Immunity from NK Cell Subsets Is Important for Vaccine-Mediated Protection in HPV+ Cancers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tumor Cell Lines
2.3. In Vivo Tumor Challenge
2.4. Intranasal Vaccination
2.5. Magnetic Resonance Imaging (MRI)
2.6. Immune Cell Isolation
2.7. Flow Cytometry
2.8. Statistical Analysis
3. Results
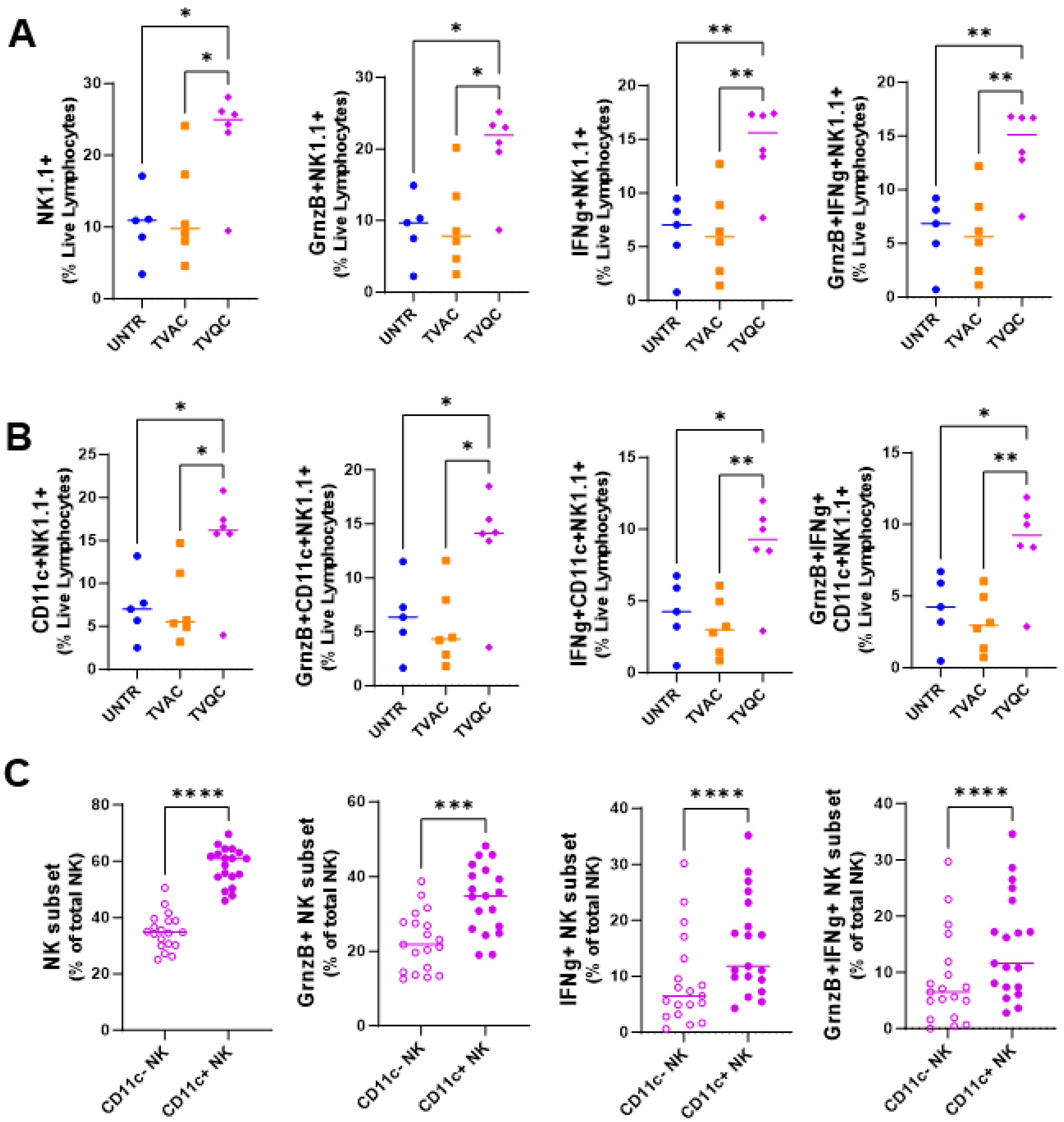
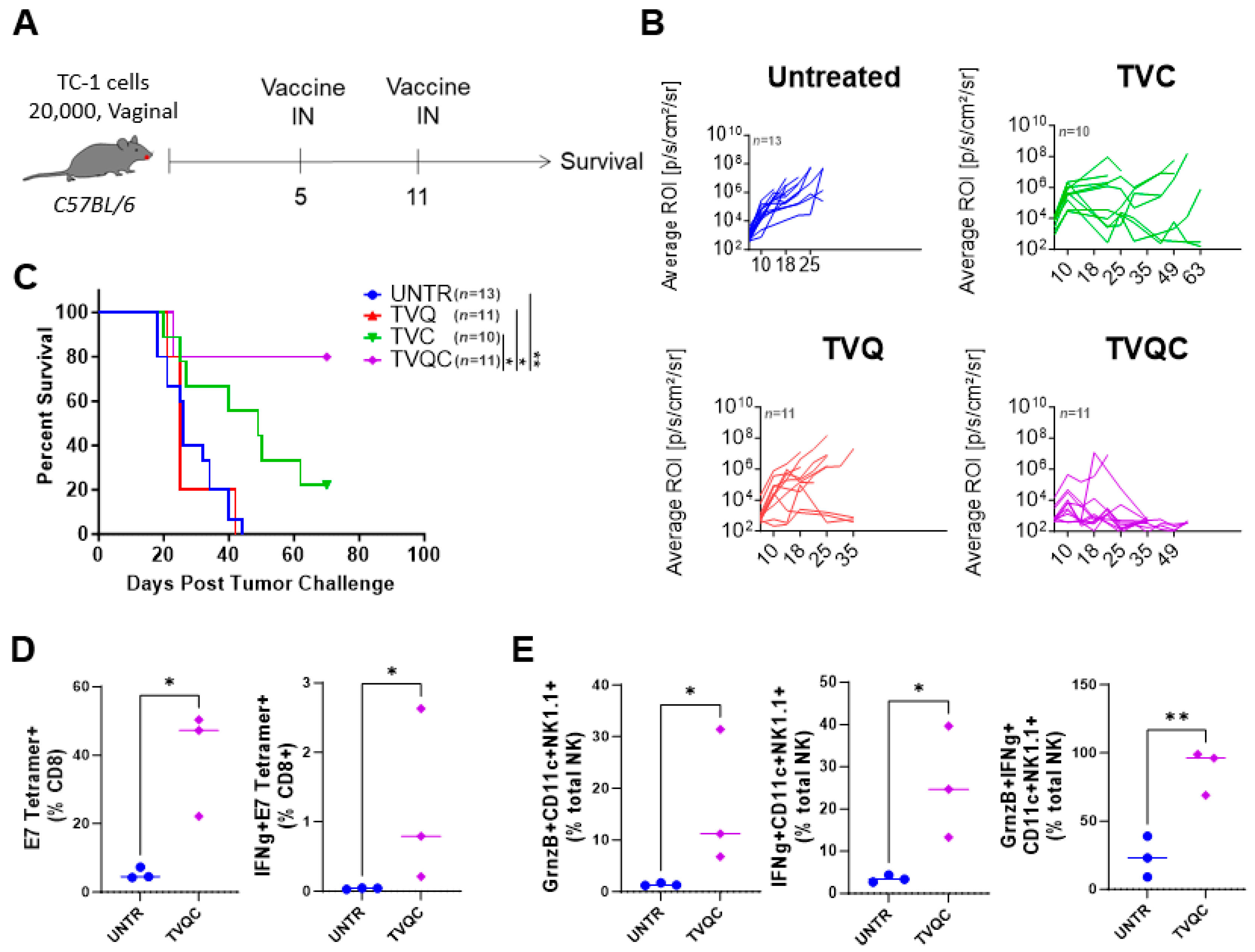
3.1. Association of Innate Immunity from NKDCs with Vaccine-Mediated Protection in the Oral HPV Tumor Mouse Model
3.2. Both HPV E7-Specific and NKDC Responses Are Associated with TVQC-Mediated Protection against HPV Vaginal Tumors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berman, T.A.; Schiller, J.T. Human papillomavirus in cervical cancer and oropharyngeal cancer: One cause, two diseases. Cancer 2017, 123, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.A.; Jhingran, A.; Oaknin, A.; Denny, L. Cervical cancer. Lancet 2019, 393, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Gillison, M.L. Human papillomavirus in head and neck cancer: Its role in pathogenesis and clinical implications. Clin. Cancer Res. 2009, 15, 6758–6762. [Google Scholar] [CrossRef] [PubMed]
- Gillison, M.L.; Chaturvedi, A.K.; Anderson, W.F.; Fakhry, C. Epidemiology of Human Papillomavirus–Positive Head and Neck Squamous Cell Carcinoma. J. Clin. Oncol. 2015, 33, 3235–3242. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Jeang, J.; Cheng, K.; Cheng, T.; Yang, B.; Wu, T.-C.; Hung, C.-F. Current state in the development of candidate therapeutic HPV vaccines. Expert Rev. Vaccines 2016, 15, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Maraj, B.; Tran, N.P.; Knoff, J.; Chen, A.; Alvarez, R.D.; Hung, C.-F.; Wu, T.-C. Emerging human papillomavirus vaccines. Expert Opin. Emerg. Drugs 2012, 17, 469–492. [Google Scholar] [CrossRef] [PubMed]
- van Poelgeest, M.I.; Welters, M.J.; van Esch, E.M.; Stynenbosch, L.F.; Kerpershoek, G.; van Persijn van Meerten, E.L.; van den Hende, M.; Löwik, M.J.G.; Berends-van der Meer, D.M.A.; Fathers, L.M. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. J. Transl. Med. 2013, 11, 88. [Google Scholar] [CrossRef]
- Akira, S. Innate Immunity and Adjuvants. Philos. Trans. R Soc. Lond. B Biol. Sci. 2011, 366, 2748–2755. [Google Scholar] [CrossRef]
- Dar, T.B.; Henson, R.M.; Shiao, S.L. Targeting Innate Immunity to Enhance the Efficacy of Radiation Therapy. Front. Immunol. 2018, 9, 3077. [Google Scholar] [CrossRef]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Villegas, F.R.; Coca, S.; Villarrubia, V.G.; Jiménez, R.; Chillón, M.J.; Jareño, J.; Zuil, M.; Callol, L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer 2002, 35, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, S.N.; Reighard, S.D.; Gyurova, I.E.; A Cranert, S.A.; E Mahl, S.E.; Karmele, E.P.; McNally, J.P.; Moran, M.T.; Brooks, T.R.; Yaqoob, F.; et al. Roles of natural killer cells in antiviral immunity. Curr. Opin. Virol. 2016, 16, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Pashine, A.; Valiante, N.M.; Ulmer, J.B. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 2005, 11 (Suppl. 4), S63–S68. [Google Scholar] [CrossRef] [PubMed]
- Hermans, I.F.; Silk, J.D.; Gileadi, U.; Salio, M.; Mathew, B.; Ritter, G.; Schmidt, R.; Harris, A.L.; Old, L.; Cerundolo, V. NKT Cells Enhance CD4+ and CD8+ T Cell Responses to Soluble Antigen In Vivo through Direct Interaction with Dendritic Cells. J. Immunol. 2003, 171, 5140–5147. [Google Scholar] [CrossRef] [PubMed]
- Silk, J.D.; Hermans, I.F.; Gileadi, U.; Chong, T.W.; Shepherd, D.; Salio, M.; Mathew, B.; Schmidt, R.R.; Lunt, S.J.; Williams, K.J.; et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell–mediated immunotherapy. J. Clin. Investig. 2004, 114, 1800–1811. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.H.; Lee, J.; Kline, L.; Mathison, J.C.; Ulevitch, R.J. Toll-like receptor 9 mediates CpG-DNA signaling. J. Leukoc. Biol. 2002, 71, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Soltysik, S.; Wu, J.-Y.; Recchia, J.; Wheeler, D.A.; Newman, M.J.; Coughlin, R.T.; Kensil, C.R. Structure/function studies of QS-21 adjuvant: Assessment of triterpene aldehyde and glucuronic acid roles in adjuvant function. Vaccine 1995, 13, 1403–1410. [Google Scholar] [CrossRef]
- Marciani, D.J. Elucidating the Mechanisms of Action of Saponin-Derived Adjuvants. Trends Pharmacol. Sci. 2018, 39, 573–585. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Tortolero-Luna, G.; Follen, M.; Sastry, K.J. Inverse correlation of cellular immune responses specific to synthetic peptides from the E6 and E7 oncoproteins of HPV-16 with recurrence of cervical intraepithelial neoplasia in a cross-sectional study. Gynecol. Oncol. 2005, 99 (Suppl. 1), S251–S261. [Google Scholar] [CrossRef]
- Manuri, P.R.; Nehete, B.; Nehete, P.N.; Reisenauer, R.; Wardell, S.; Courtney, A.N.; Gambhira, R.; Lomada, D.; Chopra, A.K.; Sastry, K.J. Intranasal immunization with synthetic peptides corresponding to the E6 and E7 oncoproteins of human papillomavirus type 16 induces systemic and mucosal cellular immune responses and tumor protection. Vaccine 2007, 25, 3302–3310. [Google Scholar] [CrossRef]
- Sierra, G.; Dorta-Estremera, S.; Hegde, V.L.; Nookala, S.M.; Yanamandra, A.V.; Sastry, K.J. Intranasal Therapeutic Peptide Vaccine Promotes Efficient Induction and Trafficking of Cytotoxic T Cell Response for the Clearance of HPV Vaginal Tumors. Vaccines 2020, 8, 259. [Google Scholar] [CrossRef]
- Williams, R.; Lee, D.W.; Elzey, B.D.; Anderson, M.E.; Hostager, B.S.; Lee, J.H. Preclinical models of HPV+ and HPV− HNSCC in mice: An immune clearance of HPV+ HNSCC. Head Neck 2009, 31, 911–918. [Google Scholar] [CrossRef] [PubMed]
- Dorta-Estremera, S.; Chin, R.L.; Sierra, G.; Nicholas, C.; Yanamandra, A.V.; Nookala, S.M.; Yang, G.; Singh, S.; Curran, M.A.; Sastry, K.J. Mucosal HPV E6/E7 Peptide Vaccination in Combination with Immune Checkpoint Modulation Induces Regression of HPV(+) Oral Cancers. Cancer Res. 2018, 78, 5327–5339. [Google Scholar] [CrossRef] [PubMed]
- Dorta-Estremera, S.; Hegde, V.L.; Slay, R.B.; Sun, R.; Yanamandra, A.V.; Nicholas, C.; Nookala, S.; Sierra, G.; Curran, M.A. Targeting interferon signaling and CTLA-4 enhance the therapeutic efficacy of anti-PD-1 immunotherapy in preclinical model of HPV(+) oral cancer. J. Immunother. Cancer 2019, 7, 252. [Google Scholar] [CrossRef]
- Decrausaz, L.; Gonçalves, A.; Domingos-Pereira, S.; Pythoud, C.; Stehle, J.; Schiller, J.; Jichlinski, P.; Nardelli-Haefliger, D. A novel mucosal orthotopic murine model of human papillomavirus-associated genital cancers. Int. J. Cancer 2011, 128, 2105–2113. [Google Scholar] [CrossRef] [PubMed]
- Bartkowiak, T.; Singh, S.; Yang, G.; Galvan, G.; Haria, D.; Ai, M.; Allison, J.P.; Sastry, K.J.; Curran, M.A. Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. Proc. Natl. Acad. Sci. USA 2015, 112, E5290–E5299. [Google Scholar] [CrossRef] [PubMed]
- Steinhagen, F.; Kinjo, T.; Bode, C.; Klinman, D.M. TLR-based immune adjuvants. Vaccine 2011, 29, 3341–3355. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Kirschning, C.J.; Häcker, H.; Redecke, V.; Hausmann, S.; Akira, S.; Wagner, H.; Lipford, G.B. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 2001, 98, 9237–9242. [Google Scholar] [CrossRef] [PubMed]
- Terme, M.; Mignot, G.; Ullrich, E.; Bonmort, M.; Minard-Colin, V.; Jacquet, A.; Schultze, J.L.; Kroemer, G.; Leclerc, C.; Chaput, N.; et al. The Dendritic Cell–like Functions of IFN-Producing Killer Dendritic Cells Reside in the CD11b+ Subset and Are Licensed by Tumor Cells. Cancer Res. 2009, 69, 6590–6597. [Google Scholar] [CrossRef]
- Lin, K.Y.; Guarnieri, F.G.; Staveley-O’Carroll, K.F.; Levitsky, H.I.; August, J.T.; Pardoll, D.M.; Wu, T.C. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996, 56, 21–26. [Google Scholar]
- Moynihan, K.D.; Irvine, D.J. Roles for Innate Immunity in Combination Immunotherapies. Cancer Res. 2017, 77, 5215–5221. [Google Scholar] [CrossRef]
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infil-trating immune cells across human cancers. Nat. Med. 2015, 21, 938–945. [Google Scholar] [CrossRef]
- Chaudhry, U.I.; Plitas, G.; Burt, B.M.; Kingham, T.P.; Raab, J.R.; DeMatteo, R.P. NK Dendritic Cells Expanded in IL-15 Exhibit Antitumor Responses In Vivo. J. Immunol. 2007, 179, 4654–4660. [Google Scholar] [CrossRef]
- Taieb, J.; Chaput, N.; Ménard, C.; Apetoh, L.; Ullrich, E.; Bonmort, M.; Péquignot, M.; Casares, N.; Terme, M.; Flament, C. A novel dendritic cell subset involved in tumor immunosurveillance. Nat. Med. 2006, 12, 214–219. [Google Scholar] [CrossRef]
- Cullen, S.P.; Brunet, M.; Martin, S.J. Granzymes in cancer and immunity. Cell Death Differ. 2010, 17, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Plitas, G.; Chaudhry, U.I.; Kingham, T.P.; Raab, J.R.; DeMatteo, R.P. NK Dendritic Cells Are Innate Immune Responders to Listeria monocytogenes Infection. J. Immunol. 2007, 178, 4411–4416. [Google Scholar] [CrossRef] [PubMed]
- Hancock, G.E.; Heers, K.M.; Smith, J.D. QS-21 Synergizes with Recombinant Interleukin-12 to Create a Potent Adjuvant Formulation for the Fusion Protein of Respiratory Syncytial Virus. Viral Immunol. 2000, 13, 503–509. [Google Scholar] [CrossRef]
- Mikloska, Z.; Ruckholdt, M.; Ghadiminejad, I.; Dunckley, H.; Denis, M.; Cunningham, A.L. Monophosphoryl lipid A and QS21 increase CD8 T lymphocyte cytotoxicity to herpes simplex virus-2 infected cell proteins 4 and 27 through IFN-gamma and IL-12 production. J. Immunol. 2000, 164, 5167–5176. [Google Scholar] [CrossRef] [PubMed]
- Silla, S.; Fallarino, F.; Boon, T.; Uyttenhove, C. Enhancement by IL-12 of the cytolytic T lymphocyte (CTL) response of mice immunized with tumor-specific peptides in an adjuvant containing QS21 and MPL. Eur. Cytokine Netw. 1999, 10, 181–190. [Google Scholar]
- Lacaille-Dubois, M.-A. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review. Phytomedicine 2019, 60, 152905. [Google Scholar] [CrossRef]
- Iizuka, A.; Ikarashi, Y.; Yoshida, M.; Heike, Y.; Takeda, K.; Quinn, G.; Wakasugi, H.; Kitagawa, M.; Takaue, Y. Interleukin (IL)-4 promotes T helper type 2-biased natural killer T (NKT) cell expansion, which is regulated by NKT cell-derived interferon-gamma and IL-4. Immunology 2008, 123, 100–107. [Google Scholar] [CrossRef]
- Yin, S.; Wang, H.; Bertola, A.; Feng, D.; Xu, M.-J.; Wang, Y.; Gao, B. Activation of invariant natural killer T cells impedes liver regeneration by way of both IFN-gamma- and IL-4-dependent mechanisms. Hepatology 2014, 60, 1356–1366. [Google Scholar] [CrossRef]
- Semmling, V.; Lukacs-Kornek, V.; A Thaiss, C.; Quast, T.; Hochheiser, K.; Panzer, U.; Rossjohn, J.; Perlmutter, P.; Cao, J.; I Godfrey, D.; et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell–licensed DCs. Nat. Immunol. 2010, 11, 313–320. [Google Scholar] [CrossRef]
- Thaiss, C.A.; Semmling, V.; Franken, L.; Wagner, H.; Kurts, C. Chemokines: A New Dendritic Cell Signal for T Cell Activation. Front. Immunol. 2011, 2, 31. [Google Scholar] [CrossRef]
- Cheng, W.F.; Hung, C.F.; Lin, K.Y.; Ling, M.; Juang, J.; He, L.; Lin, C.T. CD8+ T cells, NK cells and IFN-gamma are important for control of tumor with downregulated MHC class I expression by DNA vaccination. Gene Ther. 2003, 10, 1311–1320. [Google Scholar] [CrossRef]
- Young, H.A.; Hardy, K.J. Role of interferon-gamma in immune cell regulation. J. Leukoc. Biol. 1995, 58, 373–381. [Google Scholar] [CrossRef]
- Street, D.; Kaufmann, A.M.; Vaughan, A.; Fisher, S.G.; Hunter, M.; Schreckenberger, C.; Potkul, R.K.; Gissmann, L.; Qiao, L. Interferon-gamma enhances susceptibility of cervical cancer cells to lysis by tumor-specific cytotoxic T cells. Gynecol. Oncol. 1997, 65, 265–272. [Google Scholar] [CrossRef]
- Martini, M.; Innamorati, G.; Mazzocco, M.; Ugel, S.; Bronte, V.; Krampera, M.; Mosna, F.; Cestari, T.; Colombatti, M.; Sartoris, S. IFN-gamma-mediated upmodulation of MHC class I expression activates tumor-specific immune response in a mouse model of prostate cancer. Vaccine 2010, 28, 3548–3557. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; NI, J.; Meng, H.; Li, D.; Wei, Y.; Luo, Y.; Wu, Y. Interleukin-15: A potent adjuvant enhancing the efficacy of an autologous whole-cell tumor vaccine against Lewis lung carcinoma. Mol. Med. Rep. 2014, 10, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Umemura, M.; Nishimura, H.; Saito, K.; Yajima, T.; Matsuzaki, G.; Mizuno, S.; Sugawara, I.; Yoshikai, Y. Interleukin-15 as an immune adjuvant to increase the efficacy of Mycobacterium bovis bacillus Calmette-Guerin vaccination. Infect. Immun. 2003, 71, 6045–6048. [Google Scholar] [CrossRef] [PubMed]
- Melchionda, F.; Fry, T.J.; Milliron, M.J.; McKirdy, M.A.; Tagaya, Y.; Mackall, C.L. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J. Clin. Investig. 2005, 115, 1177–1187. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Xie, S.; Chen, M.; Li, Y.; Yue, J.; Ma, J.; Shu, X.; He, Y.; Xiao, W.; Tian, Z. Advances in NK cell production. Cell. Mol. Immunol. 2022, 19, 460–481. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Nakazawa, T.; Nakamura, M.; Nishimura, F.; Matsuda, R.; Omoto, K.; Shida, Y.; Murakami, T.; Nakagawa, I.; Motoyama, Y.; et al. Ex vivo-expanded highly purified natural killer cells in combination with temozolomide induce antitumor effects in human glioblastoma cells in vitro. PLoS ONE 2019, 14, e0212455. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Hara, M.P.; Yanamandra, A.V.; Sastry, K.J. Immunity from NK Cell Subsets Is Important for Vaccine-Mediated Protection in HPV+ Cancers. Vaccines 2024, 12, 206. https://doi.org/10.3390/vaccines12020206
O’Hara MP, Yanamandra AV, Sastry KJ. Immunity from NK Cell Subsets Is Important for Vaccine-Mediated Protection in HPV+ Cancers. Vaccines. 2024; 12(2):206. https://doi.org/10.3390/vaccines12020206
Chicago/Turabian StyleO’Hara, Madison P., Ananta V. Yanamandra, and K. Jagannadha Sastry. 2024. "Immunity from NK Cell Subsets Is Important for Vaccine-Mediated Protection in HPV+ Cancers" Vaccines 12, no. 2: 206. https://doi.org/10.3390/vaccines12020206




