol TE/g Extract) | HORAC (µmol CAE/g Extract) | |
| Wine lees | RW | 237.4 ± 7.7 a | 28.6 ± 2.4 a | 3167 ± 189 a | 3680 ± 163 a | 1932 ± 130 a |
| MW RW | 266.0 ± 5.6 b | 29.5 ± 2.3 a | 3500 ± 223 a | 4776 ± 268 b | 2625 ± 135 b | |
| P | 64.0 ± 2.7 c | 6.1 ± 0.7 b | 451 ± 26 b | 837 ± 49 c | 458 ± 29 c | |
| MW P | 114.5 ± 4.7 d | 11.5 ± 1.0 c | 716 ± 41 c | 1285 ± 95 d | 776 ± 49 d | |
| Grape marc | GM | 83.9 ± 2.0 e | 1.7 ± 0.1 d | 481 ± 30 b | 746 ± 49 c | 305 ± 28 e |
| MW80 GM | 45.9 ± 1.5 f | 2.7 ± 0.3 e | 448 ± 31 b | 441 ± 34 e | 198 ± 19 f | |
| Retention Time (min) | m/z | Ionic Species | Fragment Ions | Putative Identification | Phenolic Subclass | Grape Marc | Red Wine Lees | Port Wine Lees |
|---|---|---|---|---|---|---|---|---|
| 23.2 | 169 | [M−H]− | 125 | Gallic acid | Phenolic acid | ✓ | ✓ | ✓ |
| 26.6 | 616 | [M−H]− | 466, 307, 272,167, 134 | 2-S-glutathionylcaftaric acid | Phenolic acid | ✓ | ✓ | ✓ |
| 27.7 | 577 | [M−H]− | 289 | Procyanidin dimer | Flavanol | ✓ | ✓ | ✓ |
| 28.4 | 311 | [M−H]− | 179, 149, 135 | Caftaric acid | Phenolic acid | ✓ | ✓ | ✓ |
| 28.9 | 865 | [M−H]− | 577, 289 | Procyanidin trimer | Flavanol | ✓ | ✓ | ✓ |
| 29.8 | 289 | [M−H]− | 245 | Catechin | Flavanol | ✓ | ✓ | ✓ |
| 30.1 | 465 | [M−H]+ | 303 | Delphinidin-3-O-glucoside | Anthocyanin | ✓ | ✓ | ✓ |
| 30.2 | 577 | [M−H]− | 289, 175, 129 | Procyanidin dimer | Flavanol | ✓ | ✓ | ✓ |
| 30.2 | 463 | [M−H]− | 300 | Quercetin-3-O-glucoside | Flavonol | ✓ | ✓ | ✓ |
| 31.1 | 295 | [M−H]− | 163, 149, 119 | Coutaric acid | Phenolic acid | ✓ | ✓ | ✓ |
| 31.5 | 289 | [M−H]− | 245 | Epicatechin | Flavanol | ✓ | ✓ | ✓ |
| 31.9 | 449 | [M−H]+ | 287 | Cyanidin-3-O-glucoside | Anthocyanin | ✓ | ✓ | ✓ |
| 32.1 | 561 | [M−H]+ | 399 | Vitisin A | Pyranoanthocyanin | ✓ | ✓ | ✓ |
| 32.2 | 479 | [M−H]+ | 317 | Petunidin-3-O-glucoside | Anthocyanin | ✓ | ✓ | ✓ |
| 33.0 | 479 | [M−H]− | 316 | Myricetin-3-O-glucoside | Flavonol | ✓ | ✓ | ✓ |
| 34.0 | 493 | [M−H]+ | 331 | Malvidin-3-O-glucoside | Anthocyanin | ✓ | ✓ | ✓ |
| 34.1 | 463 | [M−H]+ | 301 | Peonidin-3-O-glucoside | Anthocyanin | ✓ | ✓ | ✓ |
| 34.9 | 477 | [M−H]− | 301 | Quercetin-3-O-glucuronide | Flavonol | ✓ | ✓ | ✓ |
| 34.9 | 507 | [M−H]+ | 303 | Delphinidin-3-O-6”-p-acetylglucoside | Anthocyanin | ✓ | ✓ | ✓ |
| 36.4 | 707 | [M−H]+ | 399 | 10-carboxypyranomalvidin-3-6”-p-coumaroylglucoside | Pyranoanthocyanin | ✓ | ✓ | ✓ |
| 36.9 | 521 | [M−H]+ | 317 | Petunidin-3-O-6”-p-acetylglucoside | Anthocyanin | ✗ | ✓ | ✓ |
| 36.9 | 507 | [M−H]− | 345 | Syringetin-3-O-glucoside | Flavonol | ✓ | ✓ | ✓ |
| 37.1 | 491 | [M−H]+ | 287 | Cyanidin-3-O-6”-p-acetylglucoside | Anthocyanin | ✗ | ✗ | ✓ |
| 39.6 | 317 | [M−H]− | 179, 151, 137 | Myricetin | Flavonol | ✓ | ✓ | ✓ |
| 39.2 | 535 | [M−H]+ | 331 | Malvidin-3-O-6”-p-acetylglucoside | Anthocyanin | ✗ | ✓ | ✓ |
| 39.5 | 505 | [M−H]+ | 301 | Peonidin-3-O-6”-p-acetylglucoside | Anthocyanin | ✗ | ✓ | ✓ |
| 39.8 | 611 | [M−H]+ | 303 | Delphinidin-3-O-6”-p-coumaroylglucoside | Anthocyanin | ✓ | ✓ | ✓ |
| 41.9 | 595 | [M−H]+ | 287 | Cyanidin-3-O-6”-p-coumaroylglucoside | Anthocyanin | ✓ | ✓ | ✓ |
| 41.9 | 625 | [M−H]+ | 317 | Petunidin-3-O-6”-p-coumaroylglucoside | Anthocyanin | ✓ | ✓ | ✓ |
| 43.9 | 301 | [M−H]− | 179,151, 121, 107 | Quercetin | Flavonol | ✓ | ✓ | ✓ |
| 43.8 | 639 | [M−H]+ | 331 | Malvidin-3-O-6”-p-coumaroylglucoside | Anthocyanin | ✓ | ✓ | ✓ |
| 44.3 | 609 | [M−H]+ | 301 | Peonidin-3-O-6”-p-coumaroylglucoside | Anthocyanin | ✓ | ✓ | ✓ |
| 48.2 | 285 | [M−H]− | 125 | Kaempferol | Flavonol | ✓ | ✓ | ✓ |
| 49.2 | 315 | [M−H]− | 300, 247, 215, 165, 141 | Rhamnetin | Flavonol | ✗ | ✓ | ✓ |
| Anti-hyperpigmentation Activity | Anti-ageing Activity | |||
|---|---|---|---|---|
| Extract | IC50 Tyrosinase (mg Extract/mL) | IC50 Elastase (mg Extract/mL) | IC50 MMP-1 (mg Extract/mL) | |
| Wine lees | RW | 0.20 ± 0.01 a | 0.17 ± 0.01 a | 0.22 ± 0.01 a |
| MW RW | 0.14 ± 0.01 a | 0.11 ± 0.00 a | 0.21 ± 0.01 a | |
| P | 1.06 ± 0.07 b | 1.92 ± 0.09 b | 1.25 ± 0.03 b | |
| MW P | 0.62 ± 0.04 a,b | 0.83 ± 0.04 c | 0.65 ± 0.03 a,c | |
| Grape marc | GM | 4.03 ± 0.14 c | 0.87 ± 0.03 c | 1.08 ± 0.08 b,c |
| MW80 GM | 4.00 ± 0.14 c | 3.43 ± 0.11 d | 1.16 ± 0.06 b | |
| Phytochemical Composition | Antioxidant Activity | Anti-hyperpigmentation Activity | Anti-ageing Activity | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Extract | TPC | TAC | ORAC | HOSC | HORAC | Tyrosinase | Elastase | MMP-1 | |
| Wine lees | RW | +++ | ++++ | ++++ | ++++ | ++++ | +++++ | ++++ | +++ |
| MW RW | ++++ | +++++ | +++++ | +++++ | +++++ | +++++ | +++++ | ++++ | |
| P | + | + | - | - | + | +++ | + | + | |
| MW P | + | + | + | + | ++ | +++ | ++ | ++ | |
| Grape marc | GM | + | - | - | - | - | + | ++ | + |
| MW80 GM | - | - | - | - | - | + | - | + | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matos, M.S.; Romero-Díez, R.; Álvarez, A.; Bronze, M.R.; Rodríguez-Rojo, S.; Mato, R.B.; Cocero, M.J.; Matias, A.A. Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants 2019, 8, 355. https://doi.org/10.3390/antiox8090355
Matos MS, Romero-Díez R, Álvarez A, Bronze MR, Rodríguez-Rojo S, Mato RB, Cocero MJ, Matias AA. Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants. 2019; 8(9):355. https://doi.org/10.3390/antiox8090355
Chicago/Turabian StyleMatos, Melanie S., Rut Romero-Díez, Ana Álvarez, M. R. Bronze, Soraya Rodríguez-Rojo, Rafael B. Mato, M. J. Cocero, and Ana A. Matias. 2019. "Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential" Antioxidants 8, no. 9: 355. https://doi.org/10.3390/antiox8090355
APA StyleMatos, M. S., Romero-Díez, R., Álvarez, A., Bronze, M. R., Rodríguez-Rojo, S., Mato, R. B., Cocero, M. J., & Matias, A. A. (2019). Polyphenol-Rich Extracts Obtained from Winemaking Waste Streams as Natural Ingredients with Cosmeceutical Potential. Antioxidants, 8(9), 355. https://doi.org/10.3390/antiox8090355