New Interspecific Brassica Hybrids with High Levels of Heterosis for Fatty Acids Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Seeds Sampling and Analysis of Fatty Acids Composition:
2.3. Statistical Analysis:
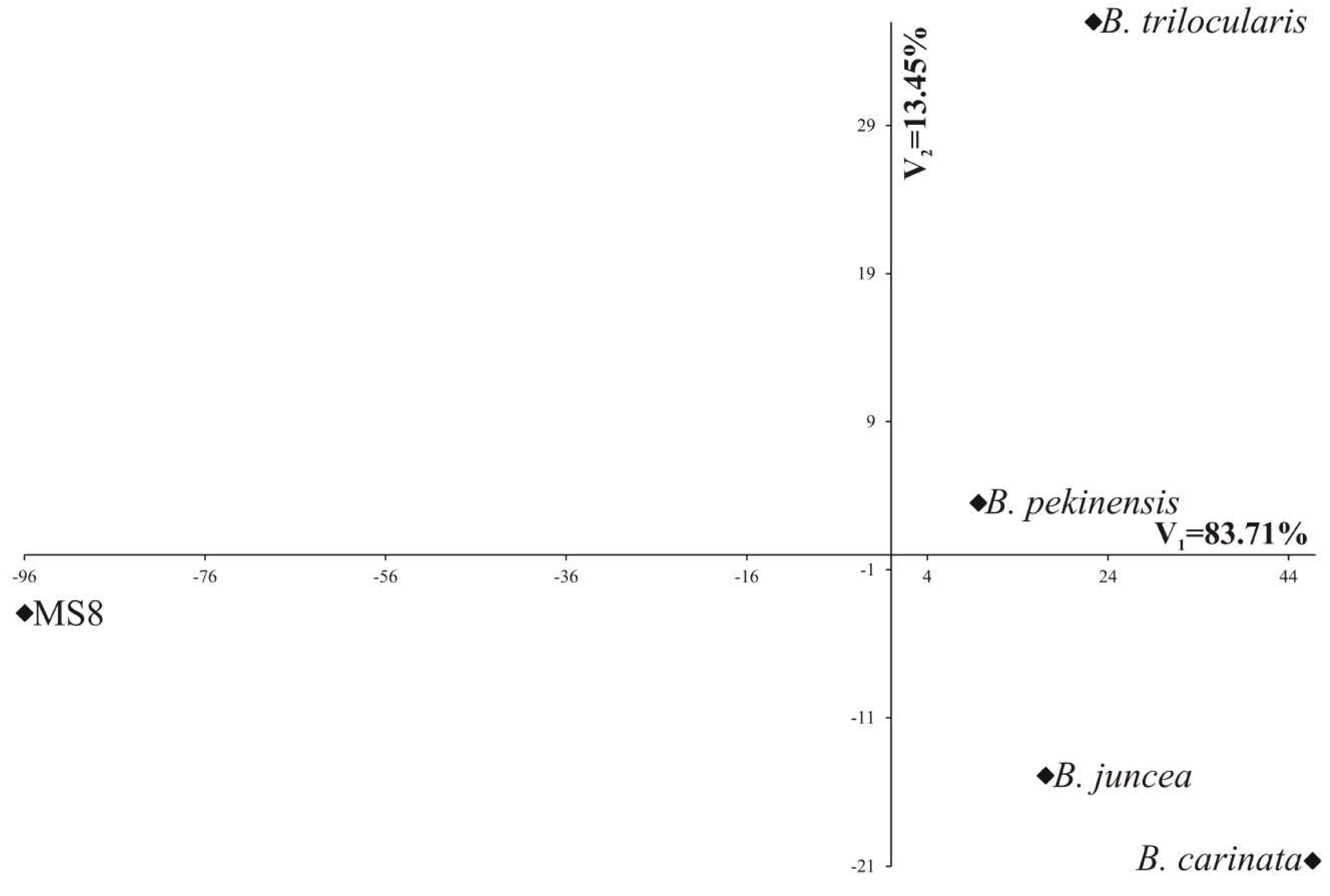
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schuster, W.; Michael, J. Untersuchungen über Inzuchtdepressionen und Heterosis effekte bei Raps (Brassica napus oleifera). Z Pflanz. 1976, 77, 55–66. [Google Scholar]
- Sernyk, J.L.; Stefansson, B.R. Heterosis in summer rape. Can. J. Plant Sci. 1983, 63, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.Y.; Heneen, W.K. Fatty acid composition of resynthesized Brassica napus L., B. campestris L. and B. alboglabra Bailey with special reference to the inheritance of erucic acid content. Heredity 1989, 63, 309–314. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.; Dey, S.S.; Bhatia, R.; Kumar, R.; Sharma, K.; Behera, T.K. Heterosis and combining ability in cytoplasmic male sterile and doubled haploid based Brassica oleracea progenies and prediction of heterosis using microsatellites. PLoS ONE 2019, 14, e0210772. [Google Scholar] [CrossRef] [PubMed]
- Fick, G.N. Genetics and breeding of sunflower. JAOCS 1983, 60, 1252–1253. [Google Scholar] [CrossRef]
- Friedt, W. Biotechnology in breeding of industrial oil crops—The present status and future prospects. Fur Sci. Technol. 1988, 90, 51–55. [Google Scholar] [CrossRef] [Green Version]
- Chen, B.Y.; Gertsson, B. Genotypes for high oleic acid content (about 80%) in the oil of rapeseed (Brassica napus L.). EUCARPIA Crucif. Newsl. 1988, 13, 46–47. [Google Scholar]
- Simopoulos, A. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.J.; Fernández, C.M.; Casas, A.; Rodríguez, L.; Pérez, A. Influence of fatty acid composition of raw materials on biodiesel properties. Bioresour. Technol. 2009, 100, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Priyamedha, S.; Singh, B.K.; Ram, B.; Kumar, A.; Singh, V.V.; Meena, M.L.; Singh, D. Development and Evaluation of Double Low Quality Lines of Indian mustard (Brassica juncea L. Czern &Coss). SABRAO J. Breed Genet. 2014, 46, 274–283. [Google Scholar]
- Ali, N.; Bakht, J.; Naveed, K.; Liaquat, M.; Khan, S.A.; Saeed, M.; Ali, S.; Hussain, I.; Khan, S.M.; Salim, M. Heterosis studies for some fatty acids composition of Indian Mustard (Brassica juncea L.). J. Anim. Plant Sci. 2015, 25, 587–592. [Google Scholar]
- Hassan, M.F.; Seyis, F.; Badani, A.G.; Pons-Kühnemann, J.; Friedt, W.; Lühs, W.; Snowdon, R.J. Surveying genetic diversity in the Brassica napus L. gene pool using SSR markers. Genet. Resour. Crop Evol. 2006, 53, 793–802. [Google Scholar] [CrossRef]
- Pal, B.P.; Sikka, S.M. Exploitation of hybrid vigour in the improvement of crop plants fruits and vegetables. Indian J. Genet. Plant Breed. 1956, 16, 95–193. [Google Scholar]
- Niemann, J.; Bocianowski, J.; Wojciechowski, A. Effects of genotype and environment on seed quality traits variability in interspecific cross-derived Brassica lines. Euphytica 2018, 214, 193. [Google Scholar] [CrossRef] [Green Version]
- Momotaz, A.; Kato, M.; Kakihara, F. Variation in seedfertility and fatty acid composition in allohexaploids between Brassica carinata and Sinapis species with the advance of generations. Breed. Sci. 2000, 2, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.S.; Wilk, M.B. An analysis of variance test for normality (complete samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Rencher, A.C. Interpretation of canonical discriminant functions, canonical variates, and principal components. Am. Stat. 1992, 46, 217–225. [Google Scholar]
- Mahalanobis, P.C. On the generalized distance in statistics. Proc. Natl. Acad. Sci. India A 1936, 12, 49–55. [Google Scholar]
- Seidler-Łożykowska, K.; Bocianowski, J. Evaluation of variability of morphological traits of selected caraway (Carum carvi L.) genotypes. Ind. Crop. Prod. 2012, 35, 140–145. [Google Scholar] [CrossRef]
- Gehringer, A.; Snowdon, R.; Spiller, T.; Basunanda, P.; Friedt, W. New oilseed rape (Brassica napus) hybrids with high levels of heterosis for seed yield under nutrient-poor conditions. Breed. Sci. 2007, 57, 315–320. [Google Scholar] [CrossRef] [Green Version]
- Qu, C.; Jia, L.; Fu, F.; Zhao, H.; Lu, K.; Wei, L.; Xu, X.; Liang, Y.; Li, S.; Wang, R.; et al. Genome-wide association mapping and identification of candidate genes for fatty acid composition in Brassica napus L. using SNP markers. BMC Genom. 2017, 18, 232. [Google Scholar] [CrossRef] [Green Version]
- Cuthbert, R.D.; Crow, G.; Mcvetty, P.B.E. Assessment of seed quality performance and heterosis for seed quality traits in hybrid high erucic acid rapeseed (HEAR). Can. J. Plant Sci. 2011, 91, 837–846. [Google Scholar] [CrossRef]
- Adamska, E.; Cegielska-Taras, T.; Kaczmarek, Z.; Szała, L. Multivariate approach to evaluating the fatty acid composition of seed oil in a doubled haploid population of winter oilseed rape (Brassica napus L.). J. Appl. Genet. 2004, 45, 419–425. [Google Scholar] [PubMed]
- Bocianowski, J.; Majchrzak, L. Analysis of effects of cover crop and tillage method combinations on the phenotypic traits of spring wheat (Triticum aestivum L.) using multivariate methods. Appl. Ecol. Env. Res. 2019, 17, 15267–15276. [Google Scholar] [CrossRef]
- Rybiński, W.; Banda, M.; Bocianowski, J.; Starzycka-Korbas, E.; Starzycki, M.; Nowosad, K. Estimation of the physicochemical variation of chickpea seeds (Cicer arietinum L.). Int. Agrophys. 2019, 33, 67–80. [Google Scholar] [CrossRef]
- Wrońska-Pilarek, D.; Jagodziński, A.M.; Bocianowski, J.; Marecik, M.; Janyszek-Sołtysiak, M. Pollen morphology and variability of Sambucus nigra L.—Adoxaceae. Biologia 2020, 75, 481–493. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Ciak, M.; Rybiński, W.; Bocianowski, J.; Börner, A. Diversity of the composition and content of soluble carbohydrates in seeds of the genus Vicia (Leguminosae). Genet. Resour. Crop Evol. 2018, 65, 541–554. [Google Scholar] [CrossRef] [Green Version]
- Radkowski, A.; Radkowska, I.; Bocianowski, J. Effect of fertilization of meadow sward with amino acids obtained from enzymatic hydrolysis on silage quality. J. Elem. 2020, 25, 259–277. [Google Scholar] [CrossRef]
- Shamsuddin, A.K.M. Genetic diversity in relation to heterosis and combining ability in spring wheat. Theor. Appl. Genet. 1985, 70, 306–308. [Google Scholar] [CrossRef] [PubMed]
- Seidler-Łożykowska, K.; Bocianowski, J.; Król, D. The evaluation of the variability of morphological and chemical traits of the selected lemon balm (Melissa officinalis L.) genotypes. Ind. Crops Prod. 2013, 49, 515–520. [Google Scholar] [CrossRef]
- Nowosad, K.; Liersch, A.; Popławska, W.; Bocianowski, J. Genotype by environment interaction for seed yield in rapeseed (Brassica napus L.) using additive main effects and multiplicative interaction model. Euphytica 2016, 208, 187–194. [Google Scholar] [CrossRef]
- Bocianowski, J.; Szulc, P.; Nowosad, K. Soil tillage methods by years interaction for dry matter of plant yield of maize (Zea mays L.) using additive main effects and multiplicative interaction model. J. Integr. Agric. 2018, 17, 2836–2839. [Google Scholar] [CrossRef] [Green Version]
- Bocianowski, J.; Nowosad, K.; Szulc, P. Soil tillage methods by years interaction for harvest index of maize (Zea mays L.) using additive main effects and multiplicative interaction model. Acta Agric. Scand. B 2019, 69, 75–81. [Google Scholar] [CrossRef]
- Wrońska-Pilarek, D.; Szkudlarz, P.; Bocianowski, J. Systematic importance of morphological features of pollen grains of species from Erica (Ericaceae) genus. PLoS ONE 2018, 13, e0204557. [Google Scholar] [CrossRef] [PubMed]
- Cegielska-Taras, T.; Adamska, E.; Szała, L.; Kaczmarek, Z. Estimation of the genetic parameters for fatty acid content in DH lines obtained from winter oilseed rape of F1 hybrid (DH O120 × DH C-1041). Rośliny Oleiste Oilseed Crop. 2005, 26, 11–17. [Google Scholar]
- Liersch, A.; Bocianowski, J.; Bartkowiak-Broda, I. Fatty acid and glucosinolate level in seeds of different types of winter oilseed rape cultivars (Brassica napus L.). Commun. Biometry Crop Sci. 2013, 8, 39–47. [Google Scholar]
- Iqbal, M.C.M.; Weerakoon, S.R.; Geethanjalie, H.D.N.; Peiris, P.K.D.; Weerasena, O.V.D.S.J. Changes in the fatty acids in seeds of interspecific hybrids between Brassica napus and Brassica juncea. Crop Pasture Sci. 2011, 62, 390–395. [Google Scholar] [CrossRef]
- Szala, L.; Kaczmarek, Z.; Adamska, E.; Cegielska-Taras, T. Assessment of winter oilseed rape DH lines using uni- and multivariate methods of quantitative genetics and mathematical methods. Biotechnol. J. Biotechnol. Comput. Biol. Bionanotechnol. 2015, 96, 171–177. [Google Scholar] [CrossRef] [Green Version]


| Genotype’s Code | Species or Cross Combination | Genotype’s Code | Species or Cross Combination |
|---|---|---|---|
| S1 | Brassica napus MS8 line | ||
| S2 | Brassica carinata | ||
| S3 | Brassica juncea | ||
| S4 | Brassica rapa ssp. pekinensis | ||
| S5 | Brassica rapa ssp. trilocularis cv. Yellow Sarson | ||
| H1 | B. napus × B. carinata (125/1) | H12 | B. napus × Brassica rapa ssp. pekinensis (14/1) |
| H2 | B. napus × B. carinata (125/2) | H13 | B. napus × Brassica rapa ssp. pekinensis (18/1) |
| H3 | B. napus × B. carinata (126/1) | H14 | B. napus × Brassica rapa ssp. pekinensis (19/1) |
| H4 | B. napus × B. carinata (126/2) | H15 | B. napus × Brassica rapa ssp. pekinensis (20/1) |
| H5 | B. napus × B. carinata (127/2) | H16 | B. napus × Brassica rapa ssp. pekinensis (24/1) |
| H6 | B. napus × B. juncea (55/1) | H17 | B. napus × Brassica rapa ssp. trilocularis (2) |
| H7 | B. napus × B. juncea (58/1) | H18 | B. napus × Brassica rapa ssp. trilocularis (5) |
| H8 | B. napus × Brassica rapa ssp. pekinensis (7/1) | H19 | B. napus × Brassica rapa ssp. trilocularis (6) |
| H9 | B. napus × Brassica rapa ssp. pekinensis (9/1) | H20 | B. napus × Brassica rapa ssp. trilocularis (43) |
| H10 | B. napus × Brassica rapa ssp. pekinensis (12/1) | H21 | B. napus × Brassica rapa ssp. trilocularis (46) |
| H11 | B. napus × Brassica rapa ssp. pekinensis(13/1) | H22 | B. napus × Brassica rapa ssp. trilocularis (47) |
| Basic Weather Parameters | Dłoń | ||
|---|---|---|---|
| 2009 | 2010 | 2011 | |
| Mean annual temperature (°C) | 9.1 | 7.9 | 9.8 |
| Sum of precipitation (mm) | 605.1 | 753.9 | 465.5 |
| Genotypes | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 | C22:0 | C22:1 | C24:0 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | Mean | s.d. | |
| S1 | 4.24 | 0.10 | 0.202 | 0.008 | 1.692 | 0.134 | 42.34 | 0.37 | 17.68 | 0.86 | 8.46 | 0.50 | 0.692 | 0.044 | 11.6 | 0.32 | 0.39 | 0.03 | 13.1 | 0.50 | 0.168 | 0.04 |
| S2 | 3.658 | 0.27 | 0.168 | 0.016 | 1.196 | 0.134 | 10.42 | 0.41 | 20.06 | 0.46 | 8.576 | 0.31 | 1.068 | 0.035 | 7.04 | 0.36 | 1.24 | 0.24 | 44.84 | 0.74 | 1.638 | 0.14 |
| S3 | 3.626 | 0.27 | 0.172 | 0.043 | 1.27 | 0.210 | 16.12 | 0.13 | 19.26 | 0.72 | 17.306 | 1.81 | 0.66 | 0.207 | 6.2 | 0.14 | 0.048 | 0.01 | 35.08 | 1.84 | 0.188 | 0.11 |
| S4 | 2.818 | 0.04 | 0.182 | 0.027 | 1.318 | 0.019 | 17.38 | 0.35 | 14.35 | 0.21 | 8.88 | 0.12 | 1.018 | 0.036 | 7.62 | 0.16 | 1.206 | 0.06 | 43.68 | 0.29 | 1.448 | 0.22 |
| S5 | 1.758 | 0.15 | 0.152 | 0.008 | 1.028 | 0.011 | 12.1 | 0.08 | 9.8 | 0.12 | 5.116 | 0.06 | 1.106 | 0.032 | 4.95 | 0.08 | 1.296 | 0.06 | 61.44 | 0.28 | 0.988 | 0.02 |
| H1 F1 | 4.556 | 0.36 | 0.216 | 0.017 | 1.612 | 0.106 | 39.2 | 3.06 | 14.9 | 1.15 | 7.246 | 1.15 | 0.75 | 0.043 | 13.18 | 0.69 | 0.412 | 0.03 | 17.61 | 1.71 | 0.2 | 0.04 |
| H1 F2 | 4.482 | 0.17 | 0.43 | 0.020 | 1.74 | 0.046 | 36.96 | 1.37 | 15.78 | 1.12 | 8.348 | 0.56 | 0.424 | 0.015 | 12.85 | 0.95 | 0.096 | 0.04 | 18.63 | 0.90 | 0.094 | 0.01 |
| H1 F3 | 4.519 | 0.17 | 0.323 | 0.016 | 1.676 | 0.044 | 38.08 | 1.30 | 15.34 | 0.75 | 7.797 | 0.46 | 0.581 | 0.019 | 13.01 | 0.71 | 0.255 | 0.03 | 18.11 | 0.94 | 0.155 | 0.03 |
| H2 F1 | 4.452 | 0.33 | 0.184 | 0.028 | 1.612 | 0.167 | 30.66 | 0.88 | 10.38 | 0.42 | 4.308 | 0.29 | 0.906 | 0.009 | 16.2 | 0.04 | 0.628 | 0.11 | 30.25 | 0.56 | 0.256 | 0.05 |
| H2 F2 | 4.488 | 0.45 | 0.202 | 0.019 | 1.694 | 0.073 | 30.76 | 1.06 | 11.82 | 0.96 | 3.944 | 0.46 | 0.902 | 0.016 | 15.97 | 0.14 | 0.578 | 0.04 | 29.2 | 0.14 | 0.218 | 0.03 |
| H2 F3 | 4.458 | 0.39 | 0.194 | 0.018 | 1.653 | 0.108 | 30.86 | 0.72 | 11.03 | 0.89 | 4.133 | 0.13 | 0.905 | 0.007 | 16.25 | 0.18 | 0.603 | 0.06 | 29.47 | 0.21 | 0.238 | 0.03 |
| H3 F1 | 4.26 | 0.19 | 0.184 | 0.009 | 1.494 | 0.070 | 33.22 | 0.82 | 13.57 | 0.65 | 6.524 | 0.76 | 0.748 | 0.041 | 15.71 | 0.19 | 0.446 | 0.01 | 23.52 | 0.53 | 0.224 | 0.03 |
| H3 F2 | 4.284 | 0.19 | 0.128 | 0.099 | 1.504 | 0.085 | 33.08 | 0.46 | 13.46 | 0.50 | 6.372 | 0.49 | 0.972 | 0.018 | 16 | 0.33 | 0.45 | 0.03 | 23.37 | 0.44 | 0.238 | 0.02 |
| H3 F3 | 4.272 | 0.19 | 0.157 | 0.046 | 1.5 | 0.076 | 33.07 | 0.58 | 13.51 | 0.57 | 6.508 | 0.53 | 0.86 | 0.026 | 15.79 | 0.07 | 0.454 | 0.01 | 23.52 | 0.46 | 0.232 | 0.03 |
| H34 F1 | 4.53 | 0.05 | 0.198 | 0.008 | 1.608 | 0.019 | 36.58 | 0.34 | 13.84 | 0.20 | 6.666 | 0.16 | 0.732 | 0.004 | 14.44 | 0.05 | 0.382 | 0.03 | 20.69 | 0.29 | 0.226 | 0.05 |
| H4 F2 | 4.482 | 0.20 | 0.142 | 0.111 | 1.586 | 0.110 | 35.29 | 1.98 | 13.74 | 0.37 | 6.632 | 0.23 | 0.972 | 0.018 | 15.26 | 1.21 | 0.402 | 0.03 | 21.1 | 1.00 | 0.232 | 0.02 |
| H4 F3 | 4.506 | 0.13 | 0.171 | 0.058 | 1.598 | 0.061 | 35.8 | 0.94 | 13.78 | 0.26 | 6.638 | 0.19 | 0.854 | 0.009 | 15 | 0.37 | 0.392 | 0.03 | 20.9 | 0.66 | 0.229 | 0.03 |
| H5 F1 | 5.888 | 0.27 | 0.246 | 0.015 | 2.16 | 0.046 | 59.67 | 3.19 | 10.18 | 1.63 | 3.382 | 0.89 | 0.83 | 0.037 | 8.81 | 0.40 | 0.43 | 0.03 | 8.04 | 0.61 | 0.252 | 0.12 |
| H5 F2 | 5.77 | 0.36 | 0.238 | 0.013 | 2.636 | 0.319 | 59.48 | 1.73 | 10.41 | 1.00 | 3.394 | 0.62 | 0.768 | 0.094 | 8.61 | 0.45 | 0.376 | 0.06 | 7.95 | 0.56 | 0.218 | 0.10 |
| H5 H3 | 5.826 | 0.22 | 0.242 | 0.013 | 2.334 | 0.187 | 59.7 | 2.15 | 10.11 | 1.07 | 3.394 | 0.56 | 0.799 | 0.034 | 8.73 | 0.37 | 0.404 | 0.03 | 8.08 | 0.44 | 0.234 | 0.08 |
| H6 F1 | 3.626 | 0.27 | 0.172 | 0.043 | 1.27 | 0.210 | 19.01 | 2.16 | 13.1 | 0.59 | 13.726 | 0.34 | 0.66 | 0.207 | 6.56 | 0.54 | 0.048 | 0.01 | 41.58 | 0.98 | 0.176 | 0.08 |
| H6 F2 | 3.678 | 0.35 | 0.278 | 0.092 | 1.314 | 0.255 | 16.77 | 0.39 | 13.92 | 0.53 | 13.07 | 0.47 | 1.386 | 0.246 | 7.43 | 0.27 | 0.44 | 0.11 | 40.79 | 0.40 | 0.886 | 0.37 |
| H6 F3 | 3.652 | 0.12 | 0.225 | 0.063 | 1.292 | 0.223 | 16.76 | 0.51 | 13.58 | 0.25 | 14.394 | 0.83 | 1.029 | 0.157 | 6.93 | 0.31 | 0.243 | 0.05 | 41.33 | 0.28 | 0.531 | 0.19 |
| H7 F1 | 4.208 | 0.15 | 0.228 | 0.036 | 1.582 | 0.111 | 61.31 | 0.85 | 18.36 | 0.73 | 9.018 | 0.44 | 0.628 | 0.029 | 2.88 | 0.37 | 0.418 | 0.04 | 1.19 | 0.19 | 0.096 | 0.04 |
| H7 F2 | 4.112 | 0.15 | 0.332 | 0.084 | 1.802 | 0.619 | 60.39 | 0.65 | 18.1 | 0.34 | 8.922 | 0.19 | 0.866 | 0.505 | 3.78 | 0.31 | 0.432 | 0.14 | 1.01 | 0.12 | 0.2 | 0.11 |
| H7 F3 | 4.065 | 0.31 | 0.37 | 0.234 | 1.736 | 0.306 | 51.84 | 19.63 | 17.31 | 2.03 | 9.919 | 1.95 | 0.852 | 0.337 | 4.06 | 1.83 | 0.407 | 0.09 | 9.2 | 18.17 | 0.174 | 0.09 |
| H8 F1 | 4.048 | 0.33 | 0.196 | 0.015 | 1.774 | 0.080 | 40.12 | 2.92 | 14.92 | 0.54 | 7.54 | 1.17 | 0.774 | 0.033 | 13.73 | 1.43 | 0.35 | 0.04 | 16.48 | 1.70 | 0.048 | 0.07 |
| H8 F2 | 3.938 | 0.43 | 0.196 | 0.015 | 1.72 | 0.162 | 39.65 | 4.41 | 14.31 | 2.01 | 7.51 | 2.30 | 0.766 | 0.047 | 14.8 | 1.31 | 0.36 | 0.05 | 16.61 | 1.92 | 0.092 | 0.06 |
| H8 F3 | 3.816 | 0.21 | 0.192 | 0.018 | 1.612 | 0.035 | 37.08 | 1.49 | 15.29 | 0.71 | 8.254 | 0.50 | 0.75 | 0.027 | 13.96 | 1.01 | 0.376 | 0.02 | 18.45 | 1.47 | 0.17 | 0.04 |
| H9 F1 | 3.586 | 0.32 | 0.188 | 0.026 | 1.6 | 0.077 | 26.65 | 5.17 | 12.86 | 2.16 | 7.286 | 0.90 | 0.81 | 0.092 | 16.24 | 1.07 | 0.396 | 0.07 | 30.13 | 7.26 | 0.22 | 0.05 |
| H9 F2 | 4.172 | 0.35 | 0.246 | 0.063 | 1.544 | 0.064 | 32.04 | 1.77 | 15.05 | 2.39 | 6.918 | 1.59 | 0.802 | 0.051 | 14.09 | 1.25 | 0.556 | 0.14 | 24.22 | 2.36 | 0.238 | 0.10 |
| H9 F3 | 4.786 | 0.34 | 0.208 | 0.015 | 1.76 | 0.096 | 31.57 | 1.02 | 11.1 | 0.81 | 4.638 | 0.78 | 0.876 | 0.038 | 16.68 | 0.21 | 0.472 | 0.04 | 27.53 | 0.60 | 0.234 | 0.05 |
| H10 F1 | 3.55 | 0.55 | 0.174 | 0.025 | 1.532 | 0.166 | 28.75 | 2.67 | 11.27 | 1.88 | 5.5 | 1.60 | 0.89 | 0.070 | 13.6 | 0.55 | 0.672 | 0.09 | 33.59 | 2.41 | 0.372 | 0.07 |
| H10 F2 | 4.826 | 0.93 | 0.214 | 0.034 | 1.718 | 0.234 | 32.26 | 2.62 | 11.45 | 0.41 | 4.832 | 1.04 | 0.858 | 0.030 | 13.9 | 2.81 | 0.52 | 0.08 | 28.92 | 1.15 | 0.284 | 0.03 |
| H10 F3 | 5.038 | 0.67 | 0.22 | 0.014 | 1.928 | 0.266 | 35.15 | 3.07 | 10.79 | 3.51 | 5.806 | 3.63 | 0.928 | 0.110 | 14.57 | 1.76 | 0.548 | 0.03 | 24.45 | 1.68 | 0.218 | 0.07 |
| H11 F1 | 4.156 | 0.62 | 0.192 | 0.024 | 1.85 | 0.197 | 46.91 | 5.54 | 15.16 | 2.34 | 7.794 | 2.51 | 0.768 | 0.119 | 11.58 | 2.17 | 0.37 | 0.10 | 11.06 | 1.71 | 0.116 | 0.17 |
| H11 F2 | 4.316 | 0.29 | 0.21 | 0.024 | 1.932 | 0.123 | 57.98 | 3.16 | 17.47 | 1.16 | 7.714 | 0.84 | 0.754 | 0.053 | 5.1 | 1.81 | 0.388 | 0.06 | 4.04 | 1.74 | 0.036 | 0.08 |
| H11 F3 | 4.302 | 0.48 | 0.198 | 0.038 | 1.918 | 0.223 | 61.05 | 3.26 | 19.82 | 1.75 | 8.258 | 1.07 | 0.734 | 0.101 | 2.26 | 0.41 | 0.404 | 0.06 | 1 | 0.48 | 0 | 0.00 |
| H12 F1 | 4.032 | 0.15 | 0.198 | 0.004 | 1.682 | 0.137 | 39.83 | 2.57 | 16.48 | 0.93 | 9.244 | 0.45 | 0.72 | 0.053 | 12.28 | 0.72 | 0.358 | 0.02 | 15 | 1.91 | 0.158 | 0.04 |
| H12 F2 | 4.246 | 0.53 | 0.204 | 0.015 | 1.96 | 0.238 | 47.58 | 2.93 | 15.31 | 1.77 | 7.164 | 2.12 | 0.8 | 0.090 | 10.84 | 2.05 | 0.376 | 0.04 | 11.41 | 1.80 | 0.07 | 0.10 |
| H12 F3 | 3.878 | 0.30 | 0.194 | 0.009 | 1.8 | 0.118 | 42.12 | 1.72 | 14.73 | 1.54 | 7.956 | 1.03 | 0.754 | 0.098 | 12.86 | 1.09 | 0.352 | 0.05 | 15.19 | 1.81 | 0.106 | 0.02 |
| H13 F1 | 4.866 | 0.80 | 0.272 | 0.119 | 1.764 | 0.202 | 46.05 | 6.03 | 13.04 | 1.12 | 6.05 | 1.53 | 0.892 | 0.263 | 12.54 | 2.04 | 0.458 | 0.11 | 13.65 | 2.79 | 0.188 | 0.06 |
| H13 F2 | 5.108 | 0.98 | 0.242 | 0.061 | 2.056 | 0.188 | 53.3 | 5.05 | 12.97 | 1.90 | 5.714 | 2.06 | 0.848 | 0.086 | 9.31 | 1.40 | 0.452 | 0.06 | 9.61 | 2.47 | 0.17 | 0.12 |
| H13 F3 | 6.118 | 0.62 | 0.25 | 0.012 | 2.172 | 0.150 | 58.94 | 3.11 | 9.8 | 1.31 | 2.89 | 0.69 | 0.952 | 0.084 | 8.69 | 1.02 | 0.586 | 0.03 | 9.04 | 1.12 | 0.224 | 0.09 |
| H14 F1 | 2.824 | 0.21 | 0.146 | 0.015 | 1.322 | 0.164 | 19.23 | 3.93 | 7.82 | 1.27 | 3.9 | 1.39 | 0.998 | 0.095 | 12.18 | 1.21 | 0.974 | 0.08 | 50.15 | 4.16 | 0.334 | 0.19 |
| H14 F2 | 3.386 | 0.12 | 0.166 | 0.013 | 1.61 | 0.206 | 30.85 | 2.59 | 13.18 | 0.57 | 7.838 | 0.49 | 0.832 | 0.075 | 16.05 | 0.87 | 0.434 | 0.05 | 25.44 | 2.04 | 0.18 | 0.11 |
| H14 F3 | 4.226 | 0.18 | 0.258 | 0.077 | 1.618 | 0.096 | 35.2 | 2.82 | 13.28 | 2.08 | 6.604 | 1.71 | 0.924 | 0.232 | 15.32 | 1.46 | 0.474 | 0.12 | 21.9 | 2.54 | 0.144 | 0.11 |
| H15 F1 | 4.73 | 0.50 | 0.21 | 0.007 | 1.844 | 0.106 | 36.77 | 2.12 | 11.06 | 1.09 | 4.502 | 0.98 | 0.862 | 0.044 | 15.98 | 1.03 | 0.456 | 0.09 | 23.22 | 1.08 | 0.166 | 0.06 |
| H15 F2 | 4.54 | 0.67 | 0.208 | 0.022 | 1.856 | 0.207 | 37.14 | 2.67 | 12.45 | 2.11 | 6.24 | 2.66 | 0.81 | 0.072 | 14.64 | 1.12 | 0.41 | 0.02 | 21.38 | 0.30 | 0.168 | 0.03 |
| H15 F3 | 4.656 | 0.72 | 0.212 | 0.026 | 1.762 | 0.228 | 38.01 | 2.24 | 12.69 | 1.92 | 6.184 | 2.07 | 0.776 | 0.059 | 14.66 | 0.79 | 0.408 | 0.05 | 20.29 | 0.57 | 0.202 | 0.09 |
| H16 F1 | 4.134 | 0.29 | 0.228 | 0.025 | 1.542 | 0.058 | 32.78 | 2.68 | 15.8 | 0.99 | 8.52 | 0.77 | 0.744 | 0.053 | 13.7 | 2.08 | 0.424 | 0.11 | 21.8 | 2.55 | 0.238 | 0.08 |
| H16 F2 | 4.144 | 0.38 | 0.22 | 0.043 | 1.716 | 0.101 | 36.26 | 2.13 | 16.26 | 1.86 | 8.064 | 1.04 | 0.77 | 0.045 | 13.21 | 1.58 | 0.432 | 0.08 | 18.65 | 1.23 | 0.212 | 0.07 |
| H16 F3 | 4.766 | 0.48 | 0.226 | 0.015 | 1.676 | 0.109 | 40.89 | 3.74 | 15.03 | 2.11 | 6.632 | 1.58 | 0.776 | 0.040 | 13.1 | 0.13 | 0.426 | 0.03 | 16.2 | 0.75 | 0.158 | 0.04 |
| H17 F1 | 4.564 | 0.15 | 0.2 | 0.012 | 1.538 | 0.023 | 42.77 | 3.50 | 14.72 | 0.28 | 7.338 | 0.61 | 0.754 | 0.053 | 10.91 | 1.11 | 0.466 | 0.15 | 16.46 | 5.08 | 0.174 | 0.05 |
| H17 F2 | 4.152 | 0.52 | 0.156 | 0.074 | 1.46 | 0.182 | 36.19 | 5.34 | 14.6 | 2.59 | 7.222 | 1.94 | 0.772 | 0.090 | 10.85 | 3.03 | 0.578 | 0.17 | 23.68 | 5.85 | 0.264 | 0.11 |
| H17 F3 | 4.654 | 0.16 | 0.196 | 0.015 | 1.596 | 0.127 | 43.64 | 1.64 | 13.95 | 1.87 | 6.79 | 1.78 | 0.784 | 0.117 | 11.01 | 0.89 | 0.48 | 0.19 | 16.64 | 5.48 | 0.164 | 0.04 |
| H18 F1 | 3.046 | 0.28 | 0.172 | 0.024 | 1.338 | 0.100 | 25.37 | 1.63 | 13.14 | 2.53 | 7.57 | 1.93 | 0.832 | 0.054 | 12.5 | 0.92 | 0.708 | 0.08 | 34.82 | 3.69 | 0.406 | 0.07 |
| H18 F2 | 3.042 | 0.22 | 0.176 | 0.026 | 1.308 | 0.134 | 23.82 | 2.67 | 12.48 | 1.84 | 7.474 | 1.74 | 0.848 | 0.078 | 11.98 | 0.92 | 0.76 | 0.03 | 37.51 | 2.49 | 0.432 | 0.14 |
| H18 F3 | 3.058 | 0.28 | 0.172 | 0.024 | 1.34 | 0.100 | 25.63 | 2.00 | 13.22 | 2.55 | 7.64 | 1.98 | 0.826 | 0.056 | 12.51 | 0.92 | 0.7 | 0.08 | 34.4 | 4.20 | 0.4 | 0.08 |
| H19 F1 | 3.13 | 0.17 | 0.154 | 0.009 | 1.414 | 0.029 | 23.46 | 6.13 | 9.26 | 2.02 | 4.396 | 1.68 | 0.94 | 0.085 | 12.82 | 1.10 | 0.868 | 0.15 | 43.03 | 8.51 | 0.372 | 0.05 |
| H19 F2 | 5.762 | 1.28 | 0.246 | 0.061 | 1.942 | 0.320 | 54.17 | 4.65 | 12.59 | 3.65 | 5.396 | 3.35 | 0.888 | 0.189 | 8.63 | 0.98 | 0.52 | 0.15 | 9.45 | 0.62 | 0.234 | 0.13 |
| H19 F3 | 3.022 | 0.19 | 0.148 | 0.013 | 1.378 | 0.071 | 20.84 | 0.61 | 8.5 | 0.38 | 3.946 | 0.70 | 0.958 | 0.046 | 13.08 | 0.52 | 0.92 | 0.04 | 46.63 | 0.66 | 0.402 | 0.03 |
| H20 F1 | 3.078 | 0.28 | 0.17 | 0.023 | 1.348 | 0.104 | 27.48 | 2.31 | 12.46 | 0.65 | 7.496 | 1.90 | 0.824 | 0.061 | 12.73 | 0.79 | 0.688 | 0.10 | 33.24 | 4.03 | 0.39 | 0.07 |
| H20 F2 | 3.134 | 0.25 | 0.178 | 0.026 | 1.336 | 0.156 | 26.01 | 6.37 | 12.82 | 2.14 | 7.602 | 1.84 | 0.832 | 0.085 | 11.68 | 0.93 | 0.714 | 0.10 | 35.12 | 7.55 | 0.412 | 0.16 |
| H20 F3 | 3.03 | 0.29 | 0.168 | 0.024 | 1.33 | 0.100 | 24.95 | 1.39 | 12.73 | 2.59 | 7.248 | 1.82 | 0.834 | 0.054 | 12.46 | 0.92 | 0.722 | 0.09 | 36.02 | 3.39 | 0.414 | 0.06 |
| H21 F1 | 4.468 | 0.33 | 0.196 | 0.015 | 1.508 | 0.056 | 42.21 | 3.82 | 13.85 | 0.41 | 7.658 | 0.64 | 0.742 | 0.030 | 12.1 | 0.79 | 0.43 | 0.08 | 16.52 | 4.94 | 0.198 | 0.10 |
| H21 F2 | 4.09 | 0.57 | 0.148 | 0.069 | 1.486 | 0.146 | 35.22 | 6.54 | 13.56 | 1.52 | 6.882 | 1.65 | 0.79 | 0.075 | 11.91 | 2.90 | 0.552 | 0.17 | 25.04 | 6.82 | 0.262 | 0.11 |
| H21 F3 | 4.476 | 0.31 | 0.196 | 0.015 | 1.55 | 0.032 | 42.36 | 4.40 | 14.29 | 1.11 | 7.33 | 0.62 | 0.766 | 0.078 | 11.34 | 0.26 | 0.466 | 0.15 | 16.96 | 6.21 | 0.162 | 0.05 |
| H22 F1 | 4.432 | 0.41 | 0.194 | 0.018 | 1.514 | 0.062 | 42.9 | 6.59 | 14.23 | 0.49 | 7.238 | 0.76 | 0.752 | 0.055 | 10.27 | 1.72 | 0.44 | 0.12 | 17.72 | 7.41 | 0.182 | 0.07 |
| H22 F2 | 4.28 | 0.66 | 0.19 | 0.023 | 1.482 | 0.215 | 38.87 | 6.26 | 13.74 | 0.73 | 7.504 | 1.59 | 0.764 | 0.074 | 10.78 | 2.91 | 0.558 | 0.16 | 21.57 | 5.32 | 0.194 | 0.03 |
| H22 F3 | 4.388 | 0.39 | 0.24 | 0.096 | 1.268 | 0.603 | 41.57 | 5.31 | 13.38 | 1.25 | 8.304 | 2.68 | 0.788 | 0.048 | 11.86 | 0.71 | 0.464 | 0.08 | 17.38 | 7.11 | 0.234 | 0.10 |
| ANOVA F | 17.27 | 5.15 | 11.51 | 54.03 | 14.55 | 15.47 | 6.32 | 48 | 33.04 | 56 | 38.81 | |||||||||||
| LSD0.001 | 0.9 | 0.1 | 0.4 | 8.15 | 3.14 | 2.939 | 0.2 | 2.51 | 0.2 | 7.91 | 0.2 | |||||||||||
| Parental Form | MS8 | B. carinata | B. juncea | B. pekinensis | B. trilocularis |
|---|---|---|---|---|---|
| MS8 | - | ||||
| B. carinata | 143.8 | - | |||
| B. juncea | 115.0 | 39.4 | - | ||
| B. pekinensis | 106.1 | 44.7 | 30.4 | - | |
| B. trilocularis | 125.0 | 62.6 | 53.2 | 36.3 | - |
| Hybrid | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C20:1 | C22:0 | C22:1 | C24:0 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| H1 F1 | 1 ** | 0.03 | 0.2 | 13 *** | −3.98 *** | −1.272 | −0.1 * | 3.9 *** | −0.4* ** | −11 *** | −0.7 *** |
| H1 F2 | 1 * | 0.25 *** | 0.3** | 11 *** | −3.09 *** | −0.17 | −0.5 *** | 3.5 *** | −0.7 *** | −10 *** | −0.8 *** |
| H1 F3 | 1 * | 0.14 *** | 0.2* | 12 *** | −3.53 *** | −0.721 | −0.3 *** | 3.7 *** | −0.6 *** | −11 *** | −0.7 *** |
| H2 F1 | 1 * | 0 | 0.2 | 4 * | −8.49 *** | -4.21 *** | 0 | 6.9 *** | −0.2 *** | 1 | −0.6 *** |
| H2 F2 | 1 * | 0.02 | 0.2* | 4 * | −7.05 *** | -4.574 *** | 0 | 6.7 *** | −0.2 *** | 0 | −0.7 *** |
| H2 F3 | 1 * | 0.01 | 0.2* | 4 * | −7.84 *** | -4.385 *** | 0 | 6.9 *** | −0.2 *** | 1 | −0.7 *** |
| H3 F1 | 0 | 0 | 0 | 7 ** | −5.3 *** | −1.994 * | −0.1 * | 6.4 *** | −0.4 *** | −5 ** | −0.7 *** |
| H3 F2 | 0 | −0.06 * | 0.1 | 7 ** | −5.41 *** | −2.146 ** | 0.1 | 6.7 *** | −0.4 *** | −6 ** | −0.7 *** |
| H3 F3 | 0 | −0.03 | 0.1 | 7 ** | −5.36 *** | −2.01 ** | 0 | 6.5 *** | −0.4 *** | −5 ** | −0.7 *** |
| H4 F1 | 1 * | 0.01 | 0.2 | 10 *** | −5.03 *** | −1.852 * | −0.1 * | 5.1 *** | −0.4 *** | −8 *** | −0.7 *** |
| H4 F2 | 1 * | −0.04 | 0.1 | 9 *** | −5.13 *** | −1.886 * | 0.1 | 5.9 *** | −0.4 *** | −8 *** | −0.7 *** |
| H4 F3 | 1 * | −0.01 | 0.2 | 9 *** | −5.09 *** | −1.88 * | 0 | 5.7 *** | −0.4 *** | −8 *** | −0.7 *** |
| H5 F1 | 2 *** | 0.06 * | 0.7*** | 33 *** | −8.7 *** | −5.136 *** | 0 | −0.5 | −0.4 *** | −21 *** | −0.7 *** |
| H5 F2 | 2 *** | 0.05 | 1.2*** | 33 *** | −8.46 *** | −5.124 *** | −0.1 | −0.7 | −0.4 *** | −21 *** | −0.7 *** |
| H5 F3 | 2 *** | 0.06 * | 0.9*** | 33 *** | −8.77 *** | −5.124 *** | −0.1 | −0.6 | −0.4 *** | −21 *** | −0.7 *** |
| H6 F1 | 0 | −0.02 | −0.2* | −10 *** | −5.37 *** | 0.843 | 0 | −2.3 *** | −0.2 *** | 17 *** | 0 |
| H6 F2 | 0 | 0.09 *** | −0.2 | −12 *** | −4.55 *** | 0.187 | 0.7 *** | −1.5 * | 0.2 *** | 17 *** | 0.7*** |
| H6 F3 | 0 | 0.04 | −0.2 | −12 *** | −4.89 *** | 1.511 * | 0.4 *** | −2 ** | 0 | 17 *** | 0.4*** |
| H7 F1 | 0 | 0.04 | 0.1 | 32 *** | −0.11 | −3.865 *** | 0 | −6 *** | 0.2 *** | −23 *** | −0.1 |
| H7 F2 | 0 | 0.15 *** | 0.3** | 31 *** | −0.37 | −3.961 *** | 0.2 ** | −5.1 *** | 0.2 *** | −23 *** | 0 |
| H7 F3 | 0 | 0.18 *** | 0.3* | 23 *** | −1.16 | −2.964 *** | 0.2 ** | −4.8 *** | 0.2 *** | −15 *** | 0 |
| H8 F1 | 1 * | 0 | 0.3** | 10 *** | −1.1 | −1.13 | −0.1 | 4.1 *** | −0.4 *** | −12 *** | −0.8 *** |
| H8 F2 | 0 | 0 | 0.2* | 10 *** | −1.7 * | −1.16 | −0.1 | 5.2 *** | −0.4 *** | −12 *** | −0.7 *** |
| H8 F3 | 0 | 0 | 0.1 | 7 *** | −0.73 | −0.416 | −0.1 | 4.4 *** | −0.4 *** | −10 *** | −0.6 *** |
| H9 F1 | 0 | 0 | 0.1 | −3 | −3.15 *** | −1.384 | 0 | 6.6 *** | −0.4 *** | 2 | −0.6 *** |
| H9 F2 | 1 ** | 0.05 * | 0 | 2 | −0.97 | −1.752 * | −0.1 | 4.5 *** | −0.2 *** | −4 | −0.6 *** |
| H9 F3 | 1 *** | 0.02 | 0.3 * | 2 | −4.92 *** | −4.032 *** | 0 | 7.1 *** | −0.3 *** | −1 | −0.6 *** |
| H10 F1 | 0 | −0.02 | 0 | −1 | −4.75 *** | −3.17 *** | 0 | 4 *** | −0.1 ** | 5* | −0.4 *** |
| H10 F2 | 1 *** | 0.02 | 0.2* | 2 | −4.56 *** | −3.838 *** | 0 | 4.3 *** | −0.3 *** | 1 | −0.5 *** |
| H10 F3 | 2 *** | 0.03 | 0.4*** | 5 * | −5.23 *** | −2.864 *** | 0.1 | 5 *** | −0.3 *** | −4 | −0.6 *** |
| H11 F1 | 1 ** | 0 | 0.3*** | 17 *** | −0.85 | −0.876 | −0.1 | 2 ** | −0.4 *** | −17 *** | −0.7 *** |
| H11 F2 | 1 *** | 0.02 | 0.4*** | 28 *** | 1.46 | −0.956 | −0.1 | −4.5 *** | −0.4 *** | −24 *** | −0.8 *** |
| H11 F3 | 1 *** | 0.01 | 0.4*** | 31 *** | 3.8 *** | −0.412 | −0.1 | −7.3 *** | −0.4 *** | −27 *** | −0.8 *** |
| H12 F1 | 1 * | 0.01 | 0.2 | 10 *** | 0.47 | 0.574 | −0.1* | 2.7 *** | −0.4 *** | −13 *** | −0.7 *** |
| H12 F2 | 1 ** | 0.01 | 0.5*** | 18 *** | −0.71 | −1.506 | −0.1 | 1.2 | −0.4 *** | −17 *** | −0.7 *** |
| H12 F3 | 0 | 0 | 0.3** | 12 *** | −1.29 | −0.714 | −0.1 | 3.3 *** | −0.4*** | −13 *** | −0.7 *** |
| H13 F1 | 1 *** | 0.08 ** | 0.3* | 16 *** | −2.98 *** | −2.62 *** | 0 | 2.9 *** | −0.3*** | −15 *** | −0.6 *** |
| H13 F2 | 2 *** | 0.05 | 0.6*** | 23 *** | −3.04 *** | −2.956 *** | 0 | −0.3 | −0.3*** | −19 *** | −0.6 *** |
| H13 F3 | 3 *** | 0.06 * | 0.7*** | 29 *** | −6.21 *** | −5.78 *** | 0.1 | −0.9 | −0.2*** | −19 *** | −0.6 *** |
| H14 F1 | −1 ** | −0.05 | −0.2 | −11 *** | −8.2 *** | −4.77 *** | 0.1* | 2.6 *** | 0.2 *** | 22 *** | −0.5 *** |
| H14 F2 | 0 | −0.03 | 0.1 | 1 | −2.83 *** | −0.832 | 0 | 6.4 *** | −0.4 *** | −3 | −0.6 *** |
| H14 F3 | 1 ** | 0.07 * | 0.1 | 5 * | −2.74 *** | −2.066 ** | 0.1 | 5.7 *** | −0.3 *** | −6 ** | −0.7 *** |
| H15 F1 | 1 *** | 0.02 | 0.3*** | 7 ** | −4.95 *** | −4.168 *** | 0 | 6.4 *** | −0.3 *** | −5 * | −0.6 *** |
| H15 F2 | 1 *** | 0.02 | 0.4*** | 7 *** | −3.57 *** | −2.43 ** | 0 | 5 *** | −0.4 *** | −7 *** | −0.6 *** |
| H15 F3 | 1 *** | 0.02 | 0.3* | 8 *** | −3.33 *** | −2.486 ** | −0.1 | 5.1 *** | −0.4 *** | −8 *** | −0.6 *** |
| H16 F1 | 1 ** | 0.04 | 0 | 3 | −0.21 | −0.15 | −0.1 | 4.1 *** | −0.4 *** | −7 ** | −0.6 *** |
| H16 F2 | 1 ** | 0.03 | 0.2* | 6 ** | 0.24 | −0.606 | −0.1 | 3.6 *** | −0.4 *** | −10 *** | −0.6 *** |
| H16 F3 | 1 *** | 0.03 | 0.2 | 11 *** | −0.98 | −2.038 ** | −0.1 | 3.5 *** | −0.4 *** | −12 *** | −0.7 *** |
| H17 F1 | 2 *** | 0.02 | 0.2 | 16 *** | 0.99 | 0.55 | −0.1* | 2.6 *** | −0.4 *** | −21 *** | −0.4 *** |
| H17 F2 | 1 *** | −0.02 | 0.1 | 9 *** | 0.86 | 0.434 | −0.1* | 2.6 *** | −0.3 *** | −14 *** | −0.3 *** |
| H17 F3 | 2 *** | 0.02 | 0.2* | 16 *** | 0.21 | 0.002 | −0.1 | 2.7 *** | −0.4 *** | −21 *** | −0.4 *** |
| H18 F1 | 0 | 0 | 0 | −2 | −0.6 | 0.782 | −0.1 | 4.2 *** | −0.1 ** | −2 | −0.2 ** |
| H18 F2 | 0 | 0 | −0.1 | −3 | −1.25 | 0.686 | −0.1 | 3.7 *** | −0.1 | 0 | −0.1 ** |
| H18 F3 | 0 | 0 | 0 | −2 | −0.52 | 0.852 | −0.1 | 4.2 *** | −0.1 ** | −3 | −0.2 *** |
| H19 F1 | 0 | −0.02 | 0.1 | −4 | −4.48 *** | −2.392 ** | 0 | 4.5 *** | 0 | 6 ** | −0.2 *** |
| H19 F2 | 3 *** | 0.07 * | 0.6*** | 27 *** | −1.15 | −1.392 | 0 | 0.4 | −0.3 *** | −28 *** | −0.3 *** |
| H19 F3 | 0 | −0.03 | 0 | −6 ** | −5.24 *** | −2.842 *** | 0.1 | 4.8 *** | 0.1 | 9 *** | −0.2 *** |
| H20 F1 | 0 | −0.01 | 0 | 0 | −1.28 | 0.708 | −0.1 | 4.5 *** | −0.2 ** | −4 | −0.2 *** |
| H20 F2 | 0 | 0 | 0 | −1 | −0.91 | 0.814 | −0.1 | 3.4 *** | −0.1 ** | −2 | −0.2 ** |
| H20 F3 | 0 | −0.01 | 0 | −2 | −1.01 | 0.46 | −0.1 | 4.2 *** | −0.1 * | −1 | −0.2 ** |
| H21 F1 | 1 *** | 0.02 | 0.1 | 15 *** | 0.12 | 0.87 | −0.2* | 3.8 *** | −0.4 *** | −21 *** | −0.4 *** |
| H21 F2 | 1 *** | −0.03 | 0.1 | 8 *** | −0.18 | 0.094 | −0.1 | 3.6 *** | −0.3 *** | −12 *** | −0.3 *** |
| H21 F3 | 1 *** | 0.02 | 0.2 | 15 *** | 0.56 | 0.542 | −0.1 * | 3.1 *** | −0.4 *** | −20 *** | −0.4 *** |
| H22 F1 | 1 *** | 0.02 | 0.2 | 16 *** | 0.49 | 0.45 | −0.1 * | 2 ** | −0.4 *** | −20 *** | −0.4 *** |
| H22 F2 | 1 *** | 0.01 | 0.1 | 12 *** | 0 | 0.716 | −0.1 * | 2.5 *** | −0.3 *** | −16 *** | −0.4 *** |
| H22 F3 | 1 *** | 0.06* | −0.1 | 14 *** | −0.36 | 1.516 * | −0.1 | 3.6 *** | −0.4 *** | −20 *** | −0.3 *** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemann, J.; Bocianowski, J.; Stuper-Szablewska, K.; Wojciechowski, T. New Interspecific Brassica Hybrids with High Levels of Heterosis for Fatty Acids Composition. Agriculture 2020, 10, 221. https://doi.org/10.3390/agriculture10060221
Niemann J, Bocianowski J, Stuper-Szablewska K, Wojciechowski T. New Interspecific Brassica Hybrids with High Levels of Heterosis for Fatty Acids Composition. Agriculture. 2020; 10(6):221. https://doi.org/10.3390/agriculture10060221
Chicago/Turabian StyleNiemann, Janetta, Jan Bocianowski, Kinga Stuper-Szablewska, and Tomasz Wojciechowski. 2020. "New Interspecific Brassica Hybrids with High Levels of Heterosis for Fatty Acids Composition" Agriculture 10, no. 6: 221. https://doi.org/10.3390/agriculture10060221





