Isotype Diversification of IgG Antibodies to HIV Gag Proteins as a Therapeutic Vaccination Strategy for HIV Infection
Abstract
:1. Introduction
2. Natural Control of HIV-1 Infection Is Associated with T-Cell Responses against HIV-1 Gag Proteins
3. IgG Antibody Responses against HIV-1 Gag Proteins, Plasmacytoid Dendritic Cells and IFN-α-Dependant Natural Killer Cell Responses May Also Contribute to Control of HIV-1 Infection
4. The Role of Non-Neutralising Antibodies in the Control of HIV-1 Infection
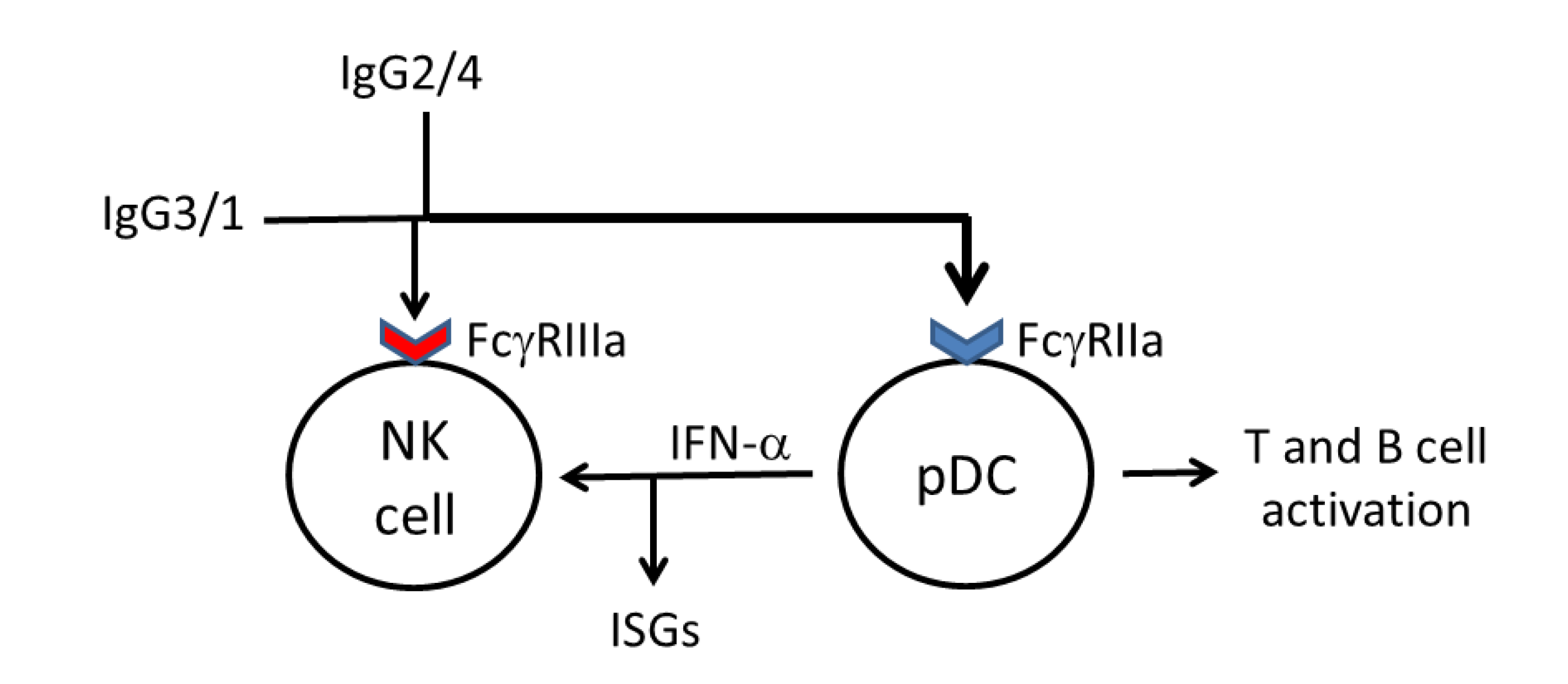
5. Diversification of IgG Antibody Responses against HIV-1 Gag Proteins May Broaden Fc Receptor Ligation and Accessory Cell Responses against HIV-1
6. Diversification of IgG Antibodies against HIV-1 Gag Proteins to Include IgG2 Antibodies May Facilitate Ligation of FcγRIIa by Complexed Antibodies
7. Isotype Diversification of IgG Antibodies to Core or Capsid Proteins of Other Persistent Viruses Is Associated with Control of Infection
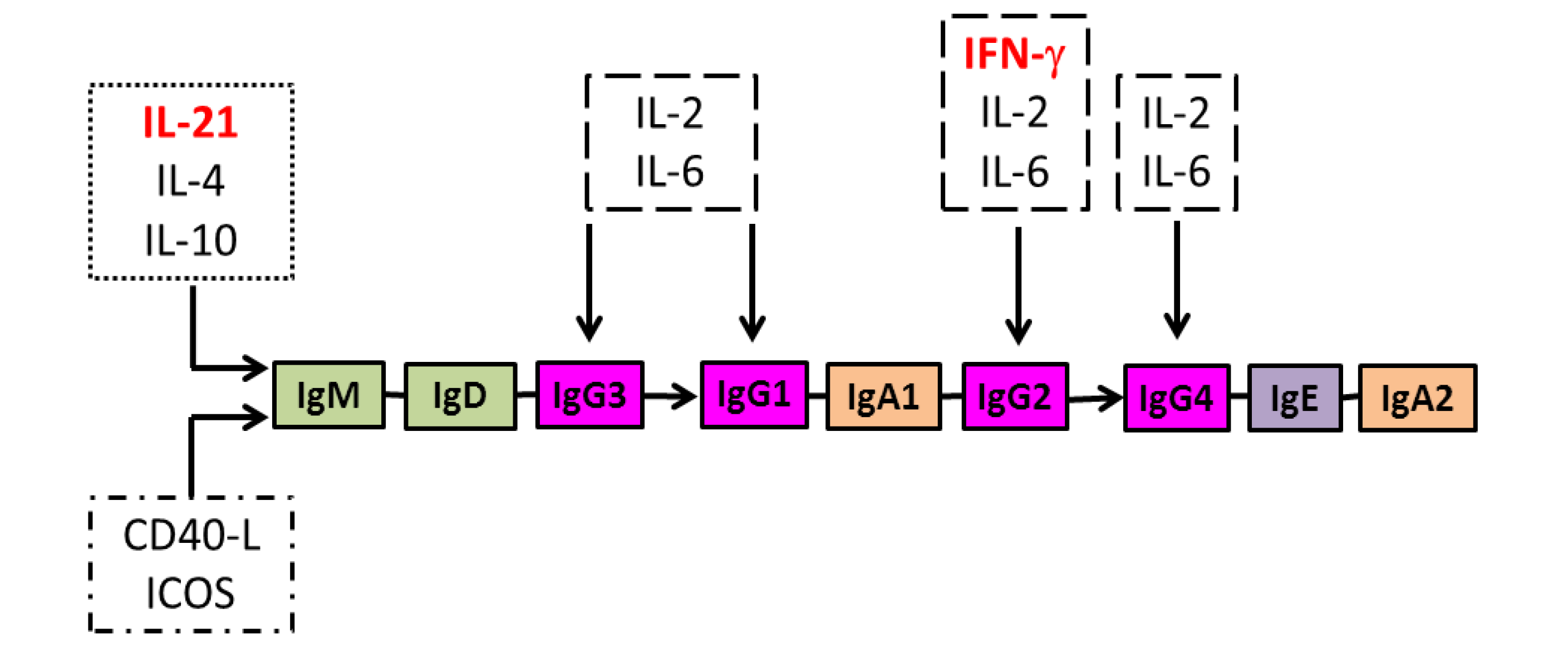
8. Regulation of IgG Antibody Isotype Diversification and the Effect of HIV Infection
| IgG3 | IgG1 | IgG2 | IgG4 |
|---|---|---|---|
| Ligation of all Fc receptors, including FcγRI in monomeric form (IgG3 > IgG1) | Restricted ligation of Fc receptors and only when complexed, particularly large complexes | Restricted ligation of Fc receptors and only when complexed, particularly large complexes | |
| Potent complement activation through the classical pathway (IgG3 > IgG1) | Weak complement activation | No complement activation | |
| Most resistant of all IgG isotypes to proteolytic degradation | Produced after chronic immune stimulation, particularly parasite infections | ||
| Predominant IgG subclass in plasma IgM-IgG complexes | Regulated similarly to IgE | ||
| Only IgG subclass to undergo covalent dimerization | May form bispecific antibodies | ||
| Predominant IgG subclass in phagocytic antibodies to polysaccharide antigens | |||
9. Potential Strategies for Enhancing Isotype Diversification of IgG Antibodies to HIV-1 Gag Proteins
10. Conclusions
Acknowledgments
Conflict of Interest
References
- Shan, L.; Deng, K.; Shroff, N.S.; Durand, C.M.; Rabi, S.A.; Yang, H.C.; Zhang, H.; Margolick, J.B.; Blankson, J.N.; Siliciano, R.F. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 2012, 36, 491–501. [Google Scholar] [CrossRef]
- Cohen, J. More woes for struggling HIV vaccine field. Science 2013, 340, 667. [Google Scholar] [CrossRef]
- Deeks, S.G.; Walker, B.D. Human immunodeficiency virus controllers: Mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 2007, 27, 406–416. [Google Scholar] [CrossRef]
- Saez-Cirion, A.; Sinet, M.; Shin, S.Y.; Urrutia, A.; Versmisse, P.; Lacabaratz, C.; Boufassa, F.; Avettand-Fenoel, V.; Rouzioux, C.; Delfraissy, J.F.; et al. Heterogeneity in HIV suppression by CD8 T-cells from HIV controllers: Association with Gag-specific CD8 T-cell responses. J. Immunol. 2009, 182, 7828–7837. [Google Scholar] [CrossRef]
- Ferre, A.L.; Lemongello, D.; Hunt, P.W.; Morris, M.M.; Garcia, J.C.; Pollard, R.B.; Yee, H.F., Jr.; Martin, J.N.; Deeks, S.G.; Shacklett, B.L. Immunodominant HIV-specific CD8+ T-cell responses are common to blood and gastrointestinal mucosa, and Gag-specific responses dominate in rectal mucosa of HIV controllers. J. Virol. 2010, 84, 10354–10365. [Google Scholar] [CrossRef]
- Vingert, B.; Benati, D.; Lambotte, O.; de Truchis, P.; Slama, L.; Jeannin, P.; Galperin, M.; Perez-Patrigeon, S.; Boufassa, F.; Kwok, W.W.; et al. HIV controllers maintain a population of highly efficient Th1 effector cells in contrast to patients treated in the long term. J. Virol. 2012, 86, 10661–10674. [Google Scholar] [CrossRef]
- Techakriengkrai, N.; Tansiri, Y.; Hansasuta, P. Poor HIV control in HLA-B*27 and B*57/58 noncontrollers is associated with limited number of polyfunctional Gag p24-specific CD8+ T-cells. AIDS 2013, 27, 17–27. [Google Scholar] [CrossRef]
- De Silva, T.I.; Peng, Y.; Leligdowicz, A.; Zaidi, I.; Li, L.; Griffin, H.; Blais, M.E.; Vincent, T.; Saraiva, M.; Yindom, L.M.; et al. Correlates of T-cell mediated viral control and phenotype of CD8+ T-cells in HIV-2, a naturally contained human retroviral infection. Blood 2013, 121, 4330–4339. [Google Scholar] [CrossRef]
- Pace, M.J.; Graf, E.H.; Agosto, L.M.; Mexas, A.M.; Male, F.; Brady, T.; Bushman, F.D.; O’Doherty, U. Directly infected resting CD4+ T-cells can produce HIV Gag without spreading infection in a model of HIV latency. PLoS Pathog. 2012, 8, e1002818. [Google Scholar] [CrossRef]
- McElrath, M.J.; de Rosa, S.C.; Moodie, Z.; Dubey, S.; Kierstead, L.; Janes, H.; Defawe, O.D.; Carter, D.K.; Hural, J.; Akondy, R.; et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: A case-cohort analysis. Lancet 2008, 372, 1894–1905. [Google Scholar] [CrossRef]
- Schooley, R.T.; Spritzler, J.; Wang, H.; Lederman, M.M.; Havlir, D.; Kuritzkes, D.R.; Pollard, R.; Battaglia, C.; Robertson, M.; Mehrotra, D.; et al. AIDS clinical trials group 5197: A placebo-controlled trial of immunization of HIV-1-infected persons with a replication-deficient adenovirus type 5 vaccine expressing the HIV-1 core protein. J. Infect. Dis. 2010, 202, 705–716. [Google Scholar] [CrossRef]
- Emu, B.; Sinclair, E.; Hatano, H.; Ferre, A.; Shacklett, B.; Martin, J.N.; McCune, J.M.; Deeks, S.G. HLA class I-restricted T-cell responses may contribute to the control of human immunodeficiency virus infection, but such responses are not always necessary for long-term virus control. J. Virol. 2008, 82, 5398–5407. [Google Scholar] [CrossRef]
- Schmidt, G.; Amiraian, K.; Frey, H.; Wethers, J.; Stevens, R.W.; Berns, D.S. Monitoring human immunodeficiency virus type 1-infected patients by ratio of antibodies to gp41 and p24. J. Clin. Microbiol. 1989, 27, 843–848. [Google Scholar]
- Fernandez-Cruz, E.; Desco, M.; Garcia-Montes, M.; Longo, N.; Gonzalez, B.; Zabay, J.M. Immunological and serological markers predictive of progression to AIDS in a cohort of HIV-infected drug users. AIDS 1990, 4, 987–994. [Google Scholar] [CrossRef]
- Mertens, T.; Ramon, A.; Kruppenbacher, J.P.; Heitmann, K.; Pika, U.; Leyssens, N.; Lievens, M. Virological examinations of patients with AIDS-related complex/Walter-Reed 5 enrolled in a double-blind placebo-controlled study with intravenous gammaglobulin administration. Vox Sang. 1990, 59, 21–29. [Google Scholar] [CrossRef]
- Allain, J.P.; Laurian, Y.; Einstein, M.H.; Braun, B.P.; Delaney, S.R.; Stephens, J.E.; Daluga, C.K.; Dahlen, S.J.; Knigge, K.M. Monitoring of specific antibodies to human immunodeficiency virus structural proteins: Clinical significance. Blood 1991, 77, 1118–1123. [Google Scholar]
- Cheingsong-Popov, R.; Panagiotidi, C.; Bowcock, S.; Aronstam, A.; Wadsworth, J.; Weber, J. Relation between humoral responses to HIV gag and env proteins at seroconversion and clinical outcome of HIV infection. Br. Med. J. 1991, 302, 23–26. [Google Scholar] [CrossRef]
- Sheppard, H.W.; Ascher, M.S.; McRae, B.; Anderson, R.E.; Lang, W.; Allain, J.P. The initial immune response to HIV and immune system activation determine the outcome of HIV disease. J. Acquir. Immune Defic. Syndr. 1991, 4, 704–712. [Google Scholar]
- Farzadegan, H.; Chmiel, J.S.; Odaka, N.; Ward, L.; Poggensee, L.; Saah, A.; Phair, J.P. Association of antibody to human immunodeficiency virus type 1 core protein (p24), CD4+ lymphocyte number, and AIDS-free time. J. Infect. Dis. 1992, 166, 1217–1222. [Google Scholar] [CrossRef]
- Chargelegue, D.; Colvin, B.T.; O’Toole, C.M. A 7-year analysis of anti-Gag (p17 and p24) antibodies in HIV-1-seropositive patients with haemophilia: Immunoglobulin G titre and avidity are early predictors of clinical course. AIDS 1993, 7, S87–S90. [Google Scholar] [CrossRef]
- Chargelegue, D.; O’Toole, C.M.; Colvin, B.T. A longitudinal study of the IgG antibody response to HIV-1 p17 gag protein in HIV-1+ patients with haemophilia: Titre and avidity. Clin. Exp. Immunol. 1993, 93, 331–336. [Google Scholar] [CrossRef]
- Zwart, G.; van der Hoek, L.; Valk, M.; Cornelissen, M.T.; Baan, E.; Dekker, J.; Koot, M.; Kuiken, C.L.; Goudsmit, J. Antibody responses to HIV-1 envelope and gag epitopes in HIV-1 seroconverters with rapid versus slow disease progression. Virology 1994, 201, 285–293. [Google Scholar] [CrossRef]
- Chargelegue, D.; Stanley, C.M.; O’Toole, C.M.; Colvin, B.T.; Steward, M.W. The affinity of IgG antibodies to gag p24 and p17 in HIV-1-infected patients correlates with disease progression. Clin. Exp. Immunol. 1995, 99, 175–181. [Google Scholar]
- Hogervorst, E.; Jurriaans, S.; de Wolf, F.; van Wijk, A.; Wiersma, A.; Valk, M.; Roos, M.; van Gemen, B.; Coutinho, R.; Miedema, F.; et al. Predictors for non- and slow progression in human immunodeficiency virus (HIV) type 1 infection: Low viral RNA copy numbers in serum and maintenance of high HIV-1 p24-specific but not V3-specific antibody levels. J. Infect. Dis. 1995, 171, 811–821. [Google Scholar] [CrossRef]
- Morand-Joubert, L.; Bludau, H.; Lerable, J.; Petit, J.C.; Lefrere, J.J. Serum anti-p24 antibody concentration has a predictive value on the decrease of CD4 lymphocyte count higher than acid-dissociated p24 antigen. J. Med. Virol. 1995, 47, 87–91. [Google Scholar] [CrossRef]
- Garland, F.C.; Garland, C.F.; Gorham, E.D.; Brodine, S.K. Western blot banding patterns of HIV rapid progressors in the U.S. Navy Seropositive Cohort: Implications for vaccine development. Navy Retroviral Working Group. Ann. Epidemiol. 1996, 6, 341–347. [Google Scholar]
- Thomas, H.I.; Wilson, S.; O’Toole, C.M.; Lister, C.M.; Saeed, A.M.; Watkins, R.P.; Morgan-Capner, P. Differential maturation of avidity of IgG antibodies to gp41, p24 and p17 following infection with HIV-1. Clin. Exp. Immunol. 1996, 103, 185–191. [Google Scholar]
- Mofenson, L.M.; Harris, D.R.; Rich, K.; Meyer, W.A., 3rd; Read, J.S.; Moye, J., Jr.; Nugent, R.P.; Korelitz, J.; Bethel, J.; Pahwa, S. Serum HIV-1 p24 antibody, HIV-1 RNA copy number and CD4 lymphocyte percentage are independently associated with risk of mortality in HIV-1-infected children. AIDS 1999, 13, 31–39. [Google Scholar] [CrossRef]
- Read, J.S.; Rich, K.C.; Korelitz, J.J.; Mofenson, L.M.; Harris, R.; Moye, J.H., Jr.; Meyer, W.A., 3rd.; Pahwa, S.G.; Bethel, J.W.; Nugent, R.P. Quantification of human immunodeficiency virus type 1 p24 antigen and antibody rivals human immunodeficiency virus type 1 RNA and CD4+ enumeration for prognosis. Pediatr. Infect. Dis. J. 2000, 19, 544–551. [Google Scholar] [CrossRef]
- Malhotra, U.; Nolin, J.; Mullins, J.I.; McElrath, M.J. Comprehensive epitope analysis of cross-cladeGag-specific T-cell responses in individuals with early HIV-1 infection in the U.S. epidemic. Vaccine 2007, 25, 381–90. [Google Scholar]
- Tomescu, C.; Duh, F.M.; Hoh, R.; Viviani, A.; Harvill, K.; Martin, M.P.; Carrington, M.; Deeks, S.G.; Montaner, L.J. Impact of protective killer inhibitory receptor/human leukocyte antigen genotypes on natural killer cell and T-cell function in HIV-1-infected controllers. AIDS 2012, 26, 1869–1878. [Google Scholar] [CrossRef]
- Barblu, L.; Machmach, K.; Gras, C.; Delfraissy, J.F.; Boufassa, F.; Leal, M.; Ruiz-Mateos, E.; Lambotte, O.; Herbeuval, J.P. Plasmacytoid dendritic cells (pDCs) from HIV controllers produce interferon-alpha and differentiate into functional killer pDCs under HIV activation. J. Infect. Dis. 2012, 206, 790–801. [Google Scholar] [CrossRef]
- Machmach, K.; Leal, M.; Gras, C.; Viciana, P.; Genebat, M.; Franco, E.; Boufassa, F.; Lambotte, O.; Herbeuval, J.P.; Ruiz-Mateos, E. Plasmacytoid dendritic cells reduce HIV production in elite controllers. J. Virol. 2012, 86, 4245–4252. [Google Scholar] [CrossRef]
- Lande, R.; Gilliet, M. Plasmacytoid dendritic cells: Key players in the initiation and regulation of immune responses. Ann. NY Acad. Sci. 2010, 1183, 89–103. [Google Scholar]
- Jost, S.; Altfeld, M. Control of human viral infections by natural killer cells. Annu. Rev. Immunol. 2013, 31, 163–194. [Google Scholar]
- Jaehn, P.S.; Zaenker, K.S.; Schmitz, J.; Dzionek, A. Functional dichotomy of plasmacytoid dendritic cells: Antigen-specific activation of T-cells versus production of type I interferon. Eur. J. Immunol. 2008, 38, 1822–1832. [Google Scholar] [CrossRef]
- Villadangos, J.A.; Young, L. Antigen-presentation properties of plasmacytoid dendritic cells. Immunity 2008, 29, 352–361. [Google Scholar] [CrossRef]
- Tel, J.; Lambeck, A.J.; Cruz, L.J.; Tacken, P.J.; de Vries, I.J.; Figdor, C.G. Human plasmacytoid dendritic cells phagocytose, process, and present exogenous particulate antigen. J. Immunol. 2010, 184, 4276–4283. [Google Scholar] [CrossRef]
- Tel, J.; Smits, E.L.; Anguille, S.; Joshi, R.N.; Figdor, C.G.; de Vries, I.J. Human plasmacytoid dendritic cells are equipped with antigen-presenting and tumoricidal capacities. Blood 2012, 120, 3936–3944. [Google Scholar] [CrossRef]
- Di Pucchio, T.; Chatterjee, B.; Smed-Sorensen, A.; Clayton, S.; Palazzo, A.; Montes, M.; Xue, Y.; Mellman, I.; Banchereau, J.; Connolly, J.E. Direct proteasome-independent cross-presentation of viral antigen by plasmacytoid dendritic cells on major histocompatibility complex class I. Nat. Immunol. 2008, 9, 551–557. [Google Scholar] [CrossRef]
- Tel, J.; Schreibelt, G.; Sittig, S.P.; Mathan, T.S.; Buschow, S.I.; Cruz, L.J.; Lambeck, A.J.; Figdor, C.G.; de Vries, I.J. Human plasmacytoid dendritic cells efficiently cross-present exogenous Ags to CD8+ T-cells despite lower Ag uptake than myeloid dendritic cell subsets. Blood 2013, 121, 459–467. [Google Scholar] [CrossRef]
- Shaw, J.; Wang, Y.H.; Ito, T.; Arima, K.; Liu, Y.J. Plasmacytoid dendritic cells regulate B-cell growth and differentiation via CD70. Blood 2010, 115, 3051–3057. [Google Scholar] [CrossRef]
- Forthal, D.N.; Moog, C. Fc receptor-mediated antiviral antibodies. Curr. Opin. HIV AIDS 2009, 4, 388–393. [Google Scholar] [CrossRef]
- Stratov, I.; Chung, A.; Kent, S.J. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J. Virol. 2008, 82, 5450–5459. [Google Scholar] [CrossRef]
- Chung, A.W.; Isitman, G.; Navis, M.; Kramski, M.; Center, R.J.; Kent, S.J.; Stratov, I. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc. Natl. Acad. Sci. USA 2011, 108, 7505–7510. [Google Scholar]
- Forthal, D.N.; Gilbert, P.B.; Landucci, G.; Phan, T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J. Immunol. 2007, 178, 6596–6603. [Google Scholar]
- Ackerman, M.E.; Dugast, A.S.; McAndrew, E.G.; Tsoukas, S.; Licht, A.F.; Irvine, D.J.; Alter, G. Enhanced phagocytic activity of HIV-specific antibodies correlates with natural production of immunoglobulins with skewed affinity for FcγR2a and FcγR2b. J. Virol. 2013, 87, 5468–5476. [Google Scholar] [CrossRef]
- French, M.A.; Center, R.J.; Wilson, K.M.; Fleyfel, I.; Fernandez, S.; Schorcht, A.; Stratov, I.; Kramski, M.; Kent, S.J.; Kelleher, A.D. Isotype-switched immunoglobulin G antibodies to HIV Gag proteins may provide alternative or additional immune responses to “protective” human leukocyte antigen-B alleles in HIV controllers. AIDS 2013, 27, 519–528. [Google Scholar] [CrossRef]
- Forthal, D.N.; Landucci, G.; Chohan, B.; Richardson, B.A.; McClelland, R.S.; Jaoko, W.; Blish, C.; Overbaugh, J. Antibody-dependent cell-mediated virus inhibition antibody activity does not correlate with risk of HIV-1 superinfection. J. Acquir. Immune Defic. Syndr. 2013, 63, 31–33. [Google Scholar] [CrossRef]
- Wren, L.H.; Chung, A.W.; Isitman, G.; Kelleher, A.D.; Parsons, M.S.; Amin, J.; Cooper, D.A.; Stratov, I.; Navis, M.; Kent, S.J. Specific antibody-dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology 2013, 138, 116–123. [Google Scholar] [CrossRef]
- Bruhns, P.; Iannascoli, B.; England, P.; Mancardi, D.A.; Fernandez, N.; Jorieux, S.; Daeron, M. Specificity and affinity of human Fcγ receptors and their polymorphic variants for human IgG subclasses. Blood 2009, 113, 3716–3725. [Google Scholar] [CrossRef]
- Lux, A.; Yu, X.; Scanlan, C.N.; Nimmerjahn, F. Impact of immune complex size and glycosylation on IgG binding to human FcγRs. J. Immunol. 2013, 190, 4315–4323. [Google Scholar] [CrossRef]
- Bave, U.; Magnusson, M.; Eloranta, M.L.; Perers, A.; Alm, G.V.; Ronnblom, L. FcγRIIa is expressed on natural IFN-α-producing cells (plasmacytoid dendritic cells) and is required for the IFN-α production induced by apoptotic cells combined with lupus IgG. J. Immunol. 2003, 171, 3296–3302. [Google Scholar]
- Means, T.K.; Latz, E.; Hayashi, F.; Murali, M.R.; Golenbock, D.T.; Luster, A.D. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 2005, 115, 407–417. [Google Scholar]
- Lovgren, T.; Eloranta, M.L.; Kastner, B.; Wahren-Herlenius, M.; Alm, G.V.; Ronnblom, L. Induction of interferon-alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen- and Sjogren’s syndrome autoantigen-associated RNA. Arthritis Rheum. 2006, 54, 1917–1927. [Google Scholar]
- Su, K.; Yang, H.; Li, X.; Gibson, A.W.; Cafardi, J.M.; Zhou, T.; Edberg, J.C.; Kimberly, R.P. Expression profile of FcγRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J. Immunol. 2007, 178, 3272–3280. [Google Scholar]
- Dugast, A.S.; Tonelli, A.; Berger, C.T.; Ackerman, M.E.; Sciaranghella, G.; Liu, Q.; Sips, M.; Toth, I.; Piechocka-Trocha, A.; Ghebremichael, M.; et al. Decreased Fc receptor expression on innate immune cells is associated with impaired antibody-mediated cellular phagocytic activity in chronically HIV-1 infected individuals. Virology 2011, 415, 160–167. [Google Scholar] [CrossRef]
- Nimmerjahn, F.; Ravetch, J.V. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 2008, 8, 34–47. [Google Scholar] [CrossRef]
- Wang, J.P.; Asher, D.R.; Chan, M.; Kurt-Jones, E.A.; Finberg, R.W. Cutting Edge: Antibody-mediated TLR7-dependent recognition of viral RNA. J. Immunol. 2007, 178, 3363–3367. [Google Scholar]
- Leeansyah, E.; Wines, B.D.; Crowe, S.M.; Jaworowski, A. The mechanism underlying defective Fcγ receptor-mediated phagocytosis by HIV-1-infected human monocyte-derived macrophages. J. Immunol. 2007, 178, 1096–1104. [Google Scholar]
- Forthal, D.N.; Landucci, G.; Bream, J.; Jacobson, L.P.; Phan, T.B.; Montoya, B. FcγRIIa genotype predicts progression of HIV infection. J. Immunol. 2007, 179, 7916–7923. [Google Scholar]
- Poonia, B.; Kijak, G.H.; Pauza, C.D. High affinity allele for the gene of FCGR3A is risk factor for HIV infection and progression. PLoS One 2010, 5, e15562. [Google Scholar] [CrossRef]
- Forthal, D.N.; Gabriel, E.E.; Wang, A.; Landucci, G.; Phan, T.B. Association of Fcγ receptor IIIa genotype with the rate of HIV infection after gp120 vaccination. Blood 2012, 120, 2836–2842. [Google Scholar] [CrossRef]
- Stahl, D.; Sibrowski, W. IgG2 containing IgM-IgG immune complexes predominate in normal human plasma, but not in plasma of patients with warm autoimmune haemolytic anaemia. Eur. J. Haematol. 2006, 77, 191–202. [Google Scholar] [CrossRef]
- Vitharsson, G.; Jonsdottir, I.; Jonsson, S.; Valdimarsson, H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J. Infect. Dis. 1994, 170, 592–599. [Google Scholar] [CrossRef]
- Rodriguez, M.E.; van der Pol, W.L.; Sanders, L.A.; van de Winkel, J.G. Crucial role of FcγRIIa (CD32) in assessment of functional anti-Streptococcus pneumoniae antibody activity in human sera. J. Infect. Dis. 1999, 179, 423–433. [Google Scholar] [CrossRef]
- Flinsenberg, T.W.; Compeer, E.B.; Koning, D.; Klein, M.; Amelung, F.J.; van Baarle, D.; Boelens, J.J.; Boes, M. Fcγ receptor antigen targeting potentiates cross-presentation by human blood and lymphoid tissue BDCA-3+ dendritic cells. Blood 2012, 120, 5163–5172. [Google Scholar] [CrossRef]
- Yoo, E.M.; Wims, L.A.; Chan, L.A.; Morrison, S.L. Human IgG2 can form covalent dimers. J. Immunol. 2003, 170, 3134–3138. [Google Scholar]
- Meulenbroek, A.J. Human IgG Subclasses: Useful Diagnostic Markers for Immunocompetence, 3rd ed.; Sanquin: Amsterdam, The Netherlands, 2008; pp. 11–14. [Google Scholar]
- Allhorn, M.; Olin, A.I.; Nimmerjahn, F.; Collin, M. Human IgG/FcγR interactions are modulated by streptococcal IgG glycan hydrolysis. PLoS One 2008, 3, e1413. [Google Scholar] [CrossRef]
- Ngo-Giang-Huong, N.; Candotti, D.; Goubar, A.; Autran, B.; Maynart, M.; Sicard, D.; Clauvel, J.P.; Agut, H.; Costagliola, D.; Rouzioux, C. HIV type 1-specific IgG2 antibodies: Markers of helper T-cell type 1 response and prognostic marker of long-term nonprogression. AIDS Res. Hum. Retroviruses 2001, 17, 1435–1446. [Google Scholar] [CrossRef]
- Banerjee, K.; Klasse, P.J.; Sanders, R.W.; Pereyra, F.; Michael, E.; Lu, M.; Walker, B.D.; Moore, J.P. IgG subclass profiles in infected HIV type 1 controllers and chronic progressors and in uninfected recipients of Env vaccines. AIDS Res. Hum. Retroviruses 2010, 26, 445–458. [Google Scholar] [CrossRef]
- Zein, N.N.; Li, H.; Persing, D.H. Humoral immunity in acute and chronic hepatitis C infection. Gastroenterology 1999, 117, 510. [Google Scholar] [CrossRef]
- Matsumoto, K.; Yoshikawa, H.; Yasugi, T.; Nakagawa, S.; Kawana, K.; Nozawa, S.; Hoshiai, H.; Shiromizu, K.; Kanda, T.; Taketani, Y. Balance of IgG subclasses toward human papillomavirus type 16 (HPV16) L1-capsids is a possible predictor for the regression of HPV16-positive cervical intraepithelial neoplasia. Biochem. Biophys. Res. Commun. 1999, 258, 128–131. [Google Scholar] [CrossRef]
- Wang, Z.H.; Kjellberg, L.; Abdalla, H.; Wiklund, F.; Eklund, C.; Knekt, P.; Lehtinen, M.; Kallings, I.; Lenner, P.; Hallmans, G.; et al. Type specificity and significance of different isotypes of serum antibodies to human papillomavirus capsids. J. Infect. Dis. 2000, 181, 456–462. [Google Scholar] [CrossRef]
- Ma, C.S.; Deenick, E.K.; Batten, M.; Tangye, S.G. The origins, function, and regulation of T follicular helper cells. J. Exp. Med. 2012, 209, 1241–1253. [Google Scholar] [CrossRef]
- Pan-Hammarstrom, Q.; Zhao, Y.; Hammarstrom, L. Class switch recombination: A comparison between mouse and human. Adv. Immunol. 2007, 93, 1–61. [Google Scholar] [CrossRef]
- French, M. Serum IgG subclasses in normal adults. Monogr. Allergy 1986, 19, 100–107. [Google Scholar]
- Ferrari, S.; Plebani, A. Cross-talk between CD40 and CD40L: Lessons from primary immune deficiencies. Curr. Opin. Allergy Clin. Immunol. 2002, 2, 489–494. [Google Scholar] [CrossRef]
- Warnatz, K.; Bossaller, L.; Salzer, U.; Skrabl-Baumgartner, A.; Schwinger, W.; van der Burg, M.; van Dongen, J.J.; Orlowska-Volk, M.; Knoth, R.; Durandy, A.; et al. Human ICOS deficiency abrogates the germinal center reaction and provides a monogenic model for common variable immunodeficiency. Blood 2006, 107, 3045–3052. [Google Scholar] [CrossRef]
- Briere, F.; Bridon, J.M.; Chevet, D.; Souillet, G.; Bienvenu, F.; Guret, C.; Martinez-Valdez, H.; Banchereau, J. Interleukin 10 induces B lymphocytes from IgA-deficient patients to secrete IgA. J. Clin. Invest. 1994, 94, 97–104. [Google Scholar] [CrossRef]
- Avery, D.T.; Bryant, V.L.; Ma, C.S.; de Waal Malefyt, R.; Tangye, S.G. IL-21-induced isotype switching to IgG and IgA by human naive B cells is differentially regulated by IL-4. J. Immunol. 2008, 181, 1767–1779. [Google Scholar]
- Borte, S.; Pan-Hammarstrom, Q.; Liu, C.; Sack, U.; Borte, M.; Wagner, U.; Graf, D.; Hammarstrom, L. Interleukin-21 restores immunoglobulin production ex vivo in patients with common variable immunodeficiency and selective IgA deficiency. Blood 2009, 114, 4089–4098. [Google Scholar] [CrossRef]
- Cubas, R.A.; Mudd, J.C.; Savoye, A.L.; Perreau, M.; van Grevenynghe, J.; Metcalf, T.; Connick, E.; Meditz, A.; Freeman, G.J.; Abesada-Terk, G., Jr.; et al. Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat. Med. 2013, 19, 494–499. [Google Scholar] [CrossRef]
- Kitani, A.; Strober, W. Regulation of C gamma subclass germ-line transcripts in human peripheral blood B cells. J. Immunol. 1993, 151, 3478–3488. [Google Scholar]
- Kawano, Y.; Noma, T.; Yata, J. Regulation of human IgG subclass production by cytokines. IFN-γ and IL-6 act antagonistically in the induction of human IgG1 but additively in the induction of IgG2. J. Immunol. 1994, 153, 4948–4958. [Google Scholar]
- Kawano, Y.; Noma, T.; Kou, K.; Yoshizawa, I.; Yata, J. Regulation of human IgG subclass production by cytokines: Human IgG subclass production enhanced differentially by interleukin-6. Immunology 1995, 84, 278–284. [Google Scholar]
- Kawano, Y.; Noma, T. Role of interleukin-2 and interferon-γ in inducing production of IgG subclasses in lymphocytes of human newborns. Immunology 1996, 88, 40–48. [Google Scholar] [CrossRef]
- Kondo, N.; Inoue, R.; Kasahara, K.; Fukao, T.; Kaneko, H.; Tashita, H.; Teramoto, T. Reduced expression of the interferon-γ messenger RNA in IgG2 deficiency. Scand. J. Immunol. 1997, 45, 227–230. [Google Scholar]
- Buckner, C.M.; Moir, S.; Ho, J.; Wang, W.; Posada, J.G.; Kardava, L.; Funk, E.K.; Nelson, A.K.; Li, Y.; Chun, T.W.; et al. Characterization of plasmablasts in the blood of HIV-infected viremic individuals: Evidence for nonspecific immune activation. J. Virol. 2013, 87, 5800–5811. [Google Scholar] [CrossRef]
- Crum-Cianflone, N.F.; Collins, G.; Defang, G.; Iverson, E.; Eberly, L.E.; Duplessis, C.; Maguire, J.; Ganesan, A.; Agan, B.K.; Lalani, T.; et al. Immunoglobulin G subclass levels and antibody responses to the 2009 influenza A (H1N1) monovalent vaccine among human immunodeficiency virus (HIV)-infected and HIV-uninfected adults. Clin. Exp. Immunol. 2012, 168, 135–141. [Google Scholar] [CrossRef]
- Xu, W.; Santini, P.A.; Sullivan, J.S.; He, B.; Shan, M.; Ball, S.C.; Dyer, W.B.; Ketas, T.J.; Chadburn, A.; Cohen-Gould, L.; et al. HIV-1 evades virus-specific IgG2 and IgA responses by targeting systemic and intestinal B cells via long-range intercellular conduits. Nat. Immunol. 2009, 10, 1008–1017. [Google Scholar] [CrossRef]
- Raux, M.; Finkielsztejn, L.; Salmon-Ceron, D.; Bouchez, H.; Excler, J.L.; Dulioust, E.; Grouin, J.M.; Sicard, D.; Blondeau, C. IgG subclass distribution in serum and various mucosal fluids of HIV type 1-infected subjects. AIDS Res. Hum. Retroviruses 2000, 16, 583–594. [Google Scholar] [CrossRef]
- French, M.A.; Denis, K.A.; Dawkins, R.; Peter, J.B. Severity of infections in IgA deficiency: Correlation with decreased serum antibodies to pneumococcal polysaccharides and decreased serum IgG2 and/or IgG4. Clin. Exp. Immunol. 1995, 100, 47–53. [Google Scholar]
- Pallikkuth, S.; Parmigiani, A.; Silva, S.Y.; George, V.K.; Fischl, M.; Pahwa, R.; Pahwa, S. Impaired peripheral blood T-follicular helper cell function in HIV-infected nonresponders to the 2009 H1N1/09 vaccine. Blood 2012, 120, 985–993. [Google Scholar] [CrossRef]
- French, M.A.; Tanaskovic, S.; Law, M.G.; Lim, A.; Fernandez, S.; Ward, L.D.; Kelleher, A.D.; Emery, S. Vaccine-induced IgG2 anti-HIV p24 is associated with control of HIV in patients with a “high-affinity” FcγRIIa genotype. AIDS 2010, 24, 1983–1990. [Google Scholar] [CrossRef]
- Perreau, M.; Savoye, A.L.; de Crignis, E.; Corpataux, J.M.; Cubas, R.; Haddad, E.K.; de Leval, L.; Graziosi, C.; Pantaleo, G. Follicular helper T-cells serve as the major CD4 T-cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 2012, 210, 143–156. [Google Scholar]
- Oliveira, T.M.; Mineo, T.W.; Bason, M.; Day, M.J.; Machado, R.Z. IgG subclass profile of serum antibodies to Leishmania chagasi in naturally infected and vaccinated dogs. Vet. Parasitol. 2009, 162, 16–22. [Google Scholar] [CrossRef]
- Titanji, K.; Velu, V.; Chennareddi, L.; Vijay-Kumar, M.; Gewirtz, A.T.; Freeman, G.J.; Amara, R.R. Acute depletion of activated memory B cells involves the PD-1 pathway in rapidly progressing SIV-infected macaques. J. Clin. Invest. 2010, 120, 3878–3890. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
French, M.A.; Abudulai, L.N.; Fernandez, S. Isotype Diversification of IgG Antibodies to HIV Gag Proteins as a Therapeutic Vaccination Strategy for HIV Infection. Vaccines 2013, 1, 328-342. https://doi.org/10.3390/vaccines1030328
French MA, Abudulai LN, Fernandez S. Isotype Diversification of IgG Antibodies to HIV Gag Proteins as a Therapeutic Vaccination Strategy for HIV Infection. Vaccines. 2013; 1(3):328-342. https://doi.org/10.3390/vaccines1030328
Chicago/Turabian StyleFrench, Martyn A., Laila N. Abudulai, and Sonia Fernandez. 2013. "Isotype Diversification of IgG Antibodies to HIV Gag Proteins as a Therapeutic Vaccination Strategy for HIV Infection" Vaccines 1, no. 3: 328-342. https://doi.org/10.3390/vaccines1030328
APA StyleFrench, M. A., Abudulai, L. N., & Fernandez, S. (2013). Isotype Diversification of IgG Antibodies to HIV Gag Proteins as a Therapeutic Vaccination Strategy for HIV Infection. Vaccines, 1(3), 328-342. https://doi.org/10.3390/vaccines1030328




