Yeast-Based Aβ1-15 Vaccine Elicits Strong Immunogenicity and Attenuates Neuropathology and Cognitive Deficits in Alzheimer’s Disease Transgenic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Yeast-Based Vaccine
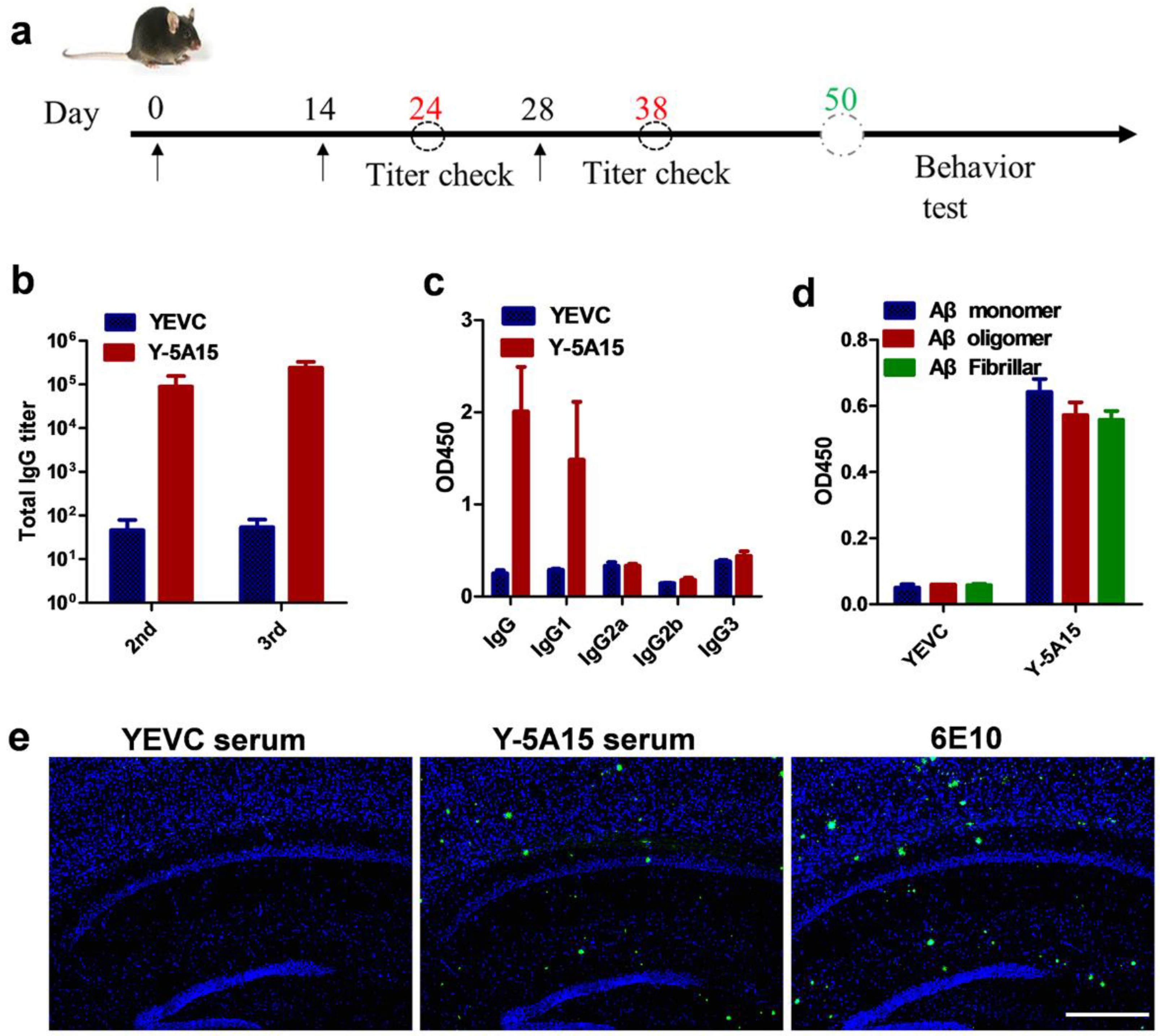
2.3. Mouse Immunization
2.4. Antibody Titer Determination
2.5. Novel Object Recognition (NOR) Test
2.6. Y-Maze Test
2.7. Morris Water Maze (MWM) Test
2.8. Cerebral Homogenate Collection
2.9. Measurement of Aβ40/42
2.10. Immunohistochemistry (IHC)
2.11. Statistical Analysis
3. Results
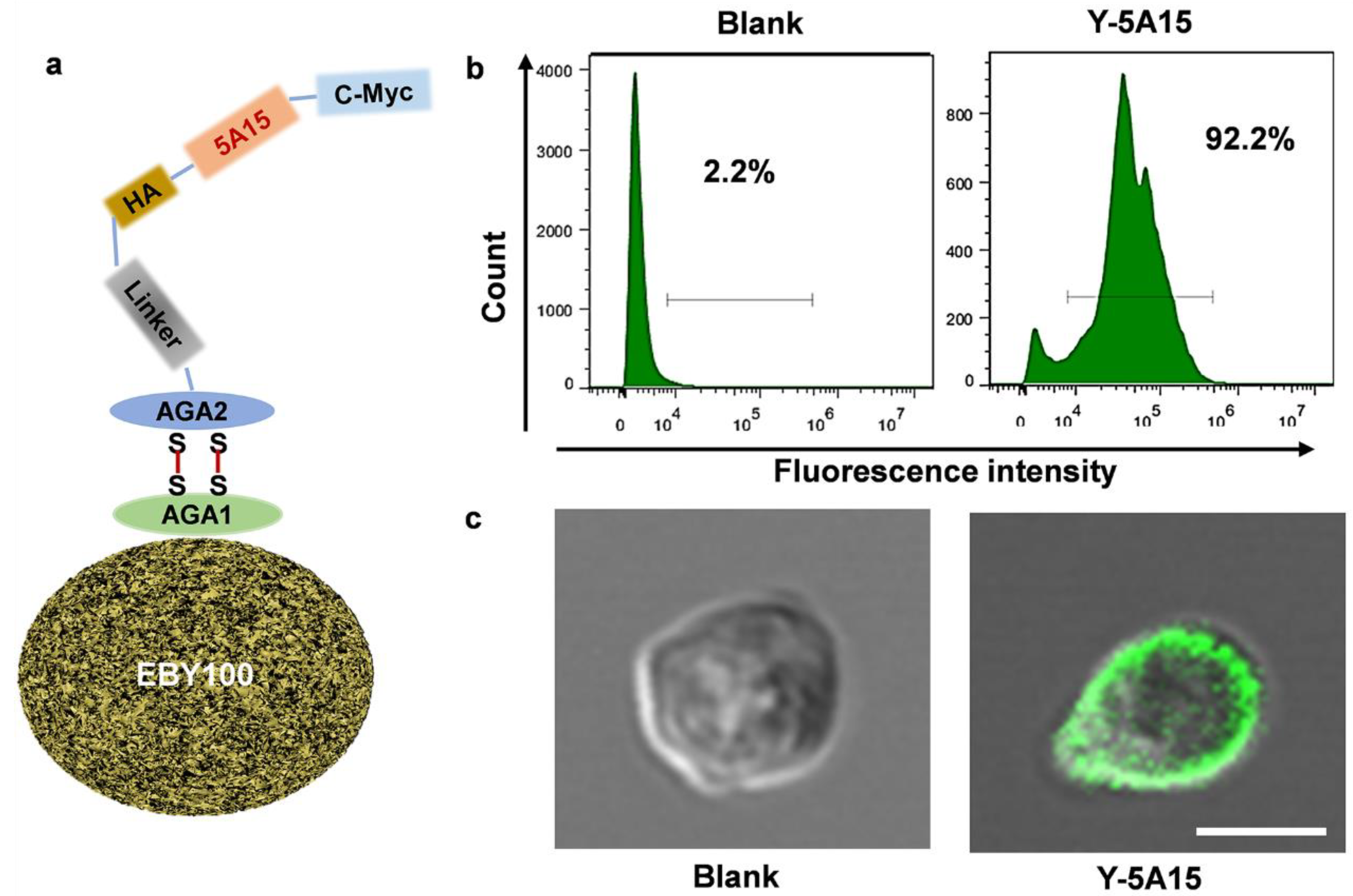
3.1. The Preparation of Y-5A15 Vaccine
3.2. Y-5A15 Vaccine Effectively Elicits High-Titer Antibodies against Aβ42 in AD Mice
3.3. Y-5A15 Vaccination Attenuates Cognitive Impairment in APP/PS1 Mice
3.4. Y-5A15 Vaccination Reduces Cerebral Aβ Levels in AD Transgenic Mice
3.5. Y-5A15 Vaccination Suppresses Astrogliosis and Microgliosis in the Brains of AD Mice
3.6. Active Immunotherapy by Y-5A15 Yeast Vaccine Rescues Synaptic Deficits in APP/PS1 Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Selkoe, D.J. The molecular pathology of Alzheimer’s disease. Neuron 1991, 6, 487–498. [Google Scholar] [CrossRef]
- Selkoe, D.J. Alzheimer’s disease: A central role for amyloid. J. Neuropathol. Exp. Neurol. 1994, 53, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Cline, E.N.; Bicca, M.A.; Viola, K.L.; Klein, W.L. The amyloid-beta oligomer hypothesis: Beginning of the third decade. J. Alzheimer’s Dis. JAD 2018, 64, S567–S610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cleary, J.P.; Walsh, D.M.; Hofmeister, J.J.; Shankar, G.M.; Kuskowski, M.A.; Selkoe, D.J.; Ashe, K.H. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat. Neurosci. 2005, 8, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Klein, W.L.; Stine, W.B., Jr.; Teplow, D.B. Small assemblies of unmodified amyloid beta-protein are the proximate neurotoxin in Alzheimer’s disease. Neurobiol. Aging 2004, 25, 569–580. [Google Scholar] [CrossRef]
- Shankar, G.M.; Li, S.M.; Mehta, T.H.; Garcia-Munoz, A.; Shepardson, N.E.; Smith, I.; Brett, F.M.; Farrell, M.A.; Rowan, M.J.; Lemere, C.A.; et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat. Med. 2008, 14, 837–842. [Google Scholar] [CrossRef] [Green Version]
- Selkoe, D.J. Light at the End of the Amyloid Tunnel. Biochemistry 2018, 57, 5921–5922. [Google Scholar] [CrossRef] [Green Version]
- Ferrer, I.; Rovira, M.B.; Guerra, M.L.S.; Rey, M.J.; Costa-Jussa, F. Neuropathology and pathogenesis of encephalitis following amyloid-beta immunization in Alzheimer’s disease. Brain Pathol. 2004, 14, 11–20. [Google Scholar] [CrossRef]
- Nicoll, J.A.R.; Wilkinson, D.; Holmes, C.; Steart, P.; Markham, H.; Weller, R.O. Neuropathology of human Alzheimer disease after immunization with amyloid-beta peptide: A case report. Nat. Med. 2003, 9, 448–452. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Jennings, G.T.; Vogel, M. A vaccine against Alzheimer’s disease: Anything left but faith? Expert Opin. Biol. Ther. 2019, 19, 73–78. [Google Scholar] [CrossRef]
- Holmes, C.; Boche, D.; Wilkinson, D.; Yadegarfar, G.; Hopkins, V.; Bayer, A.; Jones, R.W.; Bullock, R.; Love, S.; Neal, J.W.; et al. Long-term effects of A beta(42) immunisation in Alzheimer’s disease: Follow-up of a randomised, placebo-controlled phase I trial. Lancet 2008, 372, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Gilman, S.; Koller, M.; Black, R.S.; Jenkins, L.; Griffith, S.G.; Fox, N.C.; Eisner, L.; Kirby, L.; Rovira, M.B.; Forette, F.; et al. Clinical effects of Abeta immunization (AN1792) in patients with AD in an interrupted trial. Neurology 2005, 64, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Sevigny, J.; Chiao, P.; Bussiere, T.; Weinreb, P.H.; Williams, L.; Maier, M.; Dunstan, R.; Salloway, S.; Chen, T.; Ling, Y.; et al. The antibody aducanumab reduces Abeta plaques in Alzheimer’s disease. Nature 2016, 537, 50–56. [Google Scholar] [CrossRef]
- von Hehn, C.; von Rosenstiel, P.; Tian, Y.; Wu, S.; Chen, T.L.; Skordos, L.; Harrison, K.; Prada, C.; Chalkias, S.; Rajagovindan, R.; et al. Baseline characteristics from ENGAGE and EMERGE: Two phase 3 studies to evaluate aducanumab in patients with early Alzheimer’s disease (P4.1-001). Neurology 2019, 92. Available online: https://n.neurology.org/content/92/15_Supplement/P4.1-001 (accessed on 26 May 2020).
- Winblad, B.; Andreasen, N.; Minthon, L.; Floesser, A.; Imbert, G.; Dumortier, T.; Maguire, R.P.; Blennow, K.; Lundmark, J.; Staufenbiel, M.; et al. Safety, tolerability, and antibody response of active Abeta immunotherapy with CAD106 in patients with Alzheimer’s disease: Randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012, 11, 597–604. [Google Scholar] [CrossRef]
- Agadjanyan, M.G.; Ghochikyan, A.; Petrushina, I.; Vasilevko, V.; Movsesyan, N.; Mkrtichyan, M.; Saing, T.; Cribbs, D.H. Prototype Alzheimer’s disease vaccine using the immunodominant B cell epitope from beta-amyloid and promiscuous T cell epitope pan HLA DR-binding peptide. J. Immunol. 2005, 174, 1580–1586. [Google Scholar] [CrossRef] [Green Version]
- Arai, H.; Suzuki, H.; Yoshiyama, T. Vanutide cridificar and the QS-21 Adjuvant in japanese subjects with mild to moderate Alzheimer’s Disease: Results from two phase 2 studies. Curr. Alzheimer Res. 2015, 12, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Geylis, V.; Kourilov, V.; Meiner, Z.; Nennesmo, I.; Bogdanovic, N.; Steinitz, M. Human monoclonal antibodies against amyloid-beta from healthy adults. Neurobiol. Aging 2005, 26, 597–606. [Google Scholar] [CrossRef]
- Wadle, A.; Held, G.; Neumann, F.; Kleber, S.; Wuellner, B.; Asemissen, A.M.; Kubuschok, B.; Scheibenbogen, C.; Breinig, T.; Meyerhans, A.; et al. Cross-presentation of HLA class I epitopes from influenza matrix protein produced in Saccharomyces cerevisiae. Vaccine 2006, 24, 6272–6281. [Google Scholar] [CrossRef]
- Howland, S.W.; Wittrup, K.D. Antigen release kinetics in the phagosome are critical to cross-presentation efficiency. J. Immunol. 2008, 180, 1576–1583. [Google Scholar] [CrossRef] [Green Version]
- Chao, G.; Lau, W.L.; Hackel, B.J.; Sazinsky, S.L.; Lippow, S.M.; Wittrup, K.D. Isolating and engineering human antibodies using yeast surface display. Nat. Protoc. 2006, 1, 755. [Google Scholar] [CrossRef]
- Zhou, W.W.; Lu, S.; Su, Y.J.; Xue, D.; Yu, X.L.; Wang, S.W.; Zhang, H.; Xu, P.X.; Xie, X.X.; Liu, R.T. Decreasing oxidative stress and neuroinflammation with a multifunctional peptide rescues memory deficits in mice with Alzheimer disease. Free Radic. Biol. Med. 2014, 74, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Pozueta, J.; Lefort, R.; Shelanski, M.L. Synaptic changes in Alzheimer’s disease and its models. Neuroscience 2013, 251, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s disease: Past, present and future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef]
- Logovinsky, V.; Satlin, A.; Lai, R.; Swanson, C.; Kaplow, J.; Osswald, G.; Basun, H.; Lannfelt, L. Safety and tolerability of BAN2401-a clinical study in Alzheimer’s disease with a protofibril selective A beta antibody. Alzheimers Res. Ther. 2016, 8. [Google Scholar] [CrossRef] [Green Version]
- Loureiro, J.C.; Pais, M.V.; Stella, F.; Radanovic, M.; Teixeira, A.L.; Forlenza, O.V.; de Souza, L.C. Passive antiamyloid immunotherapy for Alzheimer’s disease. Curr. Opin. Psychiatry 2020, 33, 284–291. [Google Scholar] [CrossRef]
- Vellas, B.; Black, R.; Thal, L.J.; Fox, N.C.; Daniels, M.; McLennan, G.; Tompkins, C.; Leibman, C.; Pomfret, M.; Grundman, M.; et al. Long-term follow-up of patients immunized with AN1792: Reduced functional decline in antibody responders. Curr. Alzheimer Res. 2009, 6, 144–151. [Google Scholar] [CrossRef] [Green Version]
- Lobello, K.; Ryan, J.M.; Liu, E.; Rippon, G.; Black, R. Targeting Beta amyloid: A clinical review of immunotherapeutic approaches in Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 628070. [Google Scholar] [CrossRef] [Green Version]
- Petrushina, I.; Davtyan, H.; Hovakimyan, A.; Davtyan, A.; Passos, G.F.; Cribbs, D.H.; Ghochikyan, A.; Agadjanyan, M.G. Comparison of efficacy of preventive and therapeutic vaccines targeting the N terminus of beta-amyloid in an animal model of Alzheimer’s disease. Mol. Ther. J. Am. Soc. Gene Ther. 2017, 25, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Lambert, M.P.; Barlow, A.K.; Chromy, B.A.; Edwards, C.; Freed, R.; Liosatos, M.; Morgan, T.E.; Rozovsky, I.; Trommer, B.; Viola, K.L.; et al. Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc. Natl. Acad. Sci. USA 1998, 95, 6448–6453. [Google Scholar] [CrossRef] [Green Version]
- Mandler, M.; Santic, R.; Gruber, P.; Cinar, Y.; Pichler, D.; Funke, S.A.; Willbold, D.; Schneeberger, A.; Schmidt, W.; Mattner, F. Tailoring the antibody response to aggregated Ass using novel Alzheimer-vaccines. PLoS ONE 2015, 10, e0115237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frautschy, S.A.; Yang, F.; Irrizarry, M.; Hyman, B.; Saido, T.C.; Hsiao, K.; Cole, G.M. Microglial response to amyloid plaques in APPsw transgenic mice. Am. J. Pathol. 1998, 152, 307–317. [Google Scholar] [PubMed]
- Gordon, M.N.; Holcomb, L.A.; Jantzen, P.T.; DiCarlo, G.; Wilcock, D.; Boyett, K.W.; Connor, K.; Melachrino, J.; O’Callaghan, J.P.; Morgan, D. Time course of the development of Alzheimer-like pathology in the doubly transgenic PS1+APP mouse. Exp. Neurol. 2002, 173, 183–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koronyo, Y.; Salumbides, B.C.; Sheyn, J.; Pelissier, L.; Li, S.; Ljubimov, V.; Moyseyev, M.; Daley, D.; Fuchs, D.T.; Pham, M.; et al. Therapeutic effects of glatiramer acetate and grafted CD115(+) monocytes in a mouse model of Alzheimer’s disease. Brain J. Neurol. 2015, 138, 2399–2422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ardiani, A.; Higgins, J.P.; Hodge, J.W. Vaccines based on whole recombinant Saccharomyces cerevisiae cells. FEMS Yeast Res. 2010, 10, 1060–1069. [Google Scholar] [CrossRef] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.-q.; Lu, S.; Zhang, L.; Huang, Y.-r.; Ji, M.; Sun, X.-y.; Liu, X.-g.; Liu, R.-t. Yeast-Based Aβ1-15 Vaccine Elicits Strong Immunogenicity and Attenuates Neuropathology and Cognitive Deficits in Alzheimer’s Disease Transgenic Mice. Vaccines 2020, 8, 351. https://doi.org/10.3390/vaccines8030351
Liu D-q, Lu S, Zhang L, Huang Y-r, Ji M, Sun X-y, Liu X-g, Liu R-t. Yeast-Based Aβ1-15 Vaccine Elicits Strong Immunogenicity and Attenuates Neuropathology and Cognitive Deficits in Alzheimer’s Disease Transgenic Mice. Vaccines. 2020; 8(3):351. https://doi.org/10.3390/vaccines8030351
Chicago/Turabian StyleLiu, Dong-qun, Shuai Lu, Lun Zhang, Ya-ru Huang, Mei Ji, Xiao-ying Sun, Xiao-ge Liu, and Rui-tian Liu. 2020. "Yeast-Based Aβ1-15 Vaccine Elicits Strong Immunogenicity and Attenuates Neuropathology and Cognitive Deficits in Alzheimer’s Disease Transgenic Mice" Vaccines 8, no. 3: 351. https://doi.org/10.3390/vaccines8030351
APA StyleLiu, D.-q., Lu, S., Zhang, L., Huang, Y.-r., Ji, M., Sun, X.-y., Liu, X.-g., & Liu, R.-t. (2020). Yeast-Based Aβ1-15 Vaccine Elicits Strong Immunogenicity and Attenuates Neuropathology and Cognitive Deficits in Alzheimer’s Disease Transgenic Mice. Vaccines, 8(3), 351. https://doi.org/10.3390/vaccines8030351




