Comparing the ‘When’ and the ‘Where’ of Electrocortical Activity in Patients with Tourette Syndrome, Body-Focused Repetitive Behaviors, and Obsessive Compulsive Disorder
Abstract
:1. Introduction
1.1. What Are the Main Contrasts and Commonalities across TS, OCD and BFRB?
1.2. Objectives and Hypotheses
2. Materials and Methods
2.1. Participants
2.2. Instruments and Clinical Assessment
2.3. Procedures
2.4. Electrophysiological Recordings
2.5. EEG Signal Extraction
2.6. Statistical Analysis
2.7. Source Localization
3. Results
3.1. Sociodemographic and Clinical Data
3.2. Event-Related Potentials
3.2.1. P200 Amplitude
3.2.2. N200 Amplitude
3.2.3. P300 Amplitude
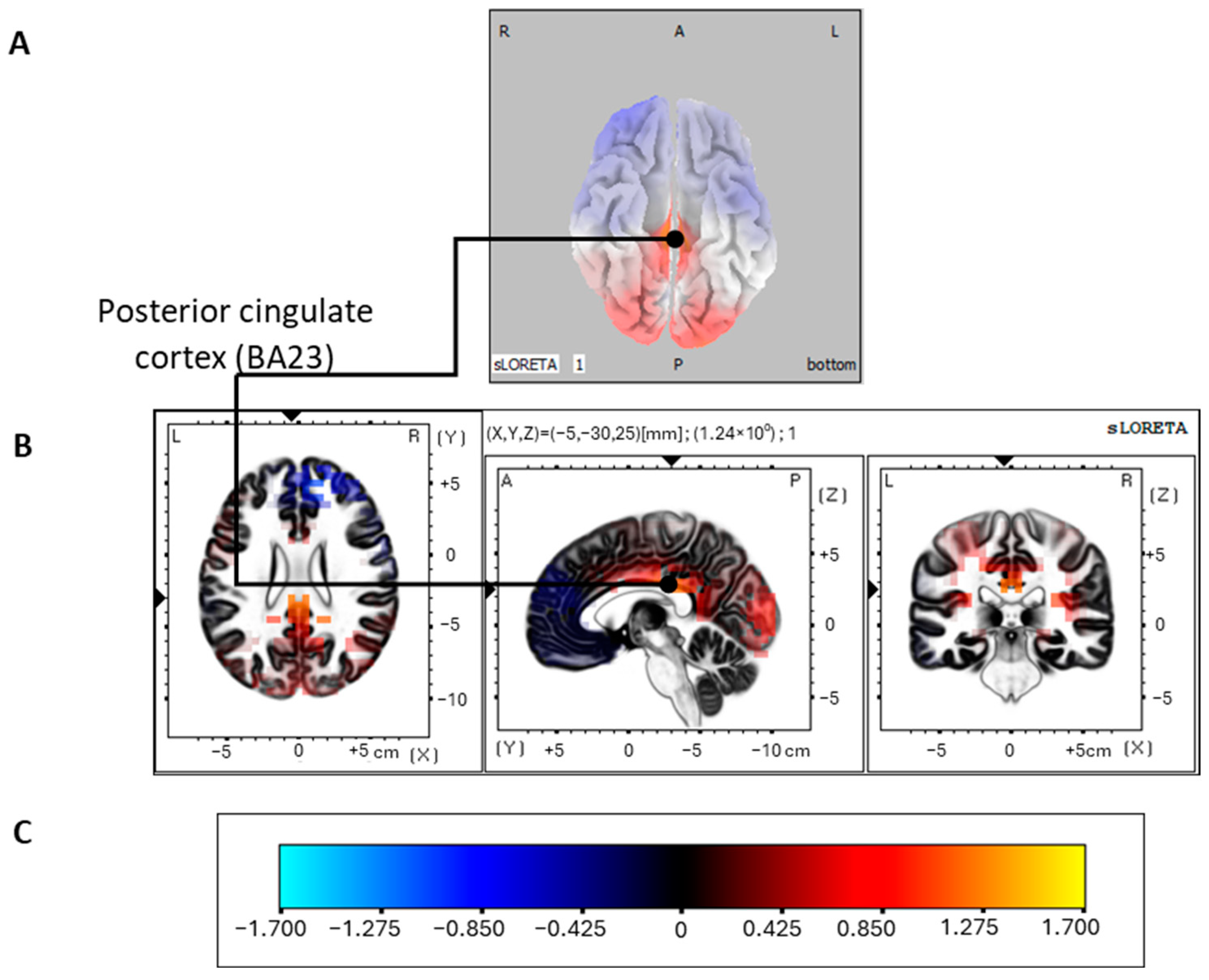
3.2.4. Source Localization of the P300 Oddball Effect
4. Discussion
4.1. Source Localization
4.2. Limits and Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cubo, E. Review of prevalence studies of tic disorders: Methodological caveats. Tremor Other Hyperkinet. Mov. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Eapen, V.; Cavanna, A.E.; Robertson, M.M. Comorbidities, Social Impact, and Quality of Life in Tourette Syndrome. Front. Psychiatry 2016, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.D.; Fast, D.K.; Burd, L.; Kerbeshian, J.; Robertson, M.M.; Sandor, P. An international perspective on Tourette syndrome: Selected findings from 3500 individuals in 22 countries. Dev. Med. Child. Neurol. 2000, 42, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.M.; Eapen, V.; Singer, H.S.; Martino, D.; Scharf, J.M.; Paschou, P.; Roessner, V.; Woods, D.W.; Hariz, M.; Mathews, C.A.; et al. Gilles de la Tourette syndrome. Nat. Rev. Dis. Primers 2017, 3, 16097. [Google Scholar] [CrossRef] [PubMed]
- Singer, H.S. Tics and Tourette Syndrome. CONTINUUM Lifelong Learn. Neurol. 2019, 25, 936–958. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Yan, J.; Yu, L.; Wang, F.; Liu, J.; Li, Y.; Cui, Y. Grey matter abnormalities in Tourette syndrome: An activation likelihood estimation meta-analysis. BMC Psychiatry 2021, 21, 184. [Google Scholar] [CrossRef] [PubMed]
- Worbe, Y.; Gerardin, E.; Hartmann, A.; Valabrégue, R.; Chupin, M.; Tremblay, L.; Vidailhet, M.; Colliot, O.; Lehéricy, S. Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain 2010, 133, 3649–3660. [Google Scholar] [CrossRef] [PubMed]
- Tinaz, S.; Malone, P.; Hallett, M.; Horovitz, S.G. Role of the right dorsal anterior insula in the urge to tic in tourette syndrome. Mov. Disord. 2015, 30, 1190–1197. [Google Scholar] [CrossRef] [PubMed]
- Neuner, I.; Werner, C.J.; Arrubla, J.; Stöcker, T.; Ehlen, C.; Wegener, H.P.; Schneider, F.; Shah, N.J. Imaging the where and when of tic generation and resting state networks in adult Tourette patients. Front. Hum. Neurosci. 2014, 8, 362. [Google Scholar] [CrossRef]
- Bohlhalter, S. Neural correlates of tic generation in Tourette syndrome: An event-related functional MRI study. Brain 2006, 129, 2029–2037. [Google Scholar] [CrossRef]
- Bhikram, T.; Arnold, P.; Crawley, A.; Abi-Jaoude, E.; Sandor, P. The functional connectivity profile of tics and obsessive-compulsive symptoms in Tourette Syndrome. J. Psychiatr. Res. 2020, 123, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Worbe, Y. Tourette syndrome: Clinical spectrum, mechanisms and personalized treatments. Curr. Opin. Neurol. 2018, 31, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.M.; Cavanna, A.E.; Eapen, V. Gilles de la Tourette Syndrome and Disruptive Behavior Disorders: Prevalence, Associations, and Explanation of the Relationships. J. Neuropsychiatry Clin. Neurosci. 2015, 27, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.K.; Grice, D.E.; Lapidus, K.A.B.; Coffey, B.J. Obsessive-Compulsive Disorder. Psychiatr. Clin. N. Am. 2014, 37, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Hirschtritt, M.E.; Bloch, M.H.; Mathews, C.A. Obsessive-Compulsive Disorder. JAMA 2017, 317, 1358. [Google Scholar] [CrossRef] [PubMed]
- De Wit, S.J.; Alonso, P.; Schweren, L.; Mataix-Cols, D.; Lochner, C.; Menchón, J.M.; Stein, D.J.; Fouche, J.-P.; Soriano-Mas, C.; Sato, J.R.; et al. Multicenter Voxel-Based Morphometry Mega-Analysis of Structural Brain Scans in Obsessive-Compulsive Disorder. Am. J. Psychiatry 2014, 171, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Rotge, J.-Y.; Langbour, N.; Guehl, D.; Bioulac, B.; Jaafari, N.; Allard, M.; Aouizerate, B.; Burbaud, P. Gray Matter Alterations in Obsessive–Compulsive Disorder: An Anatomic Likelihood Estimation Meta-Analysis. Neuropsychopharmacology 2010, 35, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.J.; Costa, D.L.C.; Lochner, C.; Miguel, E.C.; Reddy, Y.C.J.; Shavitt, R.G.; Van Den Heuvel, O.A.; Simpson, H.B. Obsessive–compulsive disorder. Nat. Rev. Dis. Primers 2019, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, J.; Hazari, N.; Venkatasubramanian, G. Neuroimaging findings in obsessive–compulsive disorder: A narrative review to elucidate neurobiological underpinnings. Indian J. Psychiatry 2019, 61, 9. [Google Scholar] [CrossRef]
- Solley, K.; Turner, C. Prevalence and correlates of clinically significant body-focused repetitive behaviors in a non-clinical sample. Compr. Psychiatry 2018, 86, 9–18. [Google Scholar] [CrossRef]
- O’Connor, K.; St-Pierre-Delorme, M.-È.; Leclerc, J.; Lavoie, M.; Blais, M.T. Meta-Cognitions in Tourette Syndrome, Tic Disorders, and Body-Focused Repetitive Disorder. Can. J. Psychiatry 2014, 59, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Peris, T.S.; Ricketts, E.J.; Bethlehem, R.A.I.; Chamberlain, S.R.; O’Neill, J.; Scharf, J.M.; Dougherty, D.D.; Deckersbach, T.; Woods, D.W.; et al. Reward processing in trichotillomania and skin picking disorder. Brain Imaging Behav. 2022, 16, 547–556. [Google Scholar] [CrossRef]
- Roos, A.; Grant, J.E.; Fouche, J.-P.; Stein, D.J.; Lochner, C. A comparison of brain volume and cortical thickness in excoriation (skin picking) disorder and trichotillomania (hair pulling disorder) in women. Behav. Brain Res. 2015, 279, 255–258. [Google Scholar] [CrossRef]
- Harries, M.D.; Chamberlain, S.R.; Redden, S.A.; Odlaug, B.L.; Blum, A.W.; Grant, J.E. A structural MRI study of excoriation (skin-picking) disorder and its relationship to clinical severity. Psychiatry Res. Neuroimaging 2017, 269, 26–30. [Google Scholar] [CrossRef]
- Schienle, A.; Potthoff, J.; Wabnegger, A. Voxel-based morphometry analysis of structural brain scans in skin-picking disorder. Compr. Psychiatry 2018, 84, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Wabnegger, A.; Schienle, A. The Role of the Cerebellum in Skin-Picking Disorder. Cerebellum 2019, 18, 91–98. [Google Scholar] [CrossRef]
- Morand-Beaulieu, S.; O’Connor, K.P.; Richard, M.; Sauvé, G.; Leclerc, J.B.; Blanchet, P.J.; Lavoie, M.E. The Impact of a Cognitive–Behavioral Therapy on Event-Related Potentials in Patients with Tic Disorders or Body-Focused Repetitive Behaviors. Front. Psychiatry 2016, 7, 180156. [Google Scholar] [CrossRef] [PubMed]
- Morand-Beaulieu, S.; Lavoie, M.E. Cognitive and motor event-related potentials in Tourette syndrome and tic disorders: A systematic review. Clin. Neurophysiol. 2019, 130, 1041–1057. [Google Scholar] [CrossRef] [PubMed]
- Folstein, J.R.; Van Petten, C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology 2008, 45, 152–170. [Google Scholar] [CrossRef]
- Van De Watering, B.J.M.; Martens, C.M.C.; Fortgens, C.; Slaets, J.P.J.; Van Woerkom, T.C.A.M. Late components of the auditory evoked potentials in Gilles de la Tourette syndrome. Clin. Neurol. Neurosurg. 1985, 87, 181–186. [Google Scholar] [CrossRef]
- Woerkom, T.C.A.M.; Roos, R.A.C.; Dijk, J.G. Altered attentional processing of background stimuli in Gilles de la Tourette syndrome: A study in auditory event-related potentials evoked in an oddball paradigm. Acta Neurol. Scand. 2009, 90, 116–123. [Google Scholar] [CrossRef]
- Van Woerkom, T.C.A.M.; Fortgens, C.; Rompel-Martens, C.M.C.; Van De Wetering, B.J.M. Auditory event-related potentials in adult patients with Gilles de la Tourette’s syndrome in the oddball paradigm. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1988, 71, 443–449. [Google Scholar] [CrossRef]
- Johannes, S.; Weber, A.; Müller-Vahl, K.R.; Kolbe, H.; Dengler, R.; Münte, T.F. Event-related brain potentials show changed attentional mechanisms in Gilles de la Tourette Syndrome. Eur. J. Neurol. 1997, 4, 152–161. [Google Scholar] [CrossRef]
- Morand-Beaulieu, S.; Perrault, M.-A.; Lavoie, M.E. Test-Retest Reliability of Event-Related Potentials Across Three Tasks. J. Psychophysiol. 2022, 36, 100–117. [Google Scholar] [CrossRef]
- Duncan-Johnson, C.C.; Donchin, E. On Quantifying Surprise: The Variation of Event-Related Potentials with Subjective Probability. Psychophysiology 1977, 14, 456–467. [Google Scholar] [CrossRef]
- Johannes, S.; Wieringa, B.M.; Nager, W.; Müller-Vahl, K.R.; Dengler, R.; Münte, T.F. Excessive action monitoring in Tourette syndrome. J. Neurol. 2002, 249, 961–966. [Google Scholar] [CrossRef]
- Johannes, S.; Wieringa, B.M.; Mantey, M.; Nager, W.; Rada, D.; Müller-Vahl, K.R.; Emrich, H.M.; Dengler, R.; Münte, T.F.; Dietrich, D. Altered inhibition of motor responses in Tourette Syndrome and Obsessive-Compulsive Disorder. Acta Neurol. Scand. 2001, 104, 36–43. [Google Scholar] [CrossRef]
- Sauvé, G.; Morand-Beaulieu, S.; O’Connor, K.; Blanchet, P.; Lavoie, M. P300 Source Localization Contrasts in Body-Focused Repetitive Behaviors and Tic Disorders. Brain Sci. 2017, 7, 76. [Google Scholar] [CrossRef]
- Johannes, S.; Wieringa, B.M.; Nager, W.; Müller-Vahl, K.R.; Dengler, R.; Münte, T.F. Electrophysiological measures and dual-task performance in Tourette syndrome indicate deficient divided attention mechanisms. Eur. J. Neurol. 2001, 8, 253–260. [Google Scholar] [CrossRef]
- Asahi, K.; Ogura, C.; Hirano, K.; Nageishi, Y. Endogenous Event-Related Potentials in Obsessive Character. Psychiatry Clin. Neurosci. 1993, 47, 63–69. [Google Scholar] [CrossRef]
- Okasha, A.; Rafaat, M.; Mahallawy, N.; Nahas, G.E.; Dawla, A.S.E.; Sayed, M.; Kholi, S.E. Cognitive dysfunction in obsessive-compulsive disorder. Acta Psychiatr. Scand. 2000, 101, 281–285. [Google Scholar] [CrossRef]
- Miyata, A.; Matsunaga, H.; Kiriike, N.; Iwasaki, Y.; Takei, Y.; Yamagami, S. Event-related potentials in patients with obsessive-compulsive disorder. Psychiatry Clin. Neurosci. 1998, 52, 513–518. [Google Scholar] [CrossRef]
- Thibault, G.; Felezeu, M.; O’Connor, K.P.; Todorov, C.; Stip, E.; Lavoie, M.E. Influence of comorbid obsessive-compulsive symptoms on brain event-related potentials in Gilles de la Tourette syndrome. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 803–815. [Google Scholar] [CrossRef]
- Towey, J.; Bruder, G.; Hollander, E.; Friedman, D.; Erhan, H.; Liebowitz, M.; Sutton, S. Endogenous event-related potentials in obsessive-compulsive disorder. Biol. Psychiatry 1990, 28, 92–98. [Google Scholar] [CrossRef]
- Towey, J.; Bruder, G.; Tenke, C.; Leite, P.; Decaria, C.; Friedman, D.; Hollander, E. Event-related potential and clinical correlates of neurodysfunction in obsessive-compulsive disorder. Psychiatry Res. 1993, 49, 167–181. [Google Scholar] [CrossRef]
- Mavrogiorgou, P.; Juckel, G.; Frodl, T.; Gallinat, J.; Hauke, W.; Zaudig, M.; Dammann, G.; Möller, H.-J.; Hegerl, U. P300 subcomponents in obsessive-compulsive disorder. J. Psychiatr. Res. 2002, 36, 399–406. [Google Scholar] [CrossRef]
- Gohle, D.; Juckel, G.; Mavrogiorgou, P.; Pogarell, O.; Mulert, C.; Rujescu, D.; Giegling, I.; Zaudig, M.; Hegerl, U. Electrophysiological evidence for cortical abnormalities in obsessive–compulsive disorder—A replication study using auditory event-related P300 subcomponents. J. Psychiatr. Res. 2008, 42, 297–303. [Google Scholar] [CrossRef]
- Sanz, M.; Molina, V.; Martin-Loeches, M.; Calcedo, A.; Rubia, F.J. Auditory P300 event related potential and serotonin reuptake inhibitor treatment in obsessive-compulsive disorder patients. Psychiatry Res. 2001, 101, 75–81. [Google Scholar] [CrossRef]
- Kim, M.-S.; Kang, S.-S.; Youn, T.; Kang, D.-H.; Kim, J.-J.; Kwon, J.S. Neuropsychological correlates of P300 abnormalities in patients with schizophrenia and obsessive–compulsive disorder. Psychiatry Res. Neuroimaging 2003, 123, 109–123. [Google Scholar] [CrossRef]
- Yamamuro, K.; Okada, K.; Kishimoto, N.; Ota, T.; Iida, J.; Kishimoto, T. A longitudinal, event-related potential pilot study of adult obsessive-compulsive disorder with 1-year follow-up. Neuropsychiatr. Dis. Treat. 2016, 12, 2463–2471. [Google Scholar] [CrossRef]
- Snorrason, Í.; Smári, J.; Ólafsson, R.P. Motor Inhibition, Reflection Impulsivity, and Trait Impulsivity in Pathological Skin Picking. Behav. Ther. 2011, 42, 521–532. [Google Scholar] [CrossRef]
- Wilhelm, S.; Keuthen, N.J.; Deckersbach, T.; Engelhard, I.M.; Forker, A.E.; Baer, L.; O’Sullivan, R.L.; Jenike, M.A. Self-injurious skin picking: Clinical characteristics and comorbidity. J. Clin. Psychiatry 1999, 60, 454–459. [Google Scholar] [CrossRef]
- O’Connor, K.; Lavoie, M.; Desaulniers, B.; Audet, J.S. Cognitive psychophysiological treatment of bodily-focused repetitive behaviors in adults: An open trial. J. Clin. Psychol. 2018, 74, 273–285. [Google Scholar] [CrossRef]
- O’Connor, K.; Audet, J.-S.; Julien, D.; Aardema, F.; Laverdure, A.; Lavoie, M. The style of planning action (STOP) questionnaire in OCD spectrum disorders. Personal. Individ. Differ. 2015, 86, 25–32. [Google Scholar] [CrossRef]
- Grachev, I.D. MRI-based morphometric topographic parcellation of human neocortex in trichotillomania. Psychiatry Clin. Neurosci. 1997, 51, 315–321. [Google Scholar] [CrossRef]
- O’Sullivan, R.L.; Rauch, S.L.; Breiter, H.C.; Grachev, I.D.; Baer, L.; Kennedy, D.N.; Keuthen, N.J.; Savage, C.R.; Manzo, P.A.; Caviness, V.S.; et al. Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biol. Psychiatry 1997, 42, 39–45. [Google Scholar] [CrossRef]
- Keuthen, N.J.; Makris, N.; Schlerf, J.E.; Martis, B.; Savage, C.R.; McMullin, K.; Seidman, L.J.; Schmahmann, J.D.; Kennedy, D.N.; Hodge, S.M.; et al. Evidence for Reduced Cerebellar Volumes in Trichotillomania. Biol. Psychiatry 2007, 61, 374–381. [Google Scholar] [CrossRef]
- Lamothe, H.; Baleyte, J.-M.; Mallet, L.; Pelissolo, A. Trichotillomania is more related to Tourette disorder than to obsessive-compulsive disorder. Braz. J. Psychiatry 2020, 42, 87–104. [Google Scholar] [CrossRef]
- Hartmann, A.; Martino, D.; Murphy, T. Gilles de la Tourette syndrome—A treatable condition? Rev. Neurol. 2016, 172, 446–454. [Google Scholar] [CrossRef]
- Sarah, H.M.; Hana, F.Z.; Hilary, E.D.; Martin, E.F. Habit reversal training in trichotillomania: Guide for the clinician. Expert Rev. Neurother. 2013, 13, 1069–1077. [Google Scholar] [CrossRef]
- Rahman, O.; McGuire, J.; Storch, E.A.; Lewin, A.B. Preliminary randomized controlled trial of habit reversal training for treatment of hair pulling in youth. J. Child Adolesc. Psychopharmacol. 2017, 27, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Chamberlain, S.R. Trichotillomania. Am. J. Psychiatry 2016, 173, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Pauls, D.L.; Abramovitch, A.; Rauch, S.L.; Geller, D.A. Obsessive–compulsive disorder: An integrative genetic and neurobiological perspective. Nat. Rev. Neurosci. 2014, 15, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Brandt, V.; Essing, J.; Jakubovski, E.; Müller-Vahl, K. Premonitory Urge and Tic Severity, Comorbidities, and Quality of Life in Chronic Tic Disorders. Mov. Disord. Clin. Pract. 2023, 10, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Brandt, V.; Otte, J.-H.; Fremer, C.; Jakubovski, E.; Müller-Vahl, K. Non-just-right experiences are more closely related to OCD than tics in Tourette patients. Sci. Rep. 2023, 13, 19627. [Google Scholar] [CrossRef] [PubMed]
- Szejko, N.; Müller-Vahl, K.R. Challenges in the Diagnosis and Assessment in Patients with Tourette Syndrome and Comorbid Obsessive-Compulsive Disorder. Neuropsychiatr. Dis. Treat. 2021, 17, 1253–1266. [Google Scholar] [CrossRef]
- Mansueto, C.S.; Keuler, D.J. Tic or compulsion? It’s Tourettic OCD. Behav. Modif. 2005, 29, 784–799. [Google Scholar] [CrossRef]
- Katz, T.C.; Bui, T.H.; Worhach, J.; Bogut, G.; Tomczak, K.K. Tourettic OCD: Current understanding and treatment challenges of a unique endophenotype. Front. Psychiatry 2022, 13, 929526. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Li, Y.; Yan, J.; Wen, F.; Wang, F.; Liu, J.; Cui, Y.; Li, Y. Transcranial Magnetic Stimulation for Obsessive-Compulsive Disorder and Tic Disorder: A Quick Review. J. Integr. Neurosci. 2022, 21, 172. [Google Scholar] [CrossRef]
- Andreou, C.; Leicht, G.; Popescu, V.; Pogarell, O.; Mavrogiorgou, P.; Rujescu, D.; Giegling, I.; Zaudig, M.; Juckel, G.; Hegerl, U.; et al. P300 in obsessive–compulsive disorder: Source localization and the effects of treatment. J. Psychiatr. Res. 2013, 47, 1975–1983. [Google Scholar] [CrossRef]
- Hyler, S. Personality Questionnaire (PDQ-4+); New York State Psychiatric Institute: New York, NY, USA, 1994. [Google Scholar]
- Rodgers, R.; Callahan, S.; Chabrol, H. Revision of the translation of certain items in the French version of PDQ-4 (Personality Diagnostic Questionnaire, Hyler, 1994). L’encephale 2004, 30, 408–409. [Google Scholar] [PubMed]
- Leckman, J.F.; Riddle, M.A.; Hardin, M.T.; Ort, S.I.; Swartz, K.L.; Stevenson, J.; Cohen, D.J. The Yale Global Tic Severity Scale: Initial Testing of a Clinician-Rated Scale of Tic Severity. J. Am. Acad. Child Adolesc. Psychiatry 1989, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Storch, E.A.; Murphy, T.K.; Geffken, G.R.; Sajid, M.; Allen, P.; Roberti, J.W.; Goodman, W.K. Reliability and validity of the Yale Global Tic Severity Scale. Psychol. Assess. 2005, 17, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Walkup, J.T.; Rosenberg, L.A.; Brown, J.; Singer, H.S. The Validity of Instruments Measuring Tic Severity in Tourette’s Syndrome. J. Am. Acad. Child Adolesc. Psychiatry 1992, 31, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Goodman, W.K. The Yale-Brown Obsessive Compulsive Scale. Arch. Gen. Psychiatry 1989, 46, 1012. [Google Scholar] [CrossRef] [PubMed]
- Steketee, G. Behavioral assessment and treatment planning with obsessive compulsive disorder: A review emphasizing clinical application. Behav. Ther. 1994, 25, 613–633. [Google Scholar] [CrossRef]
- Taylor, S. Assessment of obsessions and compulsions: Reliability, validity, and sensitivity to treatment effects. Clin. Psychol. Rev. 1995, 15, 261–296. [Google Scholar] [CrossRef]
- Beck, A.T.; Epstein, N.; Brown, G.; Steer, R.A. An inventory for measuring clinical anxiety: Psychometric properties. J. Consult. Clin. Psychol. 1988, 56, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Freeston, M.; Ladouceur, R.; Thibodeau, N.; Gagnon, F. L’inventaire d’anxiété de Beck. Propriétés psychométriques d’une traduction française. L’Encéphale Rev. Psychiatr. Clin. Biol. Thérapeutique 1994, 20, 47–55. [Google Scholar]
- Beck, A.T. An Inventory for Measuring Depression. Arch. Gen. Psychiatry 1961, 4, 561. [Google Scholar] [CrossRef]
- Beck, A.T.; Steer, R.A.; Ball, R.; Ranieri, W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess 1996, 67, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Raven, J. Mental tests used in genetic studies. In The Performance of Related Individuals on Tests Mainly Educative and Mainly Reproductive; University of London: London, UK, 1936. [Google Scholar]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Nomenclature, S.E.P. American electroencephalographic society guidelines for. J. Clin. Neurophysiol. 1991, 8, 200–202. [Google Scholar]
- Gratton, G.; Coles, M.G.; Donchin, E. A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 468–484. [Google Scholar] [CrossRef] [PubMed]
- Society, A.E. Guideline thirteen: Guidelines for standard electrode position nomenclature. J. Clin. Neurophysiol. 1994, 11, 111–113. [Google Scholar]
- Pascual-Marqui, R.D.; Michel, C.M.; Lehmann, D. Low resolution electromagnetic tomography: A new method for localizing electrical activity in the brain. Int. J. Psychophysiol. 1994, 18, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Marqui, R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): Technical details. Methods Find Exp Clin. Pharmacol. 2002, 24, 5–12. [Google Scholar] [PubMed]
- Pascual-Marqui, R.D.; Lehmann, D.; Koenig, T.; Kochi, K.; Merlo, M.C.; Hell, D.; Koukkou, M. Low resolution brain electromagnetic tomography (LORETA) functional imaging in acute, neuroleptic-naive, first-episode, productive schizophrenia. Psychiatry Res. Neuroimaging 1999, 90, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B.; et al. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef]
- Lancaster, J.L.; Woldorff, M.G.; Parsons, L.M.; Liotti, M.; Freitas, C.S.; Rainey, L.; Kochunov, P.V.; Nickerson, D.; Mikiten, S.A.; Fox, P.T. Automated Talairach Atlas labels for functional brain mapping. Hum. Brain Mapp. 2000, 10, 120–131. [Google Scholar] [CrossRef]
- Talairach, P.J.; Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System: An Approach to Cerebral Imaging; Thieme Medical Publishers, Inc.: New York, NY, USA, 1988. [Google Scholar]
- Nichols, T.E.; Holmes, A.P. Nonparametric permutation tests for functional neuroimaging: A primer with examples. Hum. Brain Mapp. 2002, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Amitai, Y.; Connors, B.W. Intrinsic Oscillations of Neocortex Generated by Layer 5 Pyramidal Neurons. Science 1991, 251, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Haalman, I.; Vaadia, E. Dynamics of neuronal interactions: Relation to behavior, firing rates, and distance between neurons. Hum. Brain Mapp. 1997, 5, 249–253. [Google Scholar] [CrossRef]
- Friston, K.J. Statistical parametric mapping and other analyses of functional imaging data. In Brain Mapping: The Methods; Academic Press: Cambridge, MA, USA, 1996; pp. 363–385. [Google Scholar]
- Brodmann, K.; Garey, J.L. Brodmann’s Localisation in the Cerebral Cortex; Springer US: New York, NY, USA, 2006; ISBN 9780387269177. [Google Scholar]
- Holmes, C.J.; Hoge, R.; Collins, L.; Woods, R.; Toga, A.W.; Evans, A.C. Enhancement of MR images using registration for signal averaging. J. Comput. Assist. Tomogr. 1998, 22, 324–333. [Google Scholar] [CrossRef]
- Hillyard, S.A.; Münte, T.F. Selective attention to color and location: An analysis with event-related brain potentials. Percept. Psychophys. 1984, 36, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Wijers, A.A.; Mulder, G.; Okita, T.; Mulder, L.J.M. Event-Related Potentials During Memory Search and Selective Attention to Letter Size and Conjunctions of Letter Size and Color. Psychophysiology 1989, 26, 529–547. [Google Scholar] [CrossRef]
- Kenemans, J.; Kok, A.; Smulders, F. Event-related potentials to conjunctions of spatial frequency and orientation as a function of stimulus parameters and response requirements. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1993, 88, 51–63. [Google Scholar] [CrossRef]
- Mangun, K.; Mangun, G.; Hillyard, S. Event-related brain potentials and scalp current density maps during color selective attention in humans. In Proceedings of the Society for Neuroscience Abstracts; Society for Neuroscience: Washington, DC, USA, 1990; p. 578. [Google Scholar]
- Potts, G.F.; Tucker, D.M. Frontal evaluation and posterior representation in target detection. Cogn. Brain Res. 2001, 11, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Luck, S.J. An Introduction to the Event-Related Potential Technique; MIT Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Zhou, L.; Wang, G.; Nan, C.; Wang, H.; Liu, Z.; Bai, H. Abnormalities in P300 components in depression: An ERP-sLORETA study. Nord. J. Psychiatry 2019, 73, 1–8. [Google Scholar] [CrossRef]
- Klawohn, J.; Santopetro, N.J.; Meyer, A.; Hajcak, G. Reduced P300 in depression: Evidence from a flanker task and impact on ERN, CRN, and Pe. Psychophysiology 2020, 57, e13520. [Google Scholar] [CrossRef]
- Nan, C.; Wang, G.; Wang, H.; Wang, X.; Liu, Z.; Xiao, L.; Bai, H.; Wu, S. The P300 component decreases in a bimodal oddball task in individuals with depression: An event-related potentials study. Clin. Neurophysiol. 2018, 129, 2525–2533. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Wang, G.; Wang, H. Abnormalities of P300 before and after antidepressant treatment in depression: An ERP-sLORETA study. Neuroreport 2018, 29, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Polich, J. Updating P300: An integrative theory of P3a and P3b. Clin. Neurophysiol. 2007, 118, 2128–2148. [Google Scholar] [CrossRef] [PubMed]
- Pribram, K.H.; McGuinness, D. Arousal, activation, and effort in the control of attention. Psychol. Rev. 1975, 82, 116–149. [Google Scholar] [CrossRef] [PubMed]
- Kok, A. Internal and external control: A two-factor model of amplitude change of event-related potentials. Acta Psychol. 1990, 74, 213–236. [Google Scholar] [CrossRef]
- Malloy, P.; Rasmussen, S.; Braden, W.; Haier, R.J. Topographic evoked potential mapping in obsessive-compulsive disorder: Evidence of frontal lobe dysfunction. Psychiatry Res. 1989, 28, 63–71. [Google Scholar] [CrossRef]
- Beech, H.R.; Ciesielski, K.T.; Gordon, P.K. Further Observations of Evoked Potentials in Obsessional Patients. Br. J. Psychiatry 1983, 142, 605–609. [Google Scholar] [CrossRef] [PubMed]
- McNally, R.J.; Kohlbeck, P.A. Reality monitoring in obsessive-compulsive disorder. Behav. Res. Ther. 1993, 31, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Zitterl, W.; Urban, C.; Linzmayer, L.; Aigner, M.; Demal, U.; Semler, B.; Zitterl-Eglseer, K. Memory deficits in patients with DSM-IV obsessive-compulsive disorder. Psychopathology 2001, 34, 113–117. [Google Scholar] [CrossRef]
- Raggi, A.; Lanza, G.; Ferri, R. A Review on P300 in Obsessive-Compulsive Disorder. Front. Psychiatry 2021, 12, 751215. [Google Scholar] [CrossRef]
- Depue, B.E.; Curran, T.; Banich, M.T. Prefrontal regions orchestrate suppression of emotional memories via a two-phase process. Science 2007, 317, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Dayan-Riva, A.; Berger, A.; Anholt, G.E. Early cognitive processes in OCD: An ERP study. J. Affect. Disord. 2019, 246, 429–436. [Google Scholar] [CrossRef]
- Towey, J.P.; Tenke, C.E.; Bruder, G.E.; Leite, P.; Friedman, D.; Liebowitz, M.; Hollander, E. Brain event-related potential correlates of overfocused attention in obsessive-compulsive disorder. Psychophysiology 1994, 31, 535–543. [Google Scholar] [CrossRef]
- Linden, D.E.J. The Functional Neuroanatomy of Target Detection: An fMRI Study of Visual and Auditory Oddball Tasks. Cereb. Cortex 1999, 9, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.A.; Skudlarski, P.; Gatenby, J.C.; Gore, J.C. Event-related fMRI of auditory and visual oddball tasks. Magn. Reson. Imaging 2000, 18, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Mulert, C.; Pogarell, O.; Juckel, G.; Rujescu, D.; Giegling, I.; Rupp, D.; Mavrogiorgou, P.; Bussfeld, P.; Gallinat, J.; Möller, H.J.; et al. The neural basis of the P300 potential. Eur. Arch. Psychiatry Clin. Neurosci. 2004, 254, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Houshmand Chatroudi, A.; Rostami, R.; Nasrabadi, A.M.; Yotsumoto, Y. Effect of inhibition indexed by auditory P300 on transmission of visual sensory information. PLoS ONE 2021, 16, e0247416. [Google Scholar] [CrossRef] [PubMed]
- Chatzichristos, C.; Kofidis, E.; Van Paesschen, W.; De Lathauwer, L.; Theodoridis, S.; Van Huffel, S. Early soft and flexible fusion of electroencephalography and functional magnetic resonance imaging via double coupled matrix tensor factorization for multisubject group analysis. Hum. Brain Mapp. 2022, 43, 1231–1255. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.R.; Sigurdsson, H.P.; Dyke, K.; Condon, M.; Jackson, G.M. The role of the cingulate cortex in the generation of motor tics and the experience of the premonitory urge-to-tic in Tourette syndrome. J. Neuropsychol. 2021, 15, 340–362. [Google Scholar] [CrossRef]
- O’Neill, J.; Piacentini, J.C.; Peterson, B.S. Cingulate role in Tourette syndrome. Handb. Clin. Neurol. 2019, 166, 165–221. [Google Scholar]
- Lane, R.D.; Chua, P.M.L.; Dolan, R.J. Common effects of emotional valence, arousal and attention on neural activation during visual processing of pictures. Neuropsychologia 1999, 37, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Lang, P.J.; Bradley, M.M.; Fitzsimmons, J.R.; Cuthbert, B.N.; Scott, J.D.; Moulder, B.; Nangia, V. Emotional arousal and activation of the visual cortex: An fMRI analysis. Psychophysiology 1998, 35, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.D.; Reiman, E.M.; Bradley, M.M.; Lang, P.J.; Ahern, G.L.; Davidson, R.J.; Schwartz, G.E. Neuroanatomical correlates of pleasant and unpleasant emotion. Neuropsychologia 1997, 35, 1437–1444. [Google Scholar] [CrossRef]
- Mitterschiffthaler, M.T.; Kumari, V.; Malhi, G.S.; Brown, R.G.; Giampietro, V.P.; Brammer, M.J.; Suckling, J.; Poon, L.; Simmons, A.; Andrew, C. Neural response to pleasant stimuli in anhedonia: An fMRI study. Neuroreport 2003, 14, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Göttlich, M.; Krämer, U.M.; Kordon, A.; Hohagen, F.; Zurowski, B. Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Hum. Brain Mapp. 2014, 35, 5617–5632. [Google Scholar] [CrossRef]
- Szeszko, P.R.; Ardekani, B.A.; Ashtari, M.; Malhotra, A.K.; Robinson, D.G.; Bilder, R.M.; Lim, K.O. White Matter Abnormalities in Obsessive-compulsive Disorder. Arch. Gen. Psychiatry 2005, 62, 782. [Google Scholar] [CrossRef]
- Moreira, P.S.; Marques, P.; Soriano-Mas, C.; Magalhães, R.; Sousa, N.; Soares, J.M.; Morgado, P. The neural correlates of obsessive-compulsive disorder: A multimodal perspective. Transl. Psychiatry 2017, 7, e1224. [Google Scholar] [CrossRef]
- Moreira, P.S.; Macoveanu, J.; Marques, P.; Coelho, A.; Magalhães, R.; Siebner, H.R.; Soares, J.M.; Sousa, N.; Morgado, P. Altered response to risky decisions and reward in patients with obsessive–compulsive disorder. J. Psychiatry Neurosci. 2020, 45, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, Z.; Zhang, Z.; Zhu, Y.; Gao, R.; Cao, X.; Tan, L.; Wang, Z.; Zhang, H.; Li, Y.; et al. The right thalamic glutamate level correlates with functional connectivity with right dorsal anterior cingulate cortex/middle occipital gyrus in unmedicated obsessive–compulsive disorder: A combined fMRI and 1H-MRS study. Aust. N. Z. J. Psychiatry 2019, 53, 207–218. [Google Scholar] [CrossRef]
- Kariuki-Nyuthe, C.; Gomez-Mancilla, B.; Stein, D.J. Obsessive compulsive disorder and the glutamatergic system. Curr. Opin. Psychiatry 2014, 27, 32–37. [Google Scholar] [CrossRef]
- Wu, K.; Hanna, G.L.; Rosenberg, D.R.; Arnold, P.D. The role of glutamate signaling in the pathogenesis and treatment of obsessive–compulsive disorder. Pharmacol. Biochem. Behav. 2012, 100, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Fan, Q.; You, C.; Wang, J.; Dong, Z.; Wang, X.; Chen, K.; Xiao, Z.; Jiang, K. Structural changes in the gray matter of unmedicated patients with obsessive-compulsive disorder: A voxel-based morphometric study. Neurosci. Bull. 2013, 29, 642–648. [Google Scholar] [CrossRef] [PubMed]
- Flasbeck, V.; Enzi, B.; Andreou, C.; Juckel, G.; Mavrogiorgou, P. P300 and delay-discounting in obsessive–compulsive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2022, 272, 327–339. [Google Scholar] [CrossRef] [PubMed]




| TS (n = 24) | BFRB (n = 16) | OCD (n = 18) | Controls (n = 59) | ANOVA | |
|---|---|---|---|---|---|
| Age (years) | 37 (11) | 41 (15) | 43 (15) | 38 (12) | ns |
| Sex (M/F) | 16/8 | 3/13 | 11/7 | 41/18 | BFRB vs. TS (p = 0.009) BFRB vs. OCD (p = 0.048) BFRB vs. Controls (p = 0.001) |
| Intelligence (Raven’s Progressive Matrices) | 82 (22) | 81 (16) | 74 (29) | 82 (19) | ns |
| Laterality | 19/2 | 12/2 | 16/2 | 58/0/1 | ns |
| Anxiety (BAI) | 9 (6) | 10.1 (7) | 15 (12) | 3.9 (4) | Controls vs. TS (p = 0.004) Controls vs. BFRB (p = 0.017) Controls vs. OCD (p = 0.004) |
| Depression (BDI) | 14 (11) | 17 (9) | 17 (8) | 3 (4) | Controls vs. TS (p ≤ 0.001) Controls vs. BFRB (p ≤ 0.001) Controls vs. OCD (p ≤ 0.001) |
| Tics severity (YGTSS) | 38 (17) | 28 (8) | - | - | T = 2.52 (p = 0.017) |
| OCD symptoms’ severity (YBOCS) | - | - | 27 (6) | - |
| Lobe | Structure | Brodmann Area | Coordinates | Voxel Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| MNI | Talairach | ||||||||
| X | Y | Z | X | Y | Z | ||||
| Limbic | Posterior cingulate cortex | 23 | −5 | −30 | 25 | −5 | −28 | 24 | 1.03794 |
| Limbic | Cingulate gyrus | 23 | 0 | −35 | 25 | 0 | −33 | 25 | 1.03262 |
| Limbic | Posterior cingulate cortex | 30 | 5 | −45 | 20 | 5 | −43 | 21 | 1.03107 |
| Limbic | Cingulate gyrus | 31 | 0 | −40 | 25 | 0 | −38 | 25 | 1.01665 |
| Limbic | Posterior cingulate cortex | 29 | 10 | −45 | 5 | 10 | −43 | 7 | 9.31017 |
| Limbic | Parahippocampal gyrus | 27 | 10 | −35 | 0 | 10 | −34 | 2 | 9.15933 |
| Parietal | Precuneus | 31 | 10 | −50 | 30 | 10 | −47 | 30 | 9.08931 |
| Limbic | Parahippocampal gyrus | 30 | 10 | −40 | 0 | 10 | −39 | 2 | 9.08438 |
| Lobe | Structure | Brodmann Area | Coordinates | Voxel Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| MNI | Talairach | ||||||||
| X | Y | Z | X | Y | Z | ||||
| Occipital | Cuneus | 18 | −25 | −95 | −5 | −25 | −92 | 0 | 1.33097 |
| Occipital | Lingual gyrus | 18 | −20 | −100 | −10 | −20 | −97 | −4 | 1.33039 |
| Occipital | Lingual gyrus | 17 | −20 | −95 | −5 | −20 | −92 | 0 | 1.31363 |
| Occipital | Medim occipital gyrus | 18 | −25 | −95 | 0 | −25 | −92 | 5 | 1.30333 |
| Occipital | Medium occipital gyrus | 19 | −25 | −90 | 5 | −25 | −87 | 9 | 1.30078 |
| Limbic | Posterior cingulate cortex | 31 | −5 | −55 | 20 | −5 | −52 | 21 | 1.29951 |
| Parietal | Precuneus | 31 | −5 | −50 | 30 | −5 | −47 | 30 | 1.28793 |
| Limbic | Posterior cingulate cortex | 23 | 0 | −50 | 25 | 0 | −47 | 25 | 1.28386 |
| Occipital | Inferior occipital gyrus | 18 | −30 | −95 | −10 | −30 | −92 | −4 | 1.27584 |
| Limbic | Cingulate gyrus | 31 | 0 | −55 | 25 | 0 | −52 | 26 | 1.27503 |
| Occipital | Cuneus | 17 | −20 | −85 | 5 | −20 | −82 | 9 | 1.26789 |
| Limbic | Posterior cingulate cortex | 30 | −5 | −50 | 20 | −5 | −48 | 21 | 1.25667 |
| Occipital | Inferior occipital gyrus | 17 | −20 | −95 | −15 | −20 | −93 | −8 | 1.25145 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desfossés-Vallée, S.; Leclerc, J.B.; Blanchet, P.; O’Connor, K.P.; Lavoie, M.E. Comparing the ‘When’ and the ‘Where’ of Electrocortical Activity in Patients with Tourette Syndrome, Body-Focused Repetitive Behaviors, and Obsessive Compulsive Disorder. J. Clin. Med. 2024, 13, 2489. https://doi.org/10.3390/jcm13092489
Desfossés-Vallée S, Leclerc JB, Blanchet P, O’Connor KP, Lavoie ME. Comparing the ‘When’ and the ‘Where’ of Electrocortical Activity in Patients with Tourette Syndrome, Body-Focused Repetitive Behaviors, and Obsessive Compulsive Disorder. Journal of Clinical Medicine. 2024; 13(9):2489. https://doi.org/10.3390/jcm13092489
Chicago/Turabian StyleDesfossés-Vallée, Sarah, Julie B. Leclerc, Pierre Blanchet, Kieron P. O’Connor, and Marc E. Lavoie. 2024. "Comparing the ‘When’ and the ‘Where’ of Electrocortical Activity in Patients with Tourette Syndrome, Body-Focused Repetitive Behaviors, and Obsessive Compulsive Disorder" Journal of Clinical Medicine 13, no. 9: 2489. https://doi.org/10.3390/jcm13092489






