The Effect of Physicochemical Modification on the Function of Antibodies Induced by Anti-Nicotine Vaccine in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of NIC7 Conjugates Using Varied Concentrations of sNHS
2.2. Hapten Load by Acid Hydrolysis
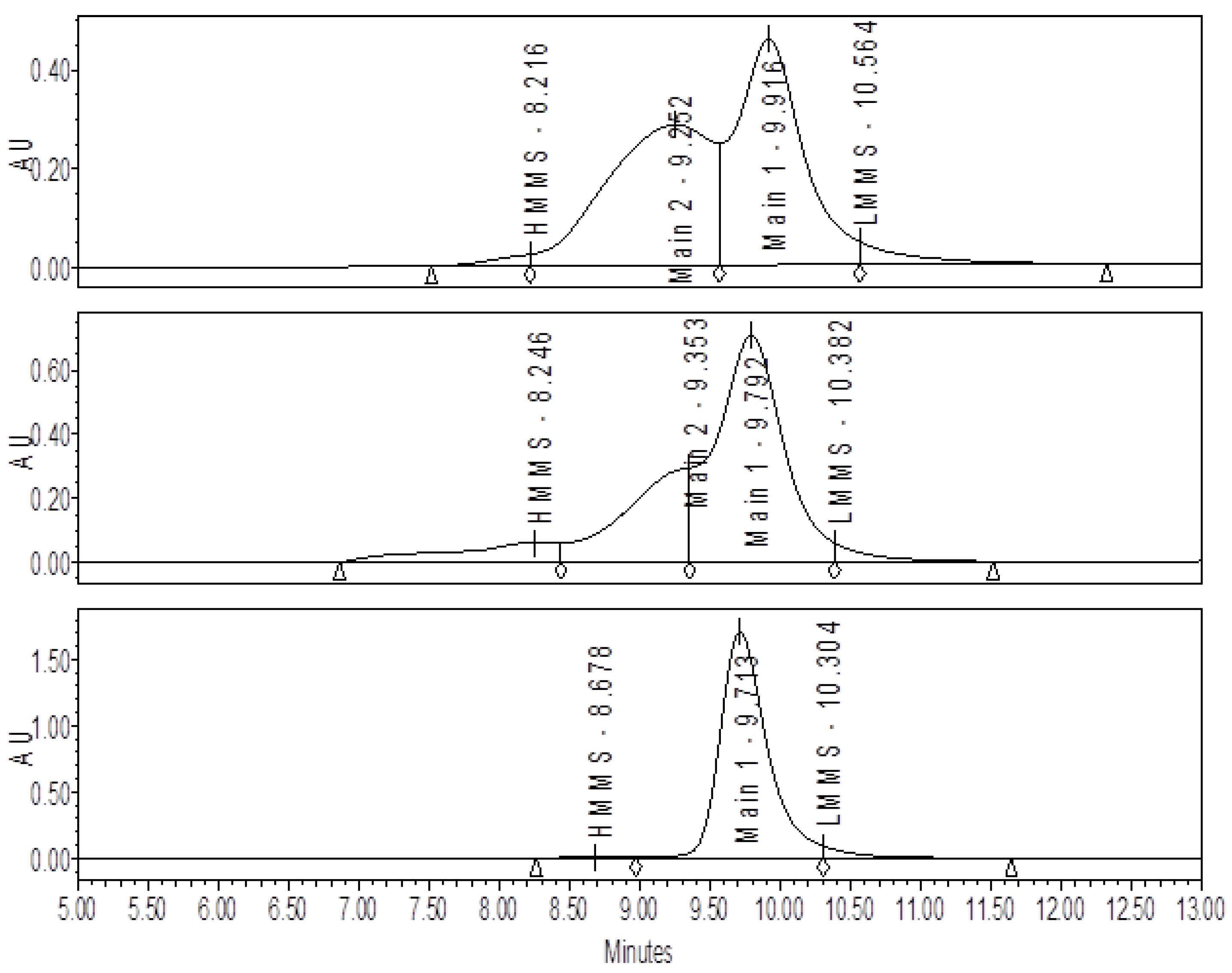
2.3. Size-Exclusion Chromatography (SE-HPLC)
2.4. Non-Reduced Capillary Gel Electrophoresis (CGE)
2.5. Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry (MALDI-MS)
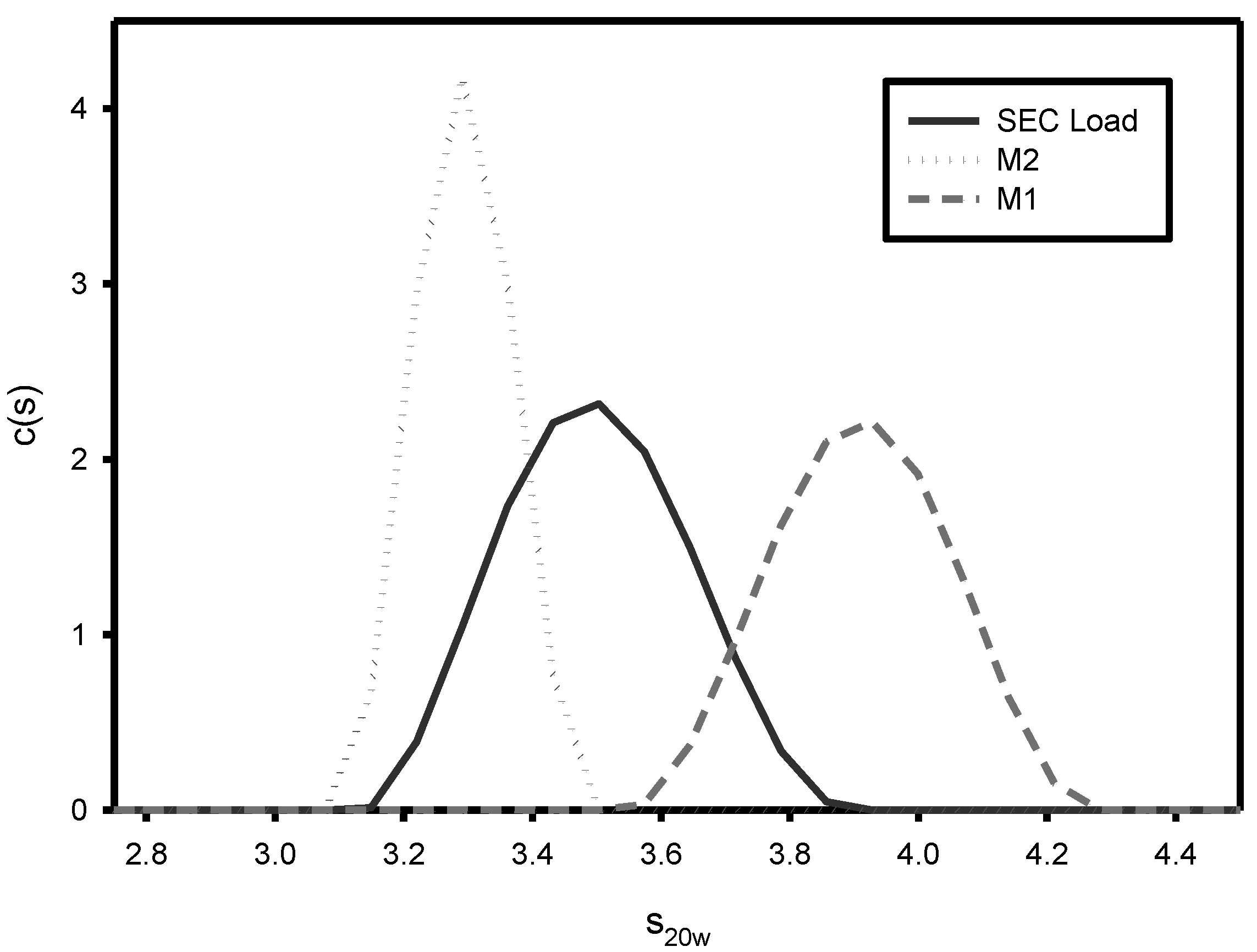
2.6. Analytical Ultracentrifugation (AUC)
2.7. Adjuvants
2.8. Animal Procedures
2.9. Anti-Nicotine Ab Titers
2.10. In Vivo Functional Assays
2.11. Statistical Analysis
3. Results
3.1. Effect of Varying sNHS/EDC Ratios on Main 1, Main 2, and Hapten Load
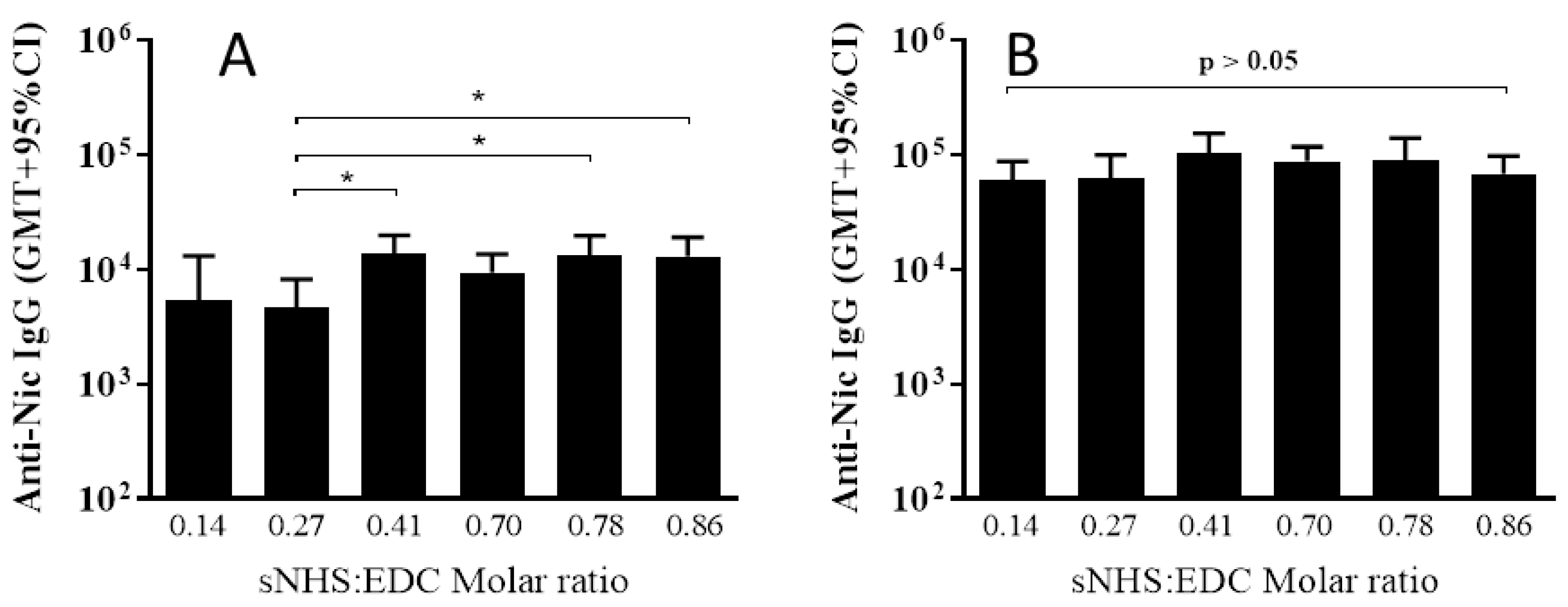
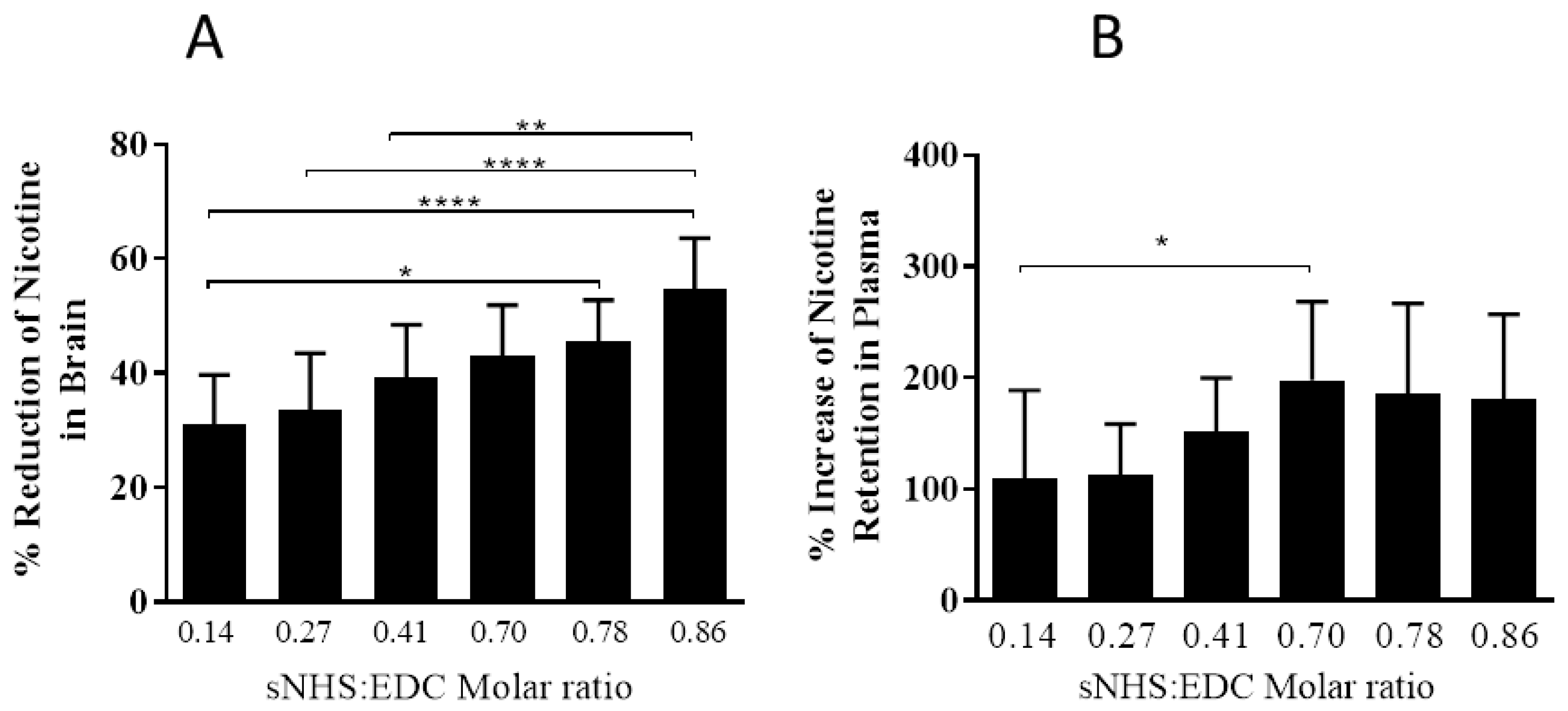
3.2. The Effect of Different sNHS/EDC Ratios on Anti-Nicotine Responses
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO Report on the Global Tobacco Epidemic. 2011: Warning about the Dangers of Tobacco. Available online: http://www.who.int/tobacco/global_report/2011/en/ (accessed on 30 November 2016).
- Tobacco—Fact Sheet No. 339. Available online: http://www.who.int/mediacentre/factsheets/fs339/en/ (accessed on 30 November 2016).
- Hughes, J.R.; Keely, J.; Naud, S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004, 99, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Nides, M.; Glover, E.D.; Reus, V.I.; Christen, A.G.; Make, B.J.; Billing, C.B.; Williams, K.E. Varenicline versus bupropion SR or placebo for smoking cessation: A pooled analysis. Am. J. Health Behav. 2008, 32, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Hatsukami, D.K.; Stead, L.F.; Gupta, P.C. Tobacco addiction. Lancet 2008, 371, 2027–2038. [Google Scholar] [CrossRef]
- Maurer, P.; Bachmann, M.F. Therapeutic vaccines for nicotine dependence. Curr. Opin. Mol. Ther. 2006, 8, 11–16. [Google Scholar] [PubMed]
- Pryde, D.C.; Jones, L.H.; Gervais, D.P.; Stead, D.R.; Blakemore, D.C.; Selby, M.D.; Brown, A.D.; Coe, J.W.; Badland, M.; Beal, D.M.; et al. Selection of a novel anti-nicotine vaccine: Influence of antigen design on antibody function in mice. PLoS ONE 2013, 8, e76557. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, J.; Weeratna, R.; Payette, P.; Jurk, M.; Schetter, C.; Laucht, M.; Wader, T.; Tluk, S.; Liu, M.; Davis, H.L.; et al. Characterization of three CpG oligodeoxynucleotide classes with distinct immunostimulatory activities. Eur. J. Immunol. 2004, 34, 251–262. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.; Thorn, J.; Mehelic, P.R.; Kolhe, P.; Bhattacharya, K.; Finneman, J.I.; Stead, D.R.; Bailey Piatchek, M.; Ningli, Z.; Chikh, G.; et al. Molecular attributes of conjugate antigen influence function of antibodies induced by anti-nicotine vaccine in mice and non-human primates. Int. Immunopharmacol. 2015, 25, 518–527. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Thorn, J.; Mehelic, P.R.; Gervais, D.P.; Stead, D.R.; Ningli, Z.; Benoit, M.; Cartier, J.; Kim, I.J.; Bhattacharya, K.; et al. Anti-nicotine vaccines: Comparison of adjuvanted CRM197 and Qb-VLP conjugate formulations for immunogenicity and function in non-human primates. Int. Immunopharmacol. 2015, 29, 663–671. [Google Scholar] [CrossRef] [PubMed]
- McCluskie, M.J.; Pryde, D.C.; Gervais, D.P.; Stead, D.R.; Zhang, N.; Benoit, M.; Robertson, K.; Kim, I.J.; Tharmanathan, T.; Merson, J.R.; et al. Enhancing immunogenicity of a 3’aminomethylnicotine-DT-conjugate anti-nicotine vaccine with CpG adjuvant in mice and non-human primates. Int. Immunopharmacol. 2013, 16, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Benowitz, N.L.; Jacob, P., III. Daily intake of nicotine during cigarette smoking. Clin. Pharmacol. Ther. 1984, 35, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Dumont, M.E.; Richards, F.M. The pH-dependent conformational change of Diphtheria toxin. J. Biol. Chem. 1988, 263, 2087–2097. [Google Scholar] [PubMed]
- Blewitt, M.G.; Chung, L.A.; London, E. Effect of pH on the conformation of Diphtheria toxin and its implication for membrane penetration. Biochemistry 1985, 24, 5458–5464. [Google Scholar] [CrossRef] [PubMed]
- Bennett, M.J.; Choe, S.; Eisenberg, D. Domain swapping: Entangling alliances between proteins. Proc. Natl. Acad. Sci. USA 1994, 91, 3127–3131. [Google Scholar] [CrossRef] [PubMed]
- Malito, E.; Bursulaya, B.; Chen, C.; Lo Surdo, P.; Picchianti, M.; Balducci, E.; Biancucci, M.; Brock, A.; Berti, F.; Bottomley, M.J.; et al. Structural basis for lack of toxicity of the diphtheria toxin mutant CRM197. Proc. Natl. Acad. Sci. USA 2012, 109, 5229–5234. [Google Scholar] [CrossRef] [PubMed]
- Hermanson, G.T. Bioconjugatio Techniques, 2nd ed.; Elsevier Inc.: London, UK, 2008; pp. 219–220. ISBN 0123705010. [Google Scholar]
- Vidunas, E.; Mathews, A.; Weaver, M.; Cai, P.; Koh, E.H.; Patel-Brown, S.; Yuan, H.; Zheng, Z.; Carriere, M.; Johnson, J.E.; et al. Production and characterization of chemically inactivated genetically engineered Clostridium difficile toxoids. J. Pharm. Sci. 2016, 105, 2032–2041. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Zhang, L.; Kaspar, A.; Rames, M.J.; Huang, L.; Woodnult, G.; Ren, G. Peptide-conjugation induces changes in human IgG1 observed by optimized negative-staining and individual-particle electron tomography. Sci. Rep. 2013, 3, e1089. [Google Scholar] [CrossRef] [PubMed]
- Totaro, K.; Liao, X.; Bhattacharya, K.; Finneman, J.I.; Sperry, J.B.; Massa, M.A.; Thorn, J.; Ho, S.V.; Pentelute, B.L. Systematic investigation of EDC/sNHS-mediated bioconjugation reactions for carboxylated peptide substrates. Bioconj. Chem. 2016, 27, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Pecetta, S.; Lo Surdo, P.; Tontini, M.; Proietti, D.; Zambonelli, C.; Bottomley, M.J.; Biagini, M.; Berti, F.; Costantino, P.; Romano, M.R. Carrier priming with CRM197 or diphtheria toxoid has a different impact on the immunogenicity of the respective glycoconjugates: Biophysical and immunochemical interpretation. Vaccine 2015, 33, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, M.L.C.; Perez, L.R.; Oviedo, M.; Costa, L.; Perdomo, V. Relationships among physico-chemical and biological tests for a synthetic Hib-TT conjugate vaccine. Vaccine 2007, 25, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Bolgiano, B.; Mawas, F.; Yost, S.E.; Crane, D.T.; Lemercinier, X.; Corbel, M. Effect of physico-chemical modification on the immunogenicity of Haemophilus. influenzae type b oligosaccharide-CRM197 conjugate vaccines. Vaccine 2001, 19, 3189–3200. [Google Scholar] [CrossRef]
- Bardotti, A.; Averani, G.; Berti, F.; Berti, S.; Carinci, V.; D’Ascenzi, S.; Fabbri, B.; Giannini, S.; Giannozzi, A.; Magagnoli, C.; et al. Physicochemical characterization of glycoconjugate vaccines for prevention of meningococcal diseases. Vaccine 2008, 26, 2284–2296. [Google Scholar] [CrossRef] [PubMed]
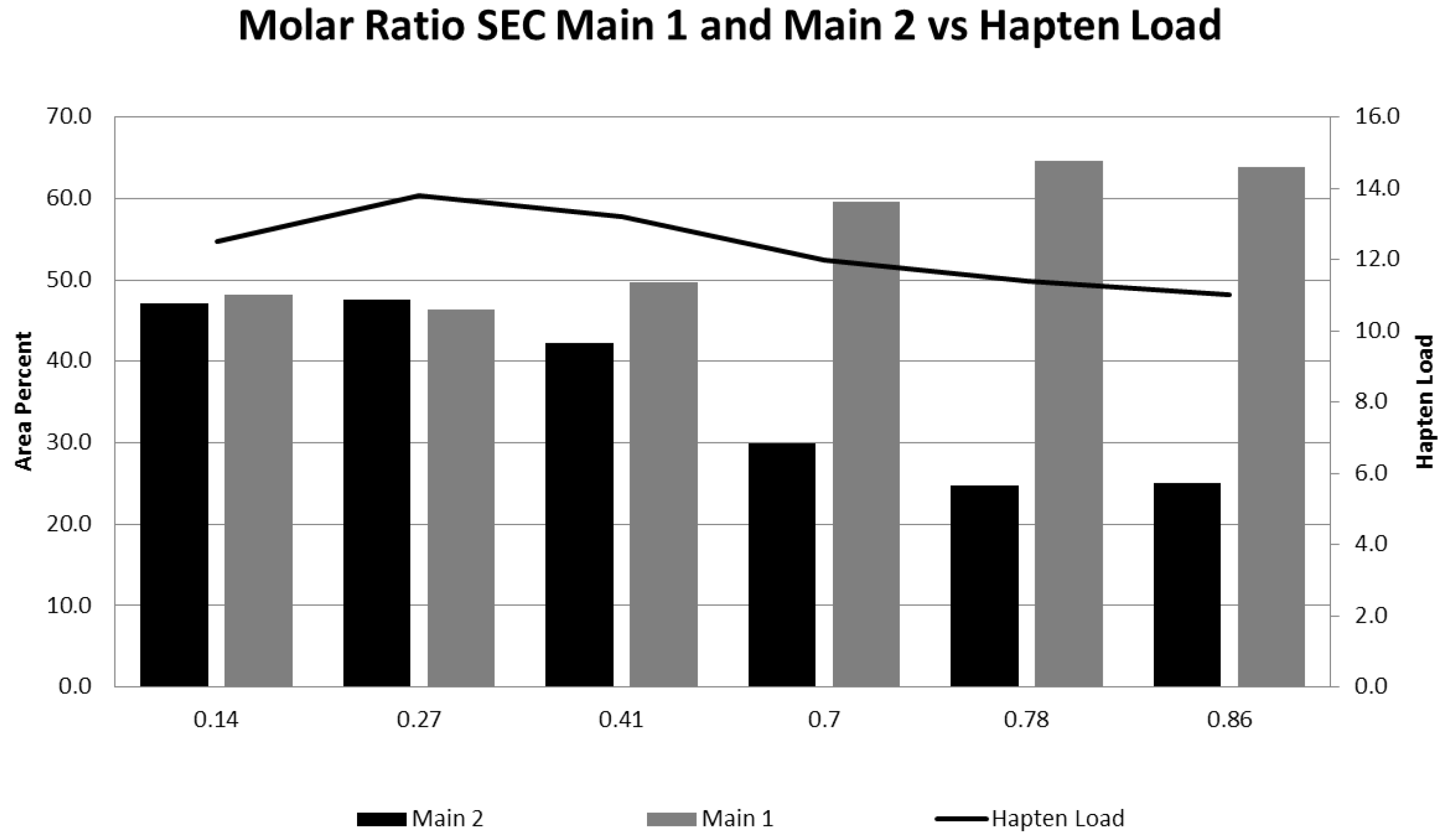
| Molar Ratio sNHS:EDC | SE-HPLC | CGE %HMMS | Hapten Load | |
|---|---|---|---|---|
| %Main 2 | %Main 1 | |||
| 0.14 | 47.1 | 48.2 | 2.3 | 13 |
| 0.27 | 47.6 | 46.4 | 2.3 | 14 |
| 0.41 | 42.3 | 49.6 | 3.0 | 13 |
| 0.70 | 29.9 | 59.6 | 1.8 | 12 |
| 0.78 | 24.8 | 64.6 | 2.0 | 11 |
| 0.86 | 25.0 | 63.9 | 2.6 | 11 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thorn, J.M.; Bhattacharya, K.; Crutcher, R.; Sperry, J.; Isele, C.; Kelly, B.; Yates, L.; Zobel, J.; Zhang, N.; Davis, H.L.; et al. The Effect of Physicochemical Modification on the Function of Antibodies Induced by Anti-Nicotine Vaccine in Mice. Vaccines 2017, 5, 11. https://doi.org/10.3390/vaccines5020011
Thorn JM, Bhattacharya K, Crutcher R, Sperry J, Isele C, Kelly B, Yates L, Zobel J, Zhang N, Davis HL, et al. The Effect of Physicochemical Modification on the Function of Antibodies Induced by Anti-Nicotine Vaccine in Mice. Vaccines. 2017; 5(2):11. https://doi.org/10.3390/vaccines5020011
Chicago/Turabian StyleThorn, Jennifer M., Keshab Bhattacharya, Renata Crutcher, Justin Sperry, Colleen Isele, Barbara Kelly, Libbey Yates, James Zobel, Ningli Zhang, Heather L. Davis, and et al. 2017. "The Effect of Physicochemical Modification on the Function of Antibodies Induced by Anti-Nicotine Vaccine in Mice" Vaccines 5, no. 2: 11. https://doi.org/10.3390/vaccines5020011
APA StyleThorn, J. M., Bhattacharya, K., Crutcher, R., Sperry, J., Isele, C., Kelly, B., Yates, L., Zobel, J., Zhang, N., Davis, H. L., & McCluskie, M. J. (2017). The Effect of Physicochemical Modification on the Function of Antibodies Induced by Anti-Nicotine Vaccine in Mice. Vaccines, 5(2), 11. https://doi.org/10.3390/vaccines5020011






