Virus-Like Particle (VLP) Plus Microcrystalline Tyrosine (MCT) Adjuvants Enhance Vaccine Efficacy Improving T and B Cell Immunogenicity and Protection against Plasmodium berghei/vivax
Abstract
:1. Introduction
2. Material and Methods
2.1. Construction, Expression and Purifications of PvTRAP Protein
2.2. Vaccines Preparation Based on PvTRAP Protein
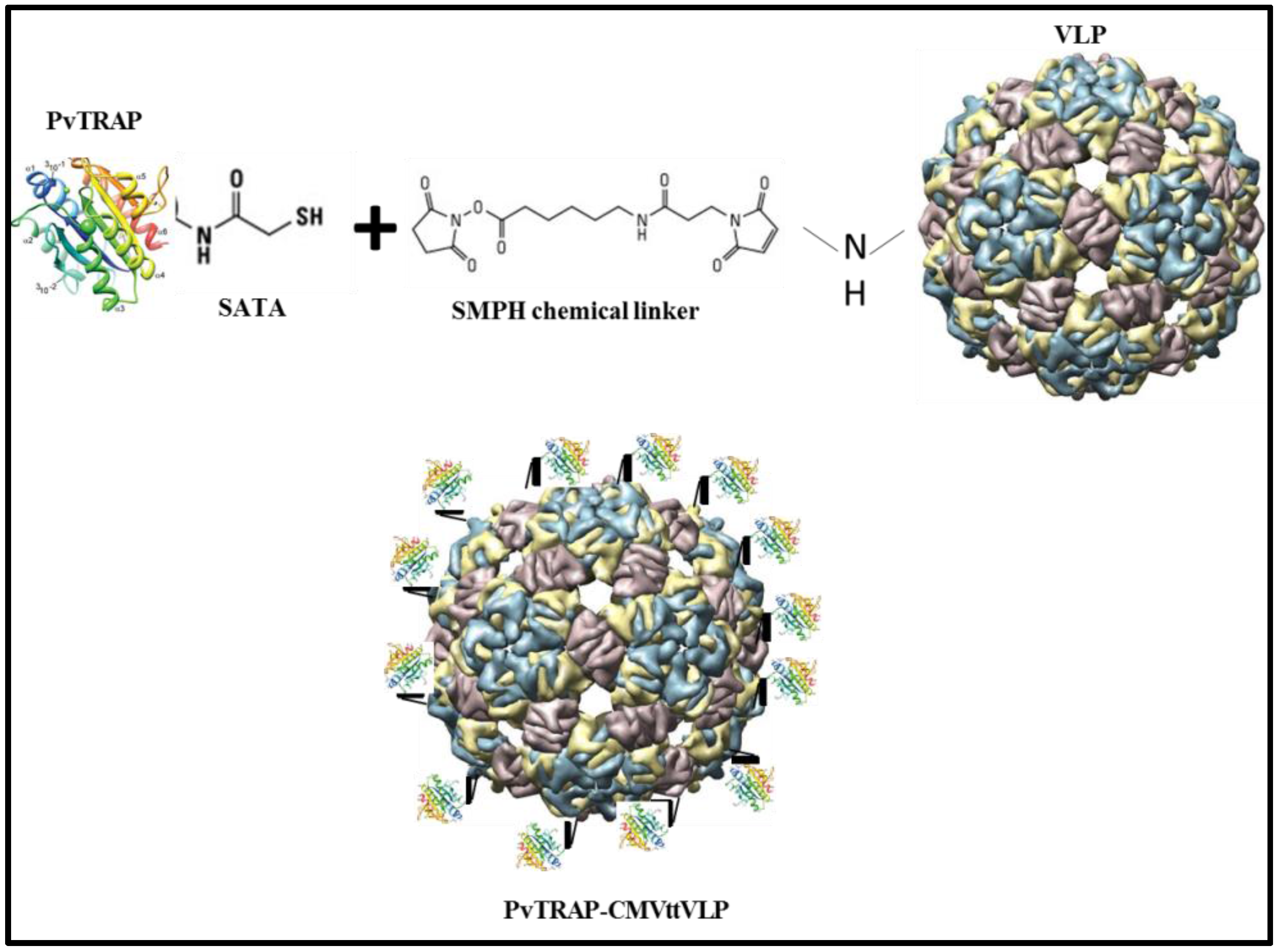
2.2.1. CMVtt-PvTRAP (Chemically Coupled) Vaccine Preparation
2.2.2. Vaccine Formulation Using MCT as Adjuvant
2.2.3. Vaccine Based on Aluminium Hydroxide Adjuvant (Alum)
2.3. Experimental Design
2.4. Antibody Production Assessment
2.5. Intracellular Cytokine Staining (ICS)
2.6. Parasite Production and Challenge
2.7. Statistical Model Applied for Predict Parasitaemia and Protection
2.8. Ethics Statement
3. Results and Discussion
3.1. Generation of Vaccine Candidates and Formulation of VLPs with MCT
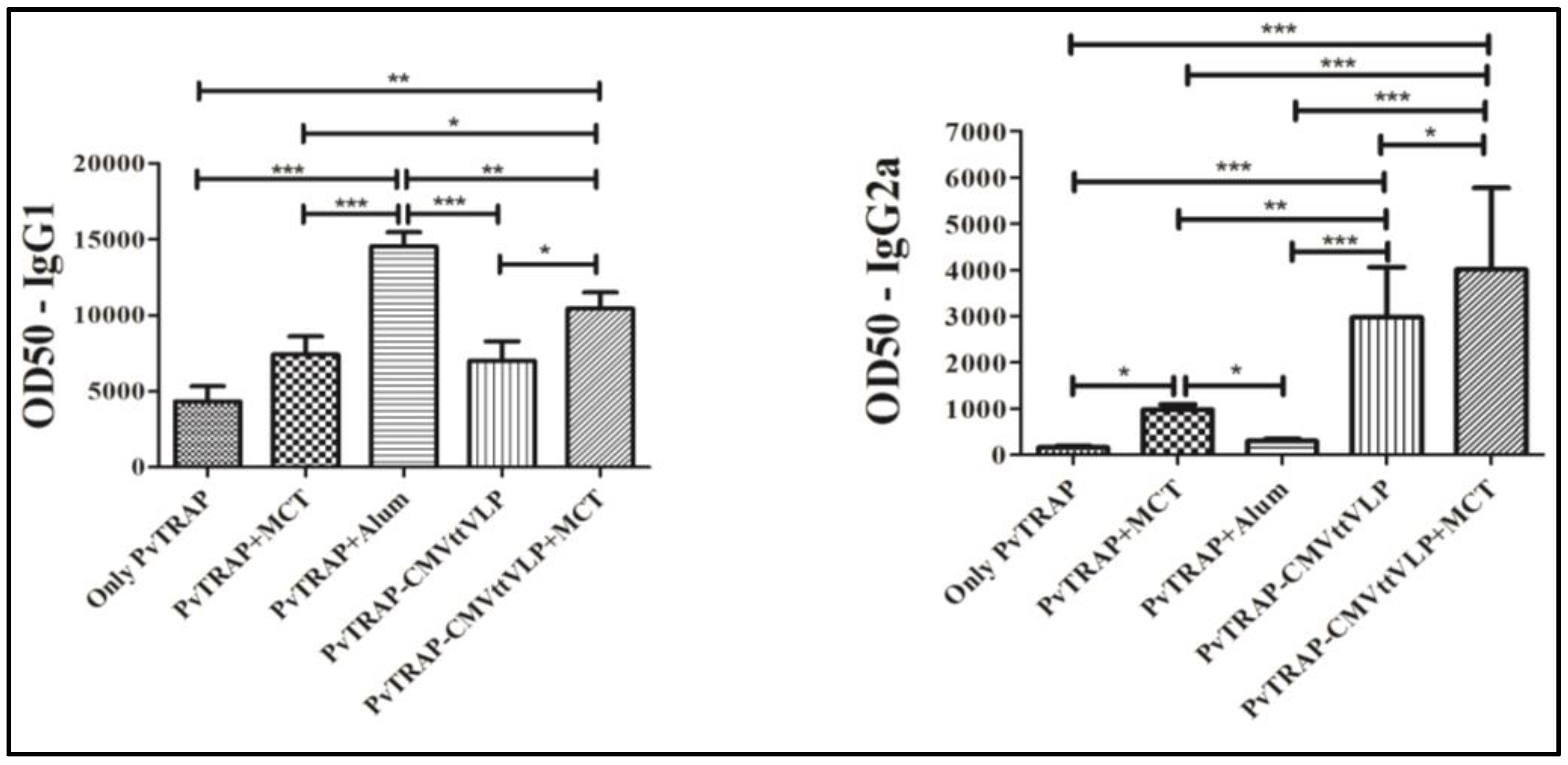
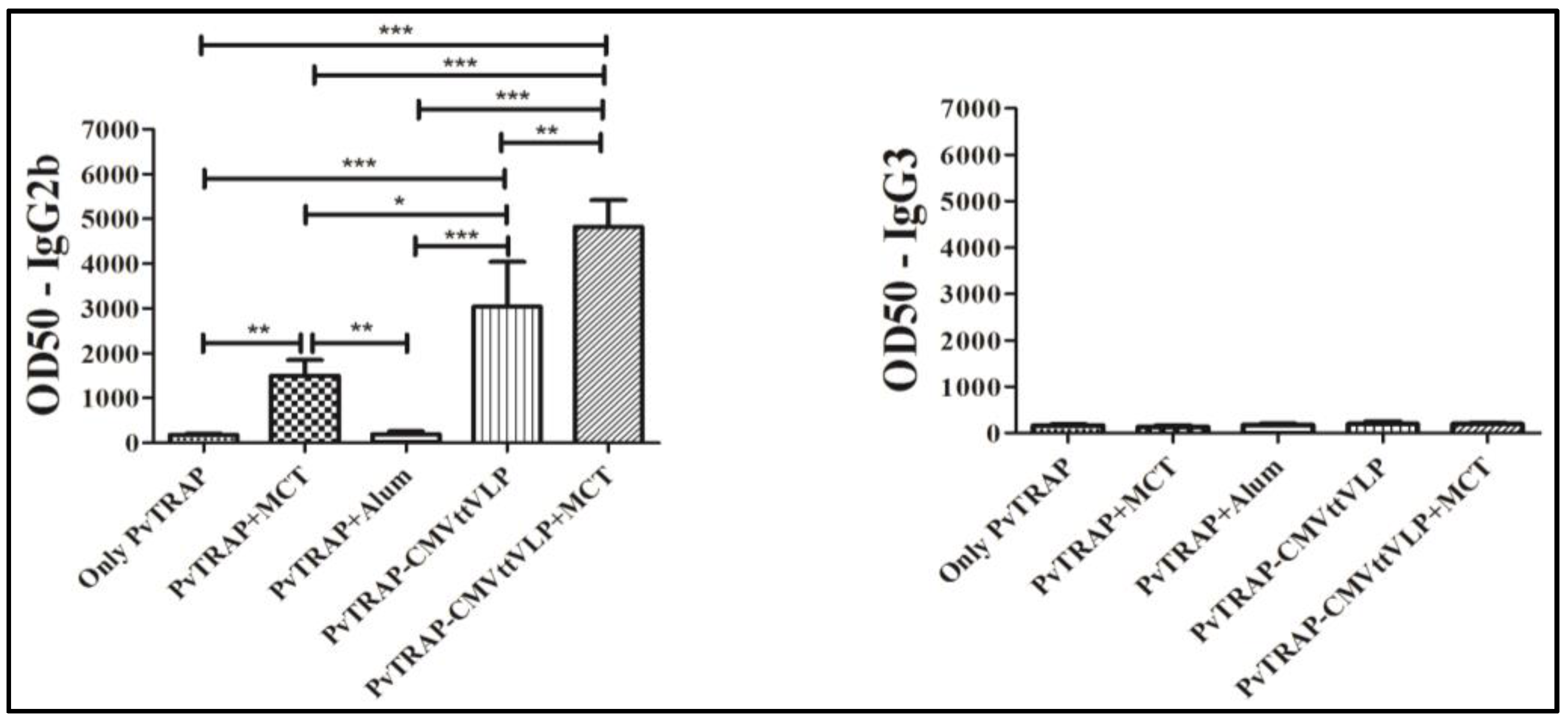
3.2. Humoral Immune Responses
3.3. Cellular Immunogenicity
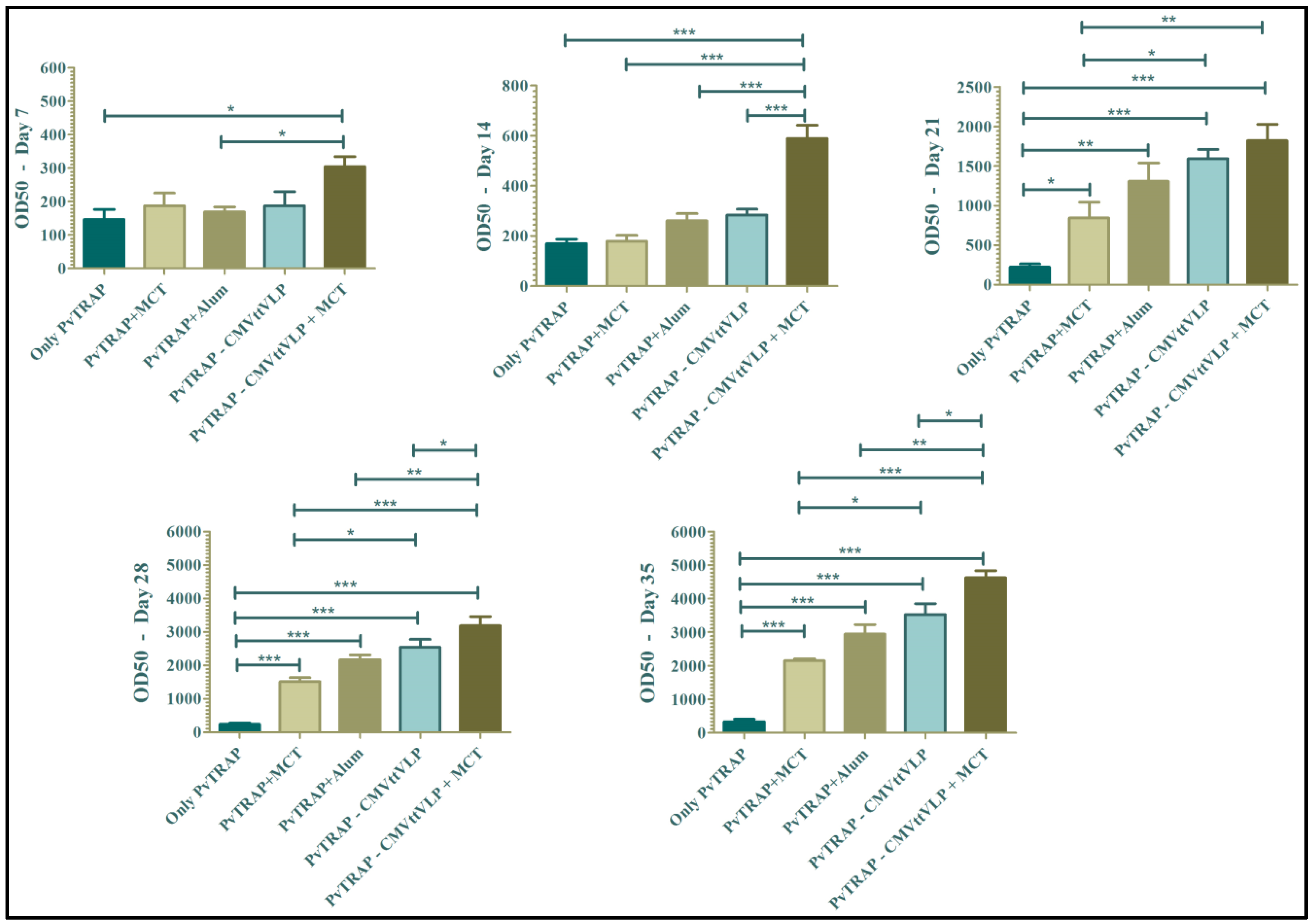
3.4. Assessment of Vaccine Efficacy
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Garner, P.; Gelband, H.; Graves, P.; Jones, K.; Maclehose, H.; Olliaro, P. Systematic Reviews in Malaria: Global Policies Need Global Reviews. Infect. Dis. Clin. N. Am. 2009, 23, 387–404. [Google Scholar] [CrossRef] [PubMed]
- Gething, P.W.; Elyazar, I.R.F.; Moyes, C.L.; Smith, D.L.; Battle, K.E.; Guerra, C.A.; Patil, A.P.; Tatem, A.J.; Howes, R.E.; Myers, M.F.; et al. A long neglected world malaria map: Plasmodium vivax endemicity in 2010. PLoS Negl. Trop. Dis. 2012, 6, e1814. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S. Epidemiological aspects of vivax and falciparum malaria: Global spectrum. Asian Pac. J. Trop. Dis. 2014, 4, S13–S26. [Google Scholar] [CrossRef]
- World Health Organization. Available online: http://www.who.int/mediacentre/factsheets/fs094/en/ (accessed on 20 December 2015).
- Bousema, T.; Drakeley, C. Epidemiology and Infectivity of Plasmodium falciparum and Plasmodium vivax Gametocytes in Relation to Malaria Control and Elimination. CMR 2011, 24, 377–410. [Google Scholar] [CrossRef] [PubMed]
- International Travel and Health. Available online: http://www.who.int/ith/diseases/malaria/en/ (accessed on 30 March 2017).
- The RTS, S Clinical Trials Partnership. Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites. PLoS Med. 2014. [CrossRef]
- Ejigiri, I.; Ragheb, D.R.T.; Pino, P.; Coppi, A.; Bennett, B.L.; Soldati-Favre, D.; Sinnis, P. Shedding of TRAP by a Rhomboid Protease from the Malaria Sporozoite Surface Is Essential for Gliding Motility and Sporozoite Infectivity. PLoS Pathog. 2012. [Google Scholar] [CrossRef] [PubMed]
- Longley, R.J.; Bauza, K.; Ewer, K.J.; Hill, A.V.S.; Spencer, A.J. Development of an In Vitro Assay and Demonstration of Plasmodium berghei Liver-Stage Inhibition by TRAP-Specific CD8+ T Cells. PLoS ONE 2015. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.J.; Cottingham, M.G.; Jenks, J.A.; Longley, R.J.; Capone, S.; Colloca, S.; Folgori, A.; Cortese, R.; Nicosia, A.; Bregu, M.; et al. Enhanced Vaccine-Induced CD8+ T Cell Responses to Malaria Antigen METRAP by Fusion to MHC Class II Invariant Chain. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed]
- White, M.T.; Griffin, J.T.; Riley, E.M.; Drakeley, C.J.; Moorman, A.M.; Sumba, P.O.; Kazura, J.W.; Ghani, A.C.; John, C.C. Efficacy model for antibody-mediated pre-erythrocytic malaria vaccines. Proc. R. Soc. B 2011, 278, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Leroux-Roels, G. Unmet needs in modern vaccinology: Adjuvants to improve the immune response. Vaccine 2010, 28, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, T.R. The mechanisms of action of vaccines containing aluminum adjuvants: An in vitro vs. in vivo paradigm. Springer Plus 2015. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.M.; Shi, Y. Alum: An old dog with new tricks. Emerg. Microbes Infect. 2016. [Google Scholar] [CrossRef] [PubMed]
- Garçon, N.; Leroux-Roels, G.; Wen-Fang, C. Understanding Modern Vaccines: Perspectives in Vaccinology; Chapter: Vaccine adjuvants; Elsevier: Amsterdam, Netherlands, 2001; Volume 1, pp. 89–113. [Google Scholar]
- Kramer, M.F.; Heath, M.D. Aluminium in allergen-specific subcutaneous immunotherapy—A German perspective. Vaccine 2014, 32, 4140–4148. [Google Scholar] [CrossRef] [PubMed]
- Bergfors, E.; Trollfors, B.; Inerot, A. Unexpectedly high incidence of persistent itching nodules and delayed hypersensitivity to aluminium in children after the use of adsorbed vaccines from a single manufacturer. Vaccine 2003, 22, 64–69. [Google Scholar] [CrossRef]
- Exley, C. Aluminium adjuvants and adverse events in sub-cutaneous allergy immunotherapy. AACI 2014, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, R.K.; Eidi, H.; Crépeaux, G.; Authier, F.J.; Cadusseau, J. Biopersistence and brain translocation of aluminum adjuvants of vaccines. Front. Neurol. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Marrack, P.; McKee, A.S.; Munks, M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Franchi, L.; Eigenbrod, T.; Muñoz-Planillo, R.; Nuñez, G. The Inflammasome: A Caspase-1 Activation Platform Regulating Immune Responses and Disease Pathogenesis. Nat. Immunol. 2009. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, P.; Richardson, D.; Wheeler, A.W. Review of L-tyrosine confirming its safe human use as an adjuvant. J. Appl. Toxicol. 2002, 22, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Leuthard, D.; Freiberger, S.N.; Weiss, S.; Duda, A.; Heath, M.D.; Skinner, M.A.; Kramer, M.F.; Kuendig, T.M.; Johansen, P. Microcrystalline Tyrosine as an Adjuvant in Allergy Immunotherapy. In ALLERGY; Wiley: Hoboken, NJ, USA, 2016; Volume 71, pp. 325–326. [Google Scholar]
- Lu, Y.; Chana, W.; Koa, B.Y.; VanLanga, C.C.; Swartz, J.R. Assessing sequence plasticity of a virus-like nanoparticle by evolution toward a versatile scaffold for vaccines and drug delivery. PNAS 2015, 112, 12360–12365. [Google Scholar] [CrossRef] [PubMed]
- Spohn, G.; Jennings, G.T.; Martina, B.E.E.; Keller, I.; Beck, M.; Pumpens, P.; Osterhaus, A.D.; Bachmann, M.F. A VLP-based vaccine targeting domain III of the West Nile virus E protein protects from lethal infection in mice. Virol. J. 2010, 7, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Zabel, F.; Mohanan, D.; Bessa, J.; Link, A.; Fettelschoss, A.; Saudan, P.; Kündig, T.M.; Bachmann, M.F. Viral Particles Drive Rapid Differentiation of Memory B Cells into Secondary Plasma Cells Producing Increased Levels of Antibodies. J. Immunol. 2014, 192, 5499–5508. [Google Scholar] [CrossRef] [PubMed]
- Bauza, K.; Malinauskas, T.; Pfander, C.; Anar, B.; Jones, E.Y.; Billker, O.; Hill, A.V.; Reyes-Sandoval, A. Efficacy of a Plasmodium vivax Malaria Vaccine Using ChAd63 and Modified Vaccinia Ankara Expressing Thrombospondin-Related Anonymous Protein as Assessed with Transgenic Plasmodium berghei Parasites. Infect. Immun. 2014, 82, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Sandoval, A.; Wyllie, D.H.; Bauza, K.; Milicic, A.; Forbes, E.K.; Rollier, C.S.; Hill, A.V. CD8+ T effector memory cells protect against liver-stage malaria. J. Immunol. 2011, 187, 1347–1357. [Google Scholar] [CrossRef] [PubMed]
- Research and Testing Using Animals. Available online: https://www.gov.uk/guidance/research-and-testing-using-animals (accessed on 30 March 2017).
- Bell, A.J.; Heath, M.D.; Hewings, S.J.; Skinner, M.A. The adsorption of allergoids and 3-O-desacyl-4′-monophosphoryl lipid A (MPL®) to microcrystalline tyrosine (MCT) in formulations for use in allergy immunotherapy. J. Inorg. Biochem. 2015, 152, 147–153. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.A.; Heath, M.D.; Kramer, M.F.; Skinner, M.A. Analysis of aluminium in rat following administration of allergen immunotherapy using either aluminium or microcrystalline-tyrosine-based adjuvants. Bioanalysis 2016, 8, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Deal, C.; Balazs, A.B.; Espinosa, D.A.; Zavala, F.; Baltimore, D.; Ketner, G. Vectored antibody gene delivery protects against Plasmodium falciparum sporozoite challenge in mice. PNAS 2014, 111, 12528–31252. [Google Scholar] [CrossRef] [PubMed]
- Ishizuka, A.S.; Lyke, K.E.; DeZure, A.; Berry, A.A.; Richie, T.L.; Mendoza, F.H.; Enama, M.E.; Gordon, I.J.; Chang, L.J.; Sarwar, U.N.; et al. Protection against malaria at 1 year and immune correlates following PfSPZ vaccination. Nat. Med. 2014, 22, 614–626. [Google Scholar] [CrossRef] [PubMed]
- Sack, B.K.; Miller, J.L.; Vaughan, A.M.; Douglass, A.; Kaushansky, A.; Mikolajczak, S.; Coppi, A.; Gonzalez-Aseguinolaza, G.; Tsuji, M.; et al. Model for In Vivo Assessment of Humoral Protection against Malaria Sporozoite Challenge by Passive Transfer of Monoclonal Antibodies and Immune Serum. Infect. Immun. 2014, 82, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Doolan, D.L.; Dobano, C.; Baird, J.K. Acquired Immunity to Malaria. Clin. Microbiol. Rev. 2009, 22, 13–36. [Google Scholar] [CrossRef] [PubMed]
- John, C.C.; Tande, A.J.; Moormann, A.M.; Sumba, P.O.; Lanar, D.E.; Min, X.M.; Kazura, J.W. Antibodies to Pre-erythrocytic Plasmodium falciparum Antigens and Risk of Clinical Malaria in Kenyan Children. J. Infect. Dis. 2008, 197, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Ndungu, F.M.; Bull, P.C.; Ross, A.; Lowe, B.S.; Kabiru, E.; Marsh, K. Naturally acquired immunoglobulin (Ig) G subclass antibodies to crude asexual Plasmodium falciparum lysates: Evidence for association with protection for IgG1 and disease for IgG2. Parasite Immunol. 2002, 24, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Pleass, R.J. Fc-receptors and immunity to malaria: From models to vaccines. Parasite Immunol. 2006, 31, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Pleass, R.J.; Holder, A.A. Antibody-based therapies for malaria. Nat. Rev. Microbiol. 2005, 3, 893–899. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.B. Protective immunity against malaria after vaccination. Parasite Immunol. 2014, 36, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Smith, N.C.; Favila-Castillo, L.; Monroy-Ostria, A.; Hirunpetcharat, C.; Good, M.F. The spleen, IgG antibody subsets and immunity to Plasmodium berghei in rats. Immunol. Cell Biol. 1997, 75, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Wipasa, J.; Elliott, S.; Xu, H.; Good, M.F. Immunity to asexual blood stage malaria and vaccine approaches. Immunol. Cell Biol. 2002, 80, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Soe, S.; Theisen, M.; Roussilhon, C.; Aye, K.S.; Druilhe, P. Association between Protection against Clinical Malaria and Antibodies to Merozoite Surface Antigens in an Area of Hyperendemicity in Myanmar: Complementarity between Responses to Merozoite Surface Protein 3 and the 220-Kilodalton Glutamate-Rich Protein. Infect. Immun. 2004, 72, 247–252. [Google Scholar] [CrossRef] [PubMed]
- De Souza, J.B.; Ling, I.T.; Ogun, S.A.; Holder, A.A.; Playfair, J.H.L. Cytokines and Antibody Subclass Associated with Protective Immunity against Blood-Stage Malaria in Mice Vaccinated with the C Terminus of Merozoite Surface Protein 1 plus a Novel Adjuvant. Infect. Immun. 1996, 64, 3532–3536. [Google Scholar] [PubMed]
- Barry, A.E.; Arnott, A. Strategies for designing and monitoring malaria vaccines targeting diverse antigens. Front. Immunol. 2014, 5, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.; Brown, G.V.; Genton, B.; Moorthy, V.S. A review of malaria vaccine clinical projects based on the WHO rainbow table. Malar. J. 2012, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kimani, D.; Jagne, Y.J.; Cox, M.; Kimani, E.; Bliss, C.M.; Gitau, E.; Ogwang, C.; Afolabi, M.O.; Bowyer, G.; Collins, K.A.; et al. Translating the Immunogenicity of Prime-boost Immunization with ChAd63 and MVA ME-TRAP From Malaria Naive to Malaria-endemic Populations. Mol. Ther. 2014, 22, 1992–2003. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Sandoval, A.; Bachmann, F.C. Plasmodium vivax malaria vaccines: Why are we where we are? Hum. Vaccin. Immunother. 2013, 12, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Alla, N.D.; Sukkar, M.Y. IFN-γ, TNF-α and IL-10 responses in children infected with malaria parasite. Khartoum Med. J. 2015, 8, 1143–1152. [Google Scholar]
- Basir, R.; Rahiman, S.F.; Hasballah, K.; Chong, W.; Talib, H.; Yam, M.; Jabbarzare, M.; Tie, T.; Othman, F.; Moklas, M.; et al. Plasmodium berghei ANKA Infection in ICR Mice as a Model of Cerebral Malaria. Iran. J. Parasitol. 2012, 7, 62–74. [Google Scholar] [PubMed]
- Vaughan, A.M.; Kappe, S.H.I.; Ploss, A.; Mikolajczak, S.A. Development of humanized mouse models to study human malaria parasite infection. Future Microbiol. 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Bejon, P.; Andrews, L.; Andersen, R.F.; Dunachie, S.; Webster, D.; Walther, M.; Gilbert, S.C.; Peto, T.; Hill, A.V. Calculation of liver-to-blood inocula, parasite growth rates, and preerythrocytic vaccine efficacy, from serial quantitative polymerase chain reaction studies of volunteers challenged with malaria sporozoites. J. Infect. Dis. 2005, 191, 619–626. [Google Scholar] [CrossRef] [PubMed]
- Ogwang, C.; Kimani, D.; Edwards, N.J.; Roberts, R.; Mwacharo, J.; Bowyer, G.; Bliss, C.; Hodgson, S.H.; Njuguna, P.; Viebig, N.K.; et al. Prime-boost vaccination with chimpanzee adenovirus and modified vaccinia Ankara encoding TRAP provides partial protection against Plasmodium falciparum infection in Kenyan adults. Sci. Transl. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Chuang, I.; Sedegah, M.; Cicatelli, S.; Spring, M.; Polhemus, M.; Tamminga, C.; Patterson, N.; Guerrero, M.; Bennett, J.W.; McGrath, S.; et al. DNA Prime/Adenovirus Boost Malaria Vaccine Encoding P. falciparum CSP and AMA1 Induces Sterile Protection Associated with Cell-Mediated Immunity. PLoS ONE 2013. [Google Scholar] [CrossRef] [PubMed]
- Ewera, K.J.; Sierra-Davidsona, K.; Salmana, A.M.; Illingwortha, J.J.; Drapera, S.J.; Biswas, S.; Hill, A.V. Progress with viral vectored malaria vaccines: A multi-stage approachinvolving “unnatural immunity”. Vaccine 2015, 33, 7444–7451. [Google Scholar] [CrossRef] [PubMed]
- Gantt, S.; Persson, C.; Rose, K.; Birkett, A.J.; Abagyan, R.; Nussenzweig, V. Antibodies against Thrombospondin-Related Anonymous Protein Do Not Inhibit Plasmodium Sporozoite Infectivity In Vivo. Infect. Immun. 2000, 68, 3667–3673. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Shen, J.; Chou, B.; Duan, X.; Tu, L.; Tetsutani, K.; Moriya, C.; Ishida, H.; Hamano, S.; Shimokawa, C.; et al. Involvement of CD81 T cells in protective immunity against murine blood-stage infection with Plasmodium yoelii 17XL strain. Eur. J. Immunol. 2010, 40, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Todryk, S.M.; Walther, M. Building better T-cell-inducing malaria vaccines. Immunology 2005, 115, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Webster, D.P.; Dunachie, S.; Vuola, J.M.; Berthoud, T.; Keating, S.; Laidlaw, S.M.; McConkey, S.J.; Poulton, I.; Andrews, L.; Andersen, R.F.; et al. Enhanced T cell-mediated protection against malaria in human challenges by using the recombinant poxviruses FP9 and modified vaccinia virus Ankara. Proc. Natl. Acad. Sci. USA 2005, 102, 4836–4841. [Google Scholar] [CrossRef] [PubMed]
- Wykes, M.N.; Horne-Debets, J.M.; Leow, C.Y.; Karunarathne, D.S. Malaria drives T cells to exhaustion. Front. Microbiology 2014. [Google Scholar] [CrossRef]
- Wheeler, A.W.; Marshall, J.S.; Ulrich, J.T. A Th1-inducing adjuvant, MPL, enhances antibody profiles in experimental animals suggesting it has the potential to improve the efficacy of allergy vaccines. Int. Arch. Allergy Immunol. 2001, 126, 135–139. [Google Scholar] [CrossRef] [PubMed]



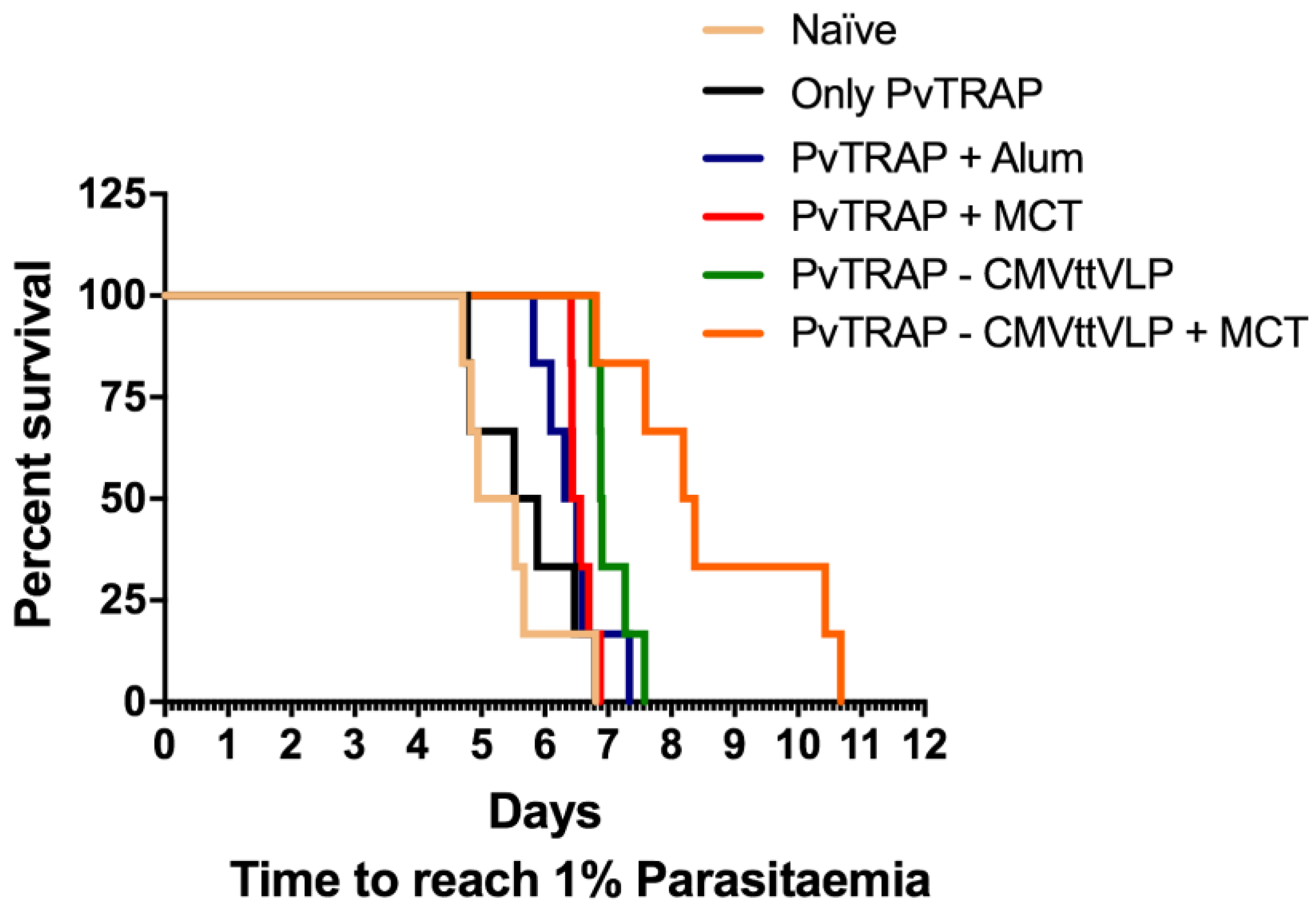
| Tukey‘s Multiple Comparison Test | Significant Summary |
|---|---|
| Naïve vs. Only PvTRAP | ns |
| Naïve vs. PvTRAP + Alum | * |
| Naïve vs. PvTRAP + MCT | ** |
| Naïve vs. PvTRAP-CMVttVLP | *** |
| Naïve vs. PvTRAP-CMVttVLP + MCT | *** |
| Only PvTRAP vs. PvTRAP + Alum | ns |
| Only PvTRAP vs. PvTRAP + MCT | * |
| Only PvTRAP vs. PvTRAP-CMVttVLP | ** |
| Only PvTRAP vs. PvTRAP-CMVttVLP + MCT | ** |
| PvTRAP + Alum vs. PvTRAP + MCT | ns |
| PvTRAP + Alum vs. PvTRAP-CMVttVLP | ** |
| PvTRAP + Alum vs. PvTRAP-CMVttVLP + MCT | *** |
| PvTRAP+MCT vs. PvTRAP-CMVttVLP | * |
| PvTRAP+MCT vs. PvTRAP-CMVttVLP + MCT | ** |
| PvTRAP-CMVttVLP vs. PvTRAP-CMVttVLP + MCT | * |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral-Miranda, G.; Heath, M.D.; Mohsen, M.O.; Gomes, A.C.; Engeroff, P.; Flaxman, A.; Leoratti, F.M.S.; El-Turabi, A.; Reyes-Sandoval, A.; Skinner, M.A.; et al. Virus-Like Particle (VLP) Plus Microcrystalline Tyrosine (MCT) Adjuvants Enhance Vaccine Efficacy Improving T and B Cell Immunogenicity and Protection against Plasmodium berghei/vivax. Vaccines 2017, 5, 10. https://doi.org/10.3390/vaccines5020010
Cabral-Miranda G, Heath MD, Mohsen MO, Gomes AC, Engeroff P, Flaxman A, Leoratti FMS, El-Turabi A, Reyes-Sandoval A, Skinner MA, et al. Virus-Like Particle (VLP) Plus Microcrystalline Tyrosine (MCT) Adjuvants Enhance Vaccine Efficacy Improving T and B Cell Immunogenicity and Protection against Plasmodium berghei/vivax. Vaccines. 2017; 5(2):10. https://doi.org/10.3390/vaccines5020010
Chicago/Turabian StyleCabral-Miranda, Gustavo, Matthew D. Heath, Mona O. Mohsen, Ariane C. Gomes, Paul Engeroff, Amy Flaxman, Fabiana M. S. Leoratti, Aadil El-Turabi, Arturo Reyes-Sandoval, Murray A. Skinner, and et al. 2017. "Virus-Like Particle (VLP) Plus Microcrystalline Tyrosine (MCT) Adjuvants Enhance Vaccine Efficacy Improving T and B Cell Immunogenicity and Protection against Plasmodium berghei/vivax" Vaccines 5, no. 2: 10. https://doi.org/10.3390/vaccines5020010






