The Respiratory Commensal Bacterium Corynebacterium pseudodiphtheriticum as a Mucosal Adjuvant for Nasal Vaccines
Abstract
:1. Introduction
2. Materials and Methods
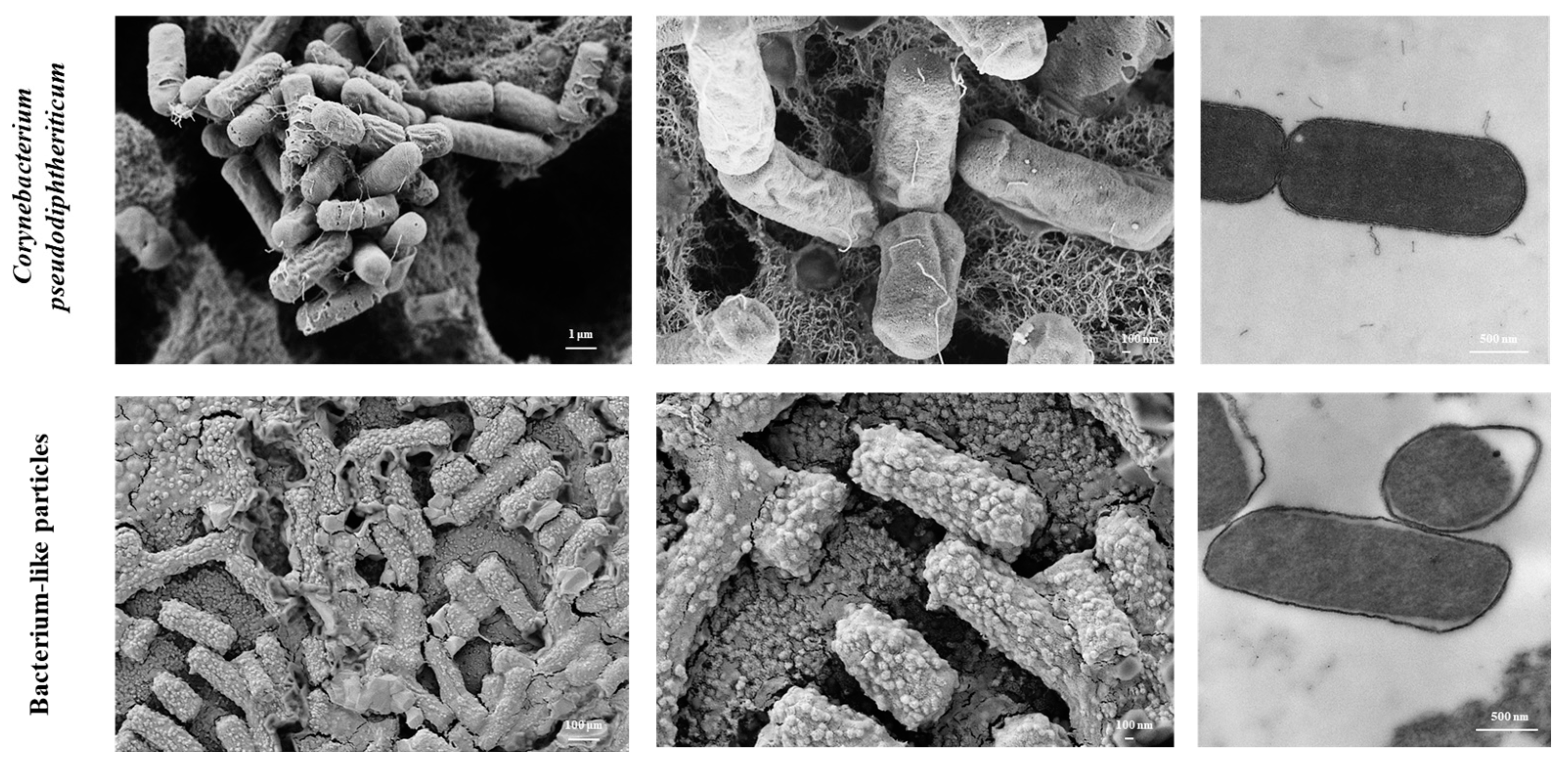
2.1. C. pseudodiphtheriticum and Obtention of BLPs
2.2. Electron Microscopy
2.3. Modulation of Alveolar Macrophages by C. pseudodiphtheriticum and Its BLPs
2.4. Measurement of Cytokines’ Production by Alveolar Macrophages
2.5. Animal Experiments
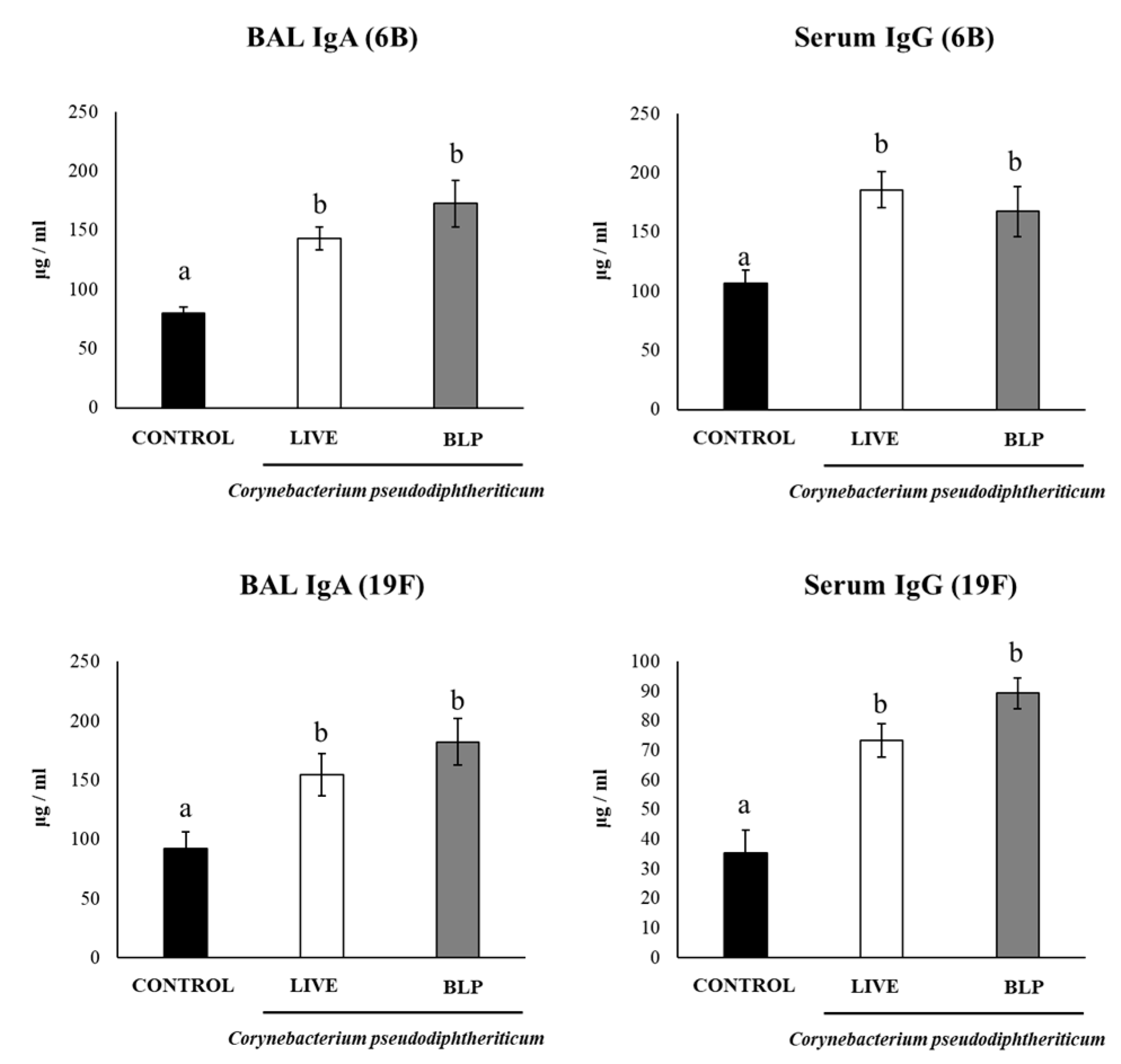
2.6. Determination of Antibodies
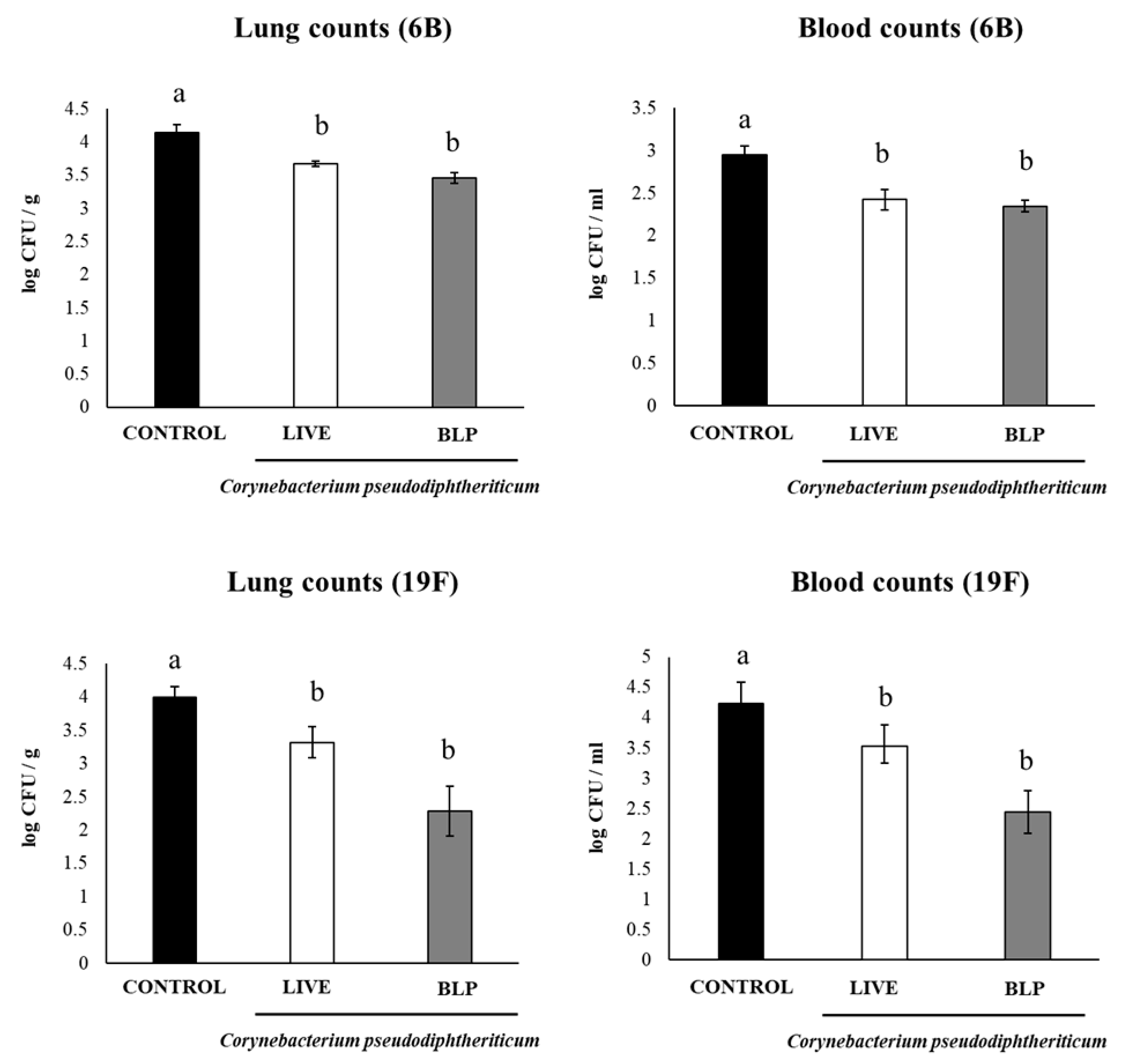
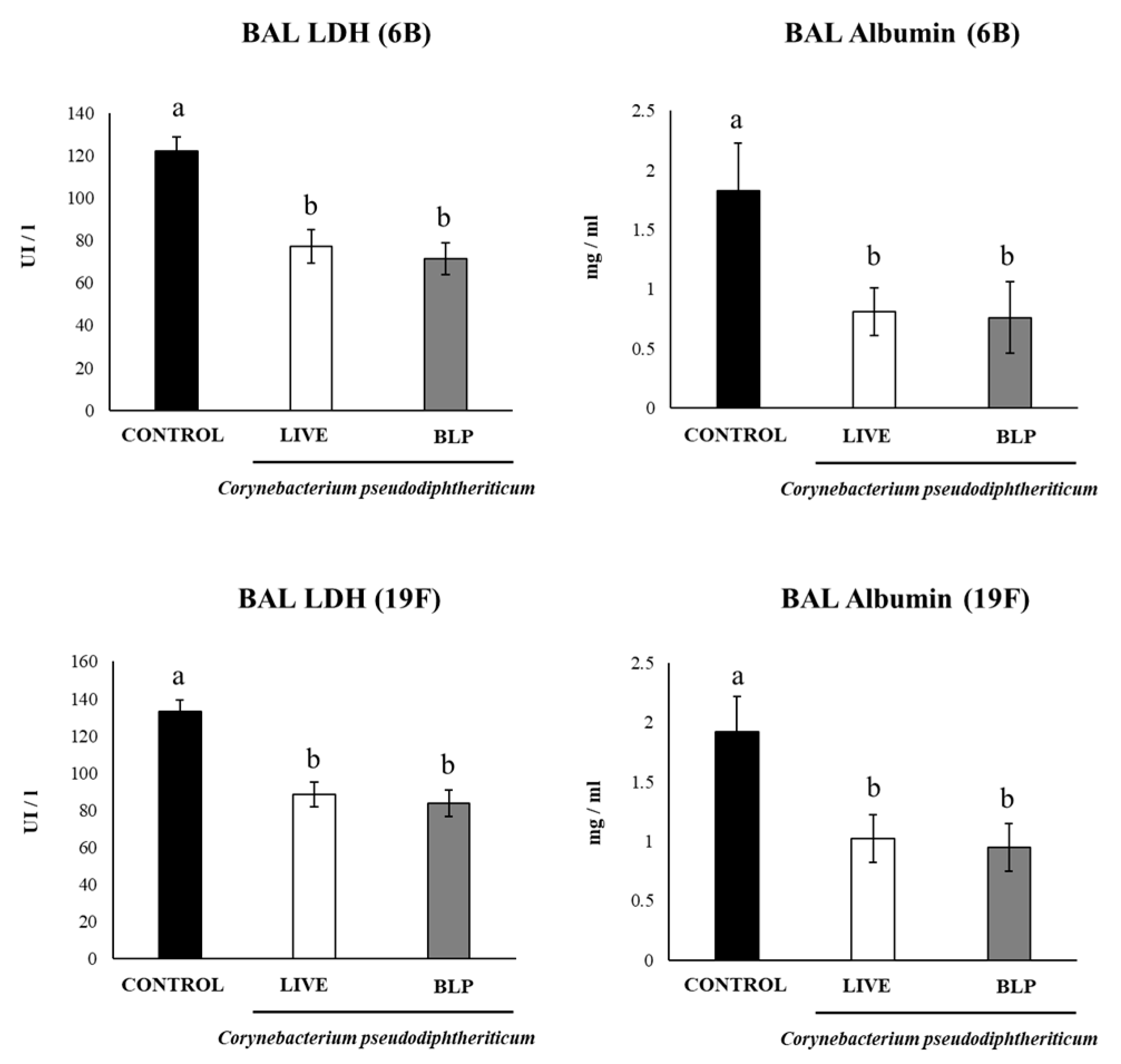
2.7. Infection Challenge Experiments
2.8. Statistical Analysis
3. Results
3.1. Obtention of BLPs from the Respiratory Commensal C. pseudodiphtheriticum
3.2. C. pseudodiphtheriticum and Its BLPs Modulate Alveolar Macrophages’ Function
3.3. C. pseudodiphtheriticum and Its BLPs Improve the Immune Response to a Pneumococcal Vaccine
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takaki, H.; Ichimiya, S.; Matsumoto, M.; Seya, T. Mucosal Immune Response in Nasal-Associated Lymphoid Tissue upon Intranasal Administration by Adjuvants. J. Innate Immun. 2018, 10, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Cai, L.; Hufnagel, S.; Cui, Z. Intranasal Vaccine: Factors to Consider in Research and Development. Int. J. Pharm. 2021, 609, 121180. [Google Scholar] [CrossRef] [PubMed]
- de Steenhuijsen Piters, W.A.A.; Binkowska, J.; Bogaert, D. Early Life Microbiota and Respiratory Tract Infections. Cell Host Microbe 2020, 28, 223–232. [Google Scholar] [CrossRef]
- Andrade, B.G.; Cuadrat, R.R.; Tonetti, F.R.; Kitazawa, H.; Villena, J. The Role of Respiratory Microbiota in the Protection against Viral Diseases: Respiratory Commensal Bacteria as next-Generation Probiotics for COVID-19. Biosci. Microbiota Food Health 2022, 41, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Clua, P.; Vizoso-Pinto, M.G.; Rodriguez, C.; Alvarez, S.; Melnikov, V.; Takahashi, H.; Kitazawa, H.; Villena, J. Respiratory Commensal Bacteria Corynebacterium pseudodiphtheriticum Improves Resistance of Infant Mice to Respiratory Syncytial Virus and Streptococcus pneumoniae Superinfection. Front. Microbiol. 2017, 8, 1613. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Moyano, R.; Raya Tonetti, F.; Tomokiyo, M.; Kanmani, P.; Vizoso-Pinto, M.G.; Kim, H.; Quilodrán-Vega, S.; Melnikov, V.; Alvarez, S.; Takahashi, H.; et al. The Ability of Respiratory Commensal Bacteria to Beneficially Modulate the Lung Innate Immune Response Is a Strain Dependent Characteristic. Microorganisms 2020, 8, 727. [Google Scholar] [CrossRef] [PubMed]
- Dentice Maidana, S.; Ortiz Moyano, R.; Vargas, J.M.; Fukuyama, K.; Kurata, S.; Melnikov, V.; Jure, M.Á.; Kitazawa, H.; Villena, J. Respiratory Commensal Bacteria Increase Protection against Hypermucoviscous Carbapenem-Resistant Klebsiella pneumoniae ST25 Infection. Pathogens 2022, 11, 1063. [Google Scholar] [CrossRef]
- Szatraj, K.; Szczepankowska, A.K.; Chmielewska-Jeznach, M. Lactic Acid Bacteria—Promising Vaccine Vectors: Possibilities, Limitations, Doubts. J. Appl. Microbiol. 2017, 123, 325–339. [Google Scholar] [CrossRef]
- Cho, S.W.; Yim, J.; Seo, S.W. Engineering Tools for the Development of Recombinant Lactic Acid Bacteria. Biotechnol. J. 2020, 15, 1900344. [Google Scholar] [CrossRef]
- Villena, J.; Kitazawa, H. The Modulation of Mucosal Antiviral Immunity by Immunobiotics: Could They Offer Any Benefit in the SARS-CoV-2 Pandemic? Front. Physiol. 2020, 11, 699. [Google Scholar] [CrossRef]
- Audouy, S.A.L.; van Roosmalen, M.L.; Neef, J.; Kanninga, R.; Post, E.; van Deemter, M.; Metselaar, H.; van Selm, S.; Robillard, G.T.; Leenhouts, K.J.; et al. Lactococcus lactis GEM Particles Displaying Pneumococcal Antigens Induce Local and Systemic Immune Responses Following Intranasal Immunization. Vaccine 2006, 24, 5434–5441. [Google Scholar] [CrossRef] [PubMed]
- Raya Tonetti, F.; Arce, L.; Salva, S.; Alvarez, S.; Takahashi, H.; Kitazawa, H.; Vizoso-Pinto, M.G.; Villena, J. Immunomodulatory Properties of Bacterium-Like Particles Obtained From Immunobiotic Lactobacilli: Prospects for Their Use as Mucosal Adjuvants. Front. Immunol. 2020, 11, 15. [Google Scholar] [CrossRef]
- Arce, L.P.; Raya Tonetti, M.F.; Raimondo, M.P.; Müller, M.F.; Salva, S.; Álvarez, S.; Baiker, A.; Villena, J.; Vizoso Pinto, M.G. Oral Vaccination with Hepatitis E Virus Capsid Protein and Immunobiotic Bacterium-Like Particles Induce Intestinal and Systemic Immunity in Mice. Probiotics Antimicro. Prot. 2020, 12, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Raya-Tonetti, F.; Müller, M.; Sacur, J.; Kitazawa, H.; Villena, J.; Vizoso-Pinto, M.G. Novel LysM Motifs for Antigen Display on Lactobacilli for Mucosal Immunization. Sci. Rep. 2021, 11, 21691. [Google Scholar] [CrossRef] [PubMed]
- Tomosada, Y.; Chiba, E.; Zelaya, H.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Nasally Administered Lactobacillus rhamnosus Strains Differentially Modulate Respiratory Antiviral Immune Responses and Induce Protection against Respiratory Syncytial Virus Infection. BMC Immunol. 2013, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Clua, P.; Kanmani, P.; Zelaya, H.; Tada, A.; Kober, A.K.M.H.; Salva, S.; Alvarez, S.; Kitazawa, H.; Villena, J. Peptidoglycan from Immunobiotic Lactobacillus rhamnosus Improves Resistance of Infant Mice to Respiratory Syncytial Viral Infection and Secondary Pneumococcal Pneumonia. Front. Immunol. 2017, 8, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Clua, P.; Tomokiyo, M.; Raya Tonetti, F.; Islam, M.A.; García Castillo, V.; Marcial, G.; Salva, S.; Alvarez, S.; Takahashi, H.; Kurata, S.; et al. The Role of Alveolar Macrophages in the Improved Protection against Respiratory Syncytial Virus and Pneumococcal Superinfection Induced by the Peptidoglycan of Lactobacillus rhamnosus CRL1505. Cells 2020, 9, 1653. [Google Scholar] [CrossRef] [PubMed]
- Raya Tonetti, F.; Clua, P.; Fukuyama, K.; Marcial, G.; Sacur, J.; Marranzino, G.; Tomokiyo, M.; Vizoso-Pinto, G.; Garcia-Cancino, A.; Kurata, S.; et al. The Ability of Postimmunobiotics from L. rhamnosus CRL1505 to Protect against Respiratory Syncytial Virus and Pneumococcal Super-Infection Is a Strain-Dependent Characteristic. Microorganisms 2022, 10, 2185. [Google Scholar] [CrossRef]
- Laiño, J.; Villena, J.; Suvorov, A.; Zelaya, H.; Moyano, R.O.; Salva, S.; Alvarez, S. Nasal Immunization with Recombinant Chimeric Pneumococcal Protein and Cell Wall from Immunobiotic Bacteria Improve Resistance of Infant Mice to Streptococcus pneumoniae Infection. PLoS ONE 2018, 13, e0206661. [Google Scholar] [CrossRef]
- Xu, M.; Li, N.; Fan, X.; Zhou, Y.; Bi, S.; Shen, A.; Wang, B. Differential Effects of Toll-Like Receptor Signaling on the Activation of Immune Responses in the Upper Respiratory Tract. Microbiol. Spectr. 2022, 10, e01144-21. [Google Scholar] [CrossRef]
- Baldridge, J. Monophosphoryl Lipid A Enhances Mucosal and Systemic Immunity to Vaccine Antigens Following Intranasal Administration. Vaccine 2000, 18, 2416–2425. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, T.; Hirano, T.; Kodama, S.; Kadowaki, Y.; Moriyama, M.; Kawano, T.; Suzuki, M. Monophosphoryl Lipid A Enhances Nontypeable Haemophilus influenzae-Specific Mucosal and Systemic Immune Responses by Intranasal Immunization. Int. J. Pediatr. Otorhinolaryngol. 2017, 97, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Ichinohe, T.; Watanabe, I.; Ito, S.; Fujii, H.; Moriyama, M.; Tamura, S.; Takahashi, H.; Sawa, H.; Chiba, J.; Kurata, T.; et al. Synthetic Double-Stranded RNA Poly(I:C) Combined with Mucosal Vaccine Protects against Influenza Virus Infection. J. Virol. 2005, 79, 2910–2919. [Google Scholar] [CrossRef] [PubMed]
- Takaki, H.; Kure, S.; Oshiumi, H.; Sakoda, Y.; Suzuki, T.; Ainai, A.; Hasegawa, H.; Matsumoto, M.; Seya, T. Toll-like Receptor 3 in Nasal CD103+ Dendritic Cells Is Involved in Immunoglobulin A Production. Mucosal Immunol. 2018, 11, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A. Liposomal Immunostimulatory DNA Sequence (ISS-ODN): An Efficient Parenteral and Mucosal Adjuvant for Influenza and Hepatitis B Vaccines. Vaccine 2002, 20, 3342–3354. [Google Scholar] [CrossRef]
- Wang, B.-Z.; Xu, R.; Quan, F.-S.; Kang, S.-M.; Wang, L.; Compans, R.W. Intranasal Immunization with Influenza VLPs Incorporating Membrane-Anchored Flagellin Induces Strong Heterosubtypic Protection. PLoS ONE 2010, 5, e13972. [Google Scholar] [CrossRef]
- Mojgani, N.; Shahali, Y.; Dadar, M. Immune Modulatory Capacity of Probiotic Lactic Acid Bacteria and Applications in Vaccine Development. Benef. Microbes 2020, 11, 213–226. [Google Scholar] [CrossRef]
- Villena, J.; Li, C.; Vizoso-Pinto, M.G.; Sacur, J.; Ren, L.; Kitazawa, H. Lactiplantibacillus plantarum as a Potential Adjuvant and Delivery System for the Development of SARS-CoV-2 Oral Vaccines. Microorganisms 2021, 9, 683. [Google Scholar] [CrossRef]
- Medina, M.; Villena, J.; Vintiñi, E.; Hebert, E.M.; Raya, R.; Alvarez, S. Nasal Immunization with Lactococcus lactis Expressing the Pneumococcal Protective Protein A Induces Protective Immunity in Mice. Infect. Immun. 2008, 76, 2696–2705. [Google Scholar] [CrossRef]
- Villena, J.; Medina, M.; Racedo, S.; Alvarez, S. Resistance of Young Mice to Pneumococcal Infection Can Be Improved by Oral Vaccination with Recombinant Lactococcus lactis. J. Microbiol. Immunol. Infect. 2010, 43, 1–10. [Google Scholar] [CrossRef]
- Nicod, L.P.; Cochand, L.; Dreher, D. Antigen Presentation in the Lung: Dendritic Cells and Macrophages. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2000, 17, 246–255. [Google Scholar]
- Kawasaki, T.; Ikegawa, M.; Kawai, T. Antigen Presentation in the Lung. Front. Immunol. 2022, 13, 2186. [Google Scholar] [CrossRef] [PubMed]
- Ina, Y.; Takada, K.; Yamamoto, M.; Morishita, M.; Yoshikawa, K. Antigen-Presenting Capacity of Alveolar Macrophages and Monocytes in Pulmonary Tuberculosis. Eur. Respir. J. 1991, 4, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Vecchiarelli, A.; Dottorini, M.; Pietrella, D.; Monari, C.; Retini, C.; Todisco, T.; Bistoni, F. Role of Human Alveolar Macrophages as Antigen-Presenting Cells in Cryptococcus neoformans Infection. Am. J. Respir. Cell. Mol. Biol. 1994, 11, 130–137. [Google Scholar] [CrossRef]
- Hussell, T.; Bell, T.J. Alveolar Macrophages: Plasticity in a Tissue-Specific Context. Nat. Rev. Immunol. 2014, 14, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Desch, A.N.; Randolph, G.J.; Murphy, K.; Gautier, E.L.; Kedl, R.M.; Lahoud, M.H.; Caminschi, I.; Shortman, K.; Henson, P.M.; Jakubzick, C.V. CD103+ Pulmonary Dendritic Cells Preferentially Acquire and Present Apoptotic Cell–Associated Antigen. J. Exp. Med. 2011, 208, 1789–1797. [Google Scholar] [CrossRef] [PubMed]
- Archambaud, C.; Salcedo, S.P.; Lelouard, H.; Devilard, E.; de Bovis, B.; Van Rooijen, N.; Gorvel, J.-P.; Malissen, B. Contrasting Roles of Macrophages and Dendritic Cells in Controlling Initial Pulmonary Brucella Infection. Eur. J. Immunol. 2010, 40, 3458–3471. [Google Scholar] [CrossRef]
- Claassen, E.; Thepen, T.; Hoeben, K.; Brevé, J.; Kraal, G. Migration of Alveolar Macrophages from Alveolar Space to Paracortical T Cell Area of the Draining Lymph Node. Dendritic Cells Fundam. Clin. Immunol. 1993, 329, 305–310. [Google Scholar]
- Kirby, A.C.; Coles, M.C.; Kaye, P.M. Alveolar Macrophages Transport Pathogens to Lung Draining Lymph Nodes. J. Immunol. 2009, 183, 1983–1989. [Google Scholar] [CrossRef]
- Kawasaki, T.; Ikegawa, M.; Yunoki, K.; Otani, H.; Ori, D.; Ishii, K.J.; Kuroda, E.; Takamura, S.; Kitabatake, M.; Ito, T.; et al. Alveolar Macrophages Instruct CD8+ T Cell Expansion by Antigen Cross-Presentation in Lung. Cell Rep. 2022, 41, 111828. [Google Scholar] [CrossRef]
- Joffre, O.P.; Segura, E.; Savina, A.; Amigorena, S. Cross-Presentation by Dendritic Cells. Nat. Rev. Immunol. 2012, 12, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Kopf, M.; Schneider, C.; Nobs, S.P. The Development and Function of Lung-Resident Macrophages and Dendritic Cells. Nat. Immunol. 2015, 16, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Boyaka, P.N.; Fujihashi, K. Host Defenses at Mucosal Surfaces. In Clinical Immunology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 285–298. [Google Scholar]
- Fujihashi, K.; Sato, S.; Kiyono, H. Mucosal Adjuvants for Vaccines to Control Upper Respiratory Infections in the Elderly. Exp. Gerontol. 2014, 54, 21–26. [Google Scholar] [CrossRef]
- Janoff, E.N.; Fasching, C.; Orenstein, J.M.; Rubins, J.B.; Opstad, N.L.; Dalmasso, A.P. Killing of Streptococcus pneumoniae by Capsular Polysaccharide–Specific Polymeric IgA, Complement, and Phagocytes. J. Clin. Investig. 1999, 104, 1139–1147. [Google Scholar] [CrossRef]
- Sun, K.; Johansen, F.-E.; Eckmann, L.; Metzger, D.W. An Important Role for Polymeric Ig Receptor-Mediated Transport of IgA in Protection against Streptococcus pneumoniae Nasopharyngeal Carriage. J. Immunol. 2004, 173, 4576–4581. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-M.; Ko, H.-J.; Shim, D.-H.; Yang, J.-Y.; Park, Y.-H.; Curtiss, R.; Kweon, M.-N. MyD88 Signaling Is Not Essential for Induction of Antigen-Specific B Cell Responses but Is Indispensable for Protection against Streptococcus pneumoniae Infection Following Oral Vaccination with Attenuated Salmonella Expressing PspA Antigen. J. Immunol. 2008, 181, 6447–6455. [Google Scholar] [CrossRef]
- Ferreira, D.M.; Darrieux, M.; Silva, D.A.; Leite, L.C.C.; Ferreira, J.M.C.; Ho, P.L.; Miyaji, E.N.; Oliveira, M.L.S. Characterization of Protective Mucosal and Systemic Immune Responses Elicited by Pneumococcal Surface Protein PspA and PspC Nasal Vaccines against a Respiratory Pneumococcal Challenge in Mice. Clin. Vaccine Immunol. 2009, 16, 636–645. [Google Scholar] [CrossRef]
- Fukuyama, Y.; King, J.D.; Kataoka, K.; Kobayashi, R.; Gilbert, R.S.; Oishi, K.; Hollingshead, S.K.; Briles, D.E.; Fujihashi, K. Secretory-IgA Antibodies Play an Important Role in the Immunity to Streptococcus pneumoniae. J. Immunol. 2010, 185, 1755–1762. [Google Scholar] [CrossRef]
- Miyasaka, T.; Akahori, Y.; Toyama, M.; Miyamura, N.; Ishii, K.; Saijo, S.; Iwakura, Y.; Kinjo, Y.; Miyazaki, Y.; Oishi, K.; et al. Dectin-2-Dependent NKT Cell Activation and Serotype-Specific Antibody Production in Mice Immunized with Pneumococcal Polysaccharide Vaccine. PLoS ONE 2013, 8, e78611. [Google Scholar] [CrossRef]
- Saeland, E.; Vidarsson, G.; Leusen, J.H.; Van Garderen, E.; Nahm, M.H.; Vile-Weekhout, H.; Walraven, V.; Stemerding, A.M.; Verbeek, J.S.; Rijkers, G.T. Central Role of Complement in Passive Protection by Human IgG1 and IgG2 Anti-Pneumococcal Antibodies in Mice. J. Immunol. 2003, 170, 6158–6164. [Google Scholar] [CrossRef]
- Bittar, F.; Cassagne, C.; Bosdure, E.; Stremler, N.; Dubus, J.-C.; Sarles, J.; Reynaud-Gaubert, M.; Raoult, D.; Rolain, J.-M. Outbreak of Corynebacterium pseudodiphtheriticum Infection in Cystic Fibrosis Patients, France. Emerg. Infect. Dis. 2010, 16, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Van Roeden, S.E.; Thijsen, S.F.; Sankatsing, S.U.C.; Limonard, G.J.M. Clinical Relevance of Corynebacterium pseudodiphtheriticum in Lower Respiratory Tract Specimens. Infect. Dis. 2015, 47, 862–868. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Ote, H.; Arai, T.; Kazama, R.; Kimura, K.; Nagata, T.; Kumasawa, J.; Kohno, M.; Kohata, H.; Nishida, K. Corynebacterium pseudodiphtheriticum as a Pathogen in Bacterial Co-Infection in COVID-19 Patients on Mechanical Ventilation. Jpn. J. Infect. Dis. 2022, 75, 202–204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hosomi, K.; Shimoyama, A.; Yoshii, K.; Nagatake, T.; Fujimoto, Y.; Kiyono, H.; Fukase, K.; Kunisawa, J. Lipopolysaccharide Derived From the Lymphoid-Resident Commensal Bacteria Alcaligenes faecalis Functions as an Effective Nasal Adjuvant to Augment IgA Antibody and Th17 Cell Responses. Front. Immunol. 2021, 12, 699349. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortiz Moyano, R.; Raya Tonetti, F.; Fukuyama, K.; Elean, M.; Tomokiyo, M.; Suda, Y.; Melnikov, V.; Kitazawa, H.; Villena, J. The Respiratory Commensal Bacterium Corynebacterium pseudodiphtheriticum as a Mucosal Adjuvant for Nasal Vaccines. Vaccines 2023, 11, 611. https://doi.org/10.3390/vaccines11030611
Ortiz Moyano R, Raya Tonetti F, Fukuyama K, Elean M, Tomokiyo M, Suda Y, Melnikov V, Kitazawa H, Villena J. The Respiratory Commensal Bacterium Corynebacterium pseudodiphtheriticum as a Mucosal Adjuvant for Nasal Vaccines. Vaccines. 2023; 11(3):611. https://doi.org/10.3390/vaccines11030611
Chicago/Turabian StyleOrtiz Moyano, Ramiro, Fernanda Raya Tonetti, Kohtaro Fukuyama, Mariano Elean, Mikado Tomokiyo, Yoshihito Suda, Vyacheslav Melnikov, Haruki Kitazawa, and Julio Villena. 2023. "The Respiratory Commensal Bacterium Corynebacterium pseudodiphtheriticum as a Mucosal Adjuvant for Nasal Vaccines" Vaccines 11, no. 3: 611. https://doi.org/10.3390/vaccines11030611






