Application of Desert DSEs to Nonhost Plants: Potential to Promote Growth and Alleviate Drought Stress of Wheat Seedlings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fungal Strains and Plant Cultivation
2.2. Experimental Design
2.3. Photosynthetic Indexes and Chlorophyll Content
2.4. Growth Parameters
2.5. DSE Colonization in Wheat Roots
2.6. Physiological Indexes
2.7. Statistical Analysis
3. Results
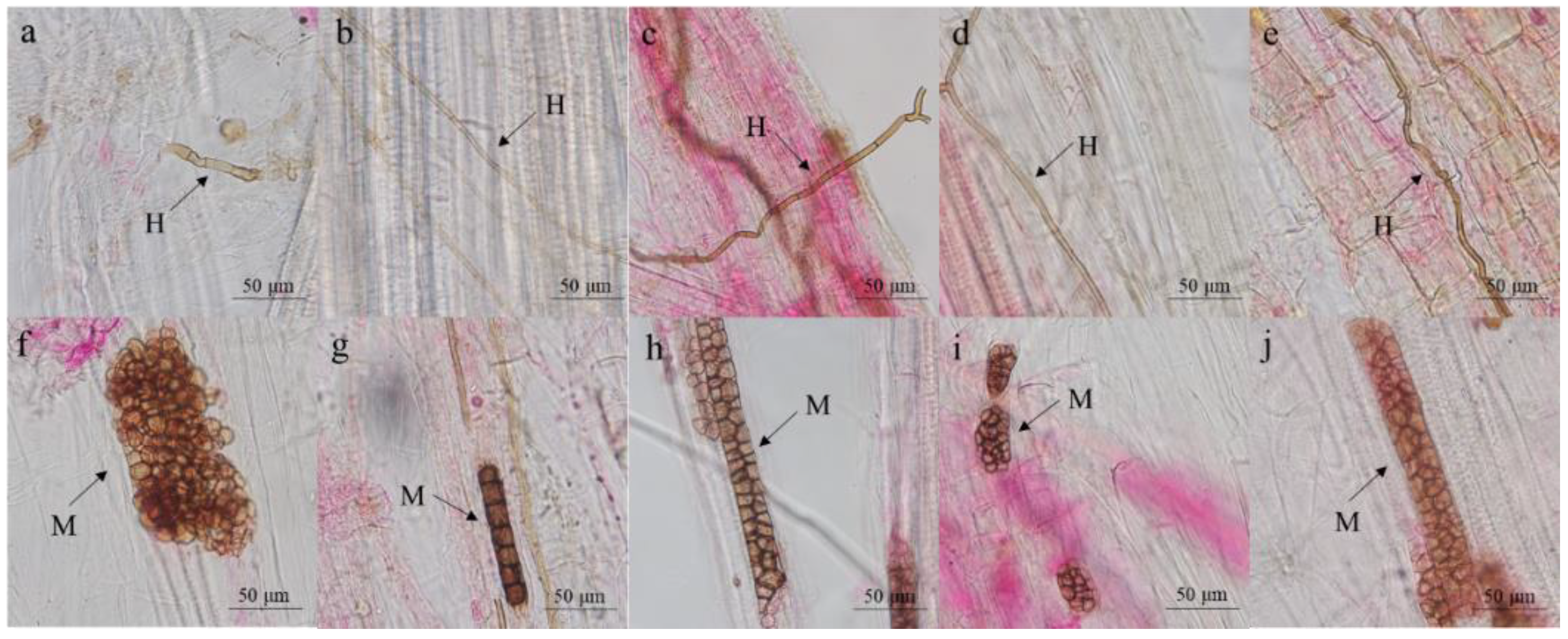
3.1. DSE in the Wheat Roots
3.2. Influences of DSE Inoculation on the Growth of Wheat Seedlings
3.3. Influences of DSE Inoculation on the Antioxidant Enzyme System of Wheat Seedlings
3.4. Influences of DSE Inoculation on the Soluble Protein, Soluble Sugar, and Proline Contents of Wheat Leaves
3.5. Influences of DSE Inoculation on Wheat Seedling Performance Based on Principal Component Analysis (PCA)
3.6. Influences of DSE on Leaf Photosynthetic Indexes and Chlorophyll Content
4. Discussion
4.1. Application Potential of DSE Inoculants for Nonhost Plants
4.2. Desert DSEs Affected the Growth of Nonhost Plants
4.3. The Influence of Desert DSEs on the Drought Resistance of Nonhost Wheat
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elkouk, A.; Pokhrel, Y.; Satoh, Y.; Bouchaou, L. Implications of changes in climate and human development on 21st-century global drought risk. J. Environ. Manag. 2022, 317, 115378. [Google Scholar] [CrossRef]
- Zachary, H.; Hoylman, R.; Bocinsky, K.; Kelsey, G.J. Drought assessment has been outpaced by climate change: Empirical arguments for a paradigm shift. Nat. Commun. 2022, 13, 2715. [Google Scholar]
- Zhao, T.; Dai, A. Uncertainties in historical changes and future projections of drought. Part II: Model-simulated historical and future drought changes. Clim. Chang. 2017, 144, 535–548. [Google Scholar] [CrossRef]
- Ghose, B. Food security and food self-sufficiency in China, from past to 2050. Food Energy Secur. 2014, 3, 86–95. [Google Scholar] [CrossRef]
- Jiang, J.; Huo, Z.; Feng, S.; Kang, S.; Wang, F.; Zhang, C. Effects of deficit irrigation with saline water on spring wheat growth and yield in arid Northwest China. J. Arid Land 2013, 5, 143–154. [Google Scholar] [CrossRef]
- Yu, X.R.; Li, B.; Wang, L.L.; Chen, X.Y.; Wang, W.J.; Gu, Y.J.; Wang, Z.; Xiong, F. Effect of drought stress on the development of endosperm starch granules and the composition and physicochemical properties of starches from soft and hard wheat. J. Sci. Food Agric. 2016, 96, 2746–2754. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, S.; Cheng, M.; Jiang, H.; Zhang, X.; Peng, C.; Lu, X.; Zhang, M.; Jin, J. Effect of drought on agronomic traits of rice and wheat: A meta-analysis. Int. J. Environ. Res. 2018, 15, 839. [Google Scholar] [CrossRef]
- Bapela, T.; Shimelis, H.; Tsilo, T.J.; Mathew, I. Genetic improvement of wheat for drought tolerance: Progress, challenges and opportunities. Plants 2022, 11, 1331. [Google Scholar] [CrossRef]
- Khadka, K.; Raizada, M.N.; Navabi, A. Recent progress in germplasm evaluation and gene mapping to enable breeding of drought-tolerant wheat. Front. Plant Sci. 2020, 11, 1149. [Google Scholar] [CrossRef]
- Baloch, M.J.; Dunwell, J.; Dennet, M.; Rajpar, I.; Jatoi, W.A.; Veesar, N.F. Evaluating spring wheat cultivars for drought tolerance through yield and physiological parameters at booting and anthesis. Afr. J. Biotechnol. 2012, 11, 11559–11565. [Google Scholar] [CrossRef]
- Ayalew, H.; Liu, H.; Börner, A.; Kobiljski, B.; Liu, C.; Yan, G. Genome-wide association mapping of major root length QTLs under PEG induced water stress in wheat. Front. Plant Sci. 2018, 9, 1759. [Google Scholar] [CrossRef]
- Trenberth, K.E.; Dai, A.; van der Schrier, G.; Jones, P.D.; Barichivich, J.; Briffa, K.R.; Sheffield, J. Global warming and changes in drought. Nat. Clim. Change 2014, 4, 17–22. [Google Scholar] [CrossRef]
- Llorens, E.; Sharon, O.; Camañes, G.; García-Agustín, P.; Sharon, A. Endophytes from wild cereals protect wheat plants from drought by alteration of physiological responses of the plants to water stress. Environ. Microbiol. 2019, 21, 3299–3312. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, M.; Germida, J.J.; Vujanovic, V. Fungal endophytes enhance wheat heat and drought tolerance in terms of grain yield and second-generation seed viability. J. Appl. Microbiol. 2014, 116, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Rezakhani, L.; Motesharezadeh, B.; Tehrani, M.M.; Etesami, H.; Mirseyed, H. Effect of silicon and phosphate-solubilizing bacteria on improved phosphorus (P) uptake is not specific to insoluble P-fertilized sorghum (Sorghum bicolor L.) plants. J. Plant Growth Regul. 2019, 39, 239–253. [Google Scholar] [CrossRef]
- Santos, M.; Ignacio, C.; Fernando, D.; Brenda, S.; Alejandro, M. Advances in the role of dark septate endophytes in the plant resistance to abiotic and biotic stresses. J. Fungi 2021, 7, 939. [Google Scholar] [CrossRef]
- Radzikowska, D.; Sulewska, H.; Bandurska, H.; Ratajczak, K.; Szymańska, G.; Kowalczewski, P.Ł.; Głowicka-Wołoszyn, R. Analysis of physiological status in response to water deficit of spelt (Triticum aestivum ssp. spelta) Cultivars in Reference to Common Wheat (Triticum aestivum ssp. vulgare). Agronomy 2022, 12, 1822. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Zhang, S.; Wang, Y. What could promote farmers to replace chemical fertilizers with organic fertilizers? J. Clean. Prod. 2018, 199, 882–890. [Google Scholar] [CrossRef]
- Yadav, A.N.; Kour, D.; Kaur, T.; Devi, R.; Yadav, A. Endophytic fungal communities and their biotechnological implications for agro-environmental sustainability. Folia Microbiol. 2022, 67, 203–232. [Google Scholar] [CrossRef]
- Rauf, M.; Awais, M.; Din, A.U.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular mechanisms of the 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing Trichoderma asperellum MAP1 in enhancing wheat tolerance to waterlogging stress. Front. Plant Sci. 2020, 11, 2213. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: New York, NY, USA, 2008. [Google Scholar]
- van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Zou, Y.N.; Kuca, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Elucidating the mechanisms underlying enhanced drought tolerance in plants mediated by arbuscular mycorrhizal fungi. Front. Microbiol. 2021, 12, 809473. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wu, Q.; Xu, L.; Druzhinina, I.S.; Stukenbrock, E.H.; Nieuwenhuis, B.P.S.; Zhong, Z.; Liu, Z.-J.; Wang, X.; Cai, F.; et al. Genomic landscape of a relict fir-associated fungus reveals rapid convergent adaptation towards endophytism. ISME J. 2022, 16, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Oberhofer, M.; Güsewell, S.; Leuchtmann, A. Effects of natural hybrid and non-hybrid Epichloë endophytes on the response of Hordelymus europaeus to drought stress. New Phytol. 2014, 201, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yao, Q.; Li, J.; Wang, Y.; Liu, X.Y.; Hu, Y.L.; Chen, J.Z. Contributions of an arbuscular mycorrhizal fungus to growth and physiology of loquat (Eriobotrya japonica) plants subjected to drought stress. Mycol. Prog. 2015, 14, 84. [Google Scholar] [CrossRef]
- Pedranzani, H.; Rodríguez-Rivera, M.; Gutiérrez, M.; Porcel, R.; Hause, B.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal symbiosis regulates physiology and performance of Digitaria eriantha plants subjected to abiotic stresses by modulating antioxidant and jasmonate levels. Mycorrhiza 2016, 26, 141–152. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Wagg, C.; Pautler, M.; Massicotte, H.B.; Peterson, R.L. The cooccurrence of ectomycorrhizal, arbuscular mycorrhizal, and dark septate fungi in seedlings of four members of the Pinaceae. Mycorrhiza 2008, 18, 103–110. [Google Scholar] [CrossRef]
- Mandyam, K.G.; Jumpponen, A. Mutualism-parasitism paradigm synthesized from results of root-endophyte models. Front. Microbiol. 2014, 5, 776. [Google Scholar]
- Ruotsalainen, A.L.; Kauppinen, M.; Wli, P.R.; Saikkonen, K.; Helander, M.; Tuomi, J. Dark septate endophytes, mutualism from by-products? Trends Plant Sci. 2022, 27, 247–254. [Google Scholar] [CrossRef]
- Newsham, K.K.; Upson, R.; Read, D.J. Mycorrhizas and dark septate root endophytes in polar regions. Fungal Ecol. 2009, 2, 10–20. [Google Scholar] [CrossRef]
- Zubek, S.; Nobis, M.; Błaszkowski, J.; Mleczko, P.; Nowak, A. Fungal root endophyte associations of plants endemic to the Pamir Alay Mountains of Central Asia. Symbiosis 2011, 54, 139–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knapp, D.G.; Németh, J.B.; Barry, K.; Hainaut, M.; Henrissat, B.; Johnson, J.; Kuo, A.; Lim, J.H.P.; Lipzen, A.; Nolan, M. Comparative genomics provides insights into the lifestyle and reveals functional heterogeneity of dark septate endophytic fungi. Sci. Rep. 2018, 8, 6321. [Google Scholar] [CrossRef] [PubMed]
- Knapp, D.G.; Imrefi, I.; Boldpurev, E.; Csíkos, S.; Akhmetova, G.; Berek-Nagy, P.J.; Otgonsuren, B.; Kovács, G.M. Root-colonizing endophytic fungi of the dominant grass Stipa krylovii from a Mongolian steppe grassland. Front. Microbiol. 2019, 10, 2565. [Google Scholar] [CrossRef] [PubMed]
- Lugo, M.A.; Menoyo, E.; Allione, L.R.; Negritto, M.A.; Henning, J.A.; Anton, A.M. Arbuscular mycorrhizas and dark septate endophytes associated with grasses from the Argentine Puna. Mycologia 2018, 110, 654–665. [Google Scholar] [CrossRef]
- Hou, L.; He, X.; Li, X.; Wang, S.; Zhao, L. Species composition and colonization of dark septate endophytes are affected by host plant species and soil depth in the Mu Us sandland, northwest China. Fungal Ecol. 2019, 39, 276–284. [Google Scholar] [CrossRef]
- Mateu, M.; Baldwin, A.; Maul, J.; Yarwood, S. Dark septate endophyte improves salt tolerance of native and invasive lineages of Phragmites australis. ISME J. 2020, 14, 1943–1954. [Google Scholar] [CrossRef]
- Zhu, L.; Li, T.; Wang, C.; Zhang, X.; Xu, L.; Xu, R.; Zhao, Z. The effects of dark septate endophyte (DSE) inoculation on tomato seedlings under Zn and Cd stress. Environ. Sci. Pollut. Res. 2018, 25, 35232–35241. [Google Scholar] [CrossRef]
- Li, M.; Hou, L.F.; Liu, J.Q.; Yang, J.Y.; Zou, Y.L.; Zhao, L.L.; He, X.L. Growth-promoting effects of dark septate endophytes on the non-mycorrhizal plant Isatis indigotica under different water conditions. Symbiosis 2021, 85, 291–303. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, T.; He, X. Mycorrhizal and dark septate endophytic fungi under the canopies of desert plants in Mu Us Sandy Land of China. Front. Agric. China 2009, 3, 164–170. [Google Scholar] [CrossRef]
- Vergara, C.; Araujo, K.E.C.; Urquiaga, S.; Santa-Catarina, C.; Schultz, N.; Araujo, E.D.S.; Balieiro, F.D.C.; Xavier, G.R.; Zilli, J.E. Dark septate endophytic fungi increase green manure-N-15 recovery efficiency, N contents, and micronutrients in rice grains. Front. Plant Sci. 2018, 9, 613. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Wang, W.; Hou, J. Plant performance of enhancing licorice with dual inoculating dark septate endophytes and Trichoderma viride mediated via effects on root development. BMC Plant Biol. 2020, 20, 325. [Google Scholar] [CrossRef]
- Gaber, D.A.; Berthelot, C.; Camehl, I.; Kovács, G.M.; Blaudez, D.; Franken, P. Salt stress tolerance of dark septate endophytes is independent of melanin accumulation. Front. Microbiol. 2020, 11, 562931. [Google Scholar] [CrossRef] [PubMed]
- Gehring, C.; Sevanto, S.; Patterson, A.; Ulrich, D.E.M.; Kuske, C.R. Ectomycorrhizal and dark septate fungal associations of pinyon pine are differentially affected by experimental drought and warming. Front. Plant Sci. 2020, 11, 582574. [Google Scholar] [CrossRef] [PubMed]
- Spagnoletti, F.N.; Chiocchio, V.M. Tolerance of dark septate endophytic fungi (DSE) to agrochemicals in vitro. Rev. Argent Microbiol. 2020, 52, 43–49. [Google Scholar] [CrossRef]
- Akhtar, N.; Wani, A.K.; Dhanjal, D.S.; Mukherjee, S. Insights into the beneficial roles of dark septate endophytes in plants under challenging environment: Resilience to biotic and abiotic stresses. World J. Microb. Biot. 2022, 38, 79. [Google Scholar] [CrossRef]
- Iqbal, Z.; Iqbal, M.S.; Hashem, A.; Abd_Allah, E.F.; Ansari, M.I. Plant defense responses to biotic stress and its interplay with fluctuating dark/light. Front. Plant Sci. 2021, 12, 631810. [Google Scholar] [CrossRef]
- Su, Z.Z.; Dai, M.D.; Zhu, J.N.; Liu, X.H.; Li, L.; Zhu, X.M.; Wang, J.-Y.; Yuan, Z.-L.; Lin, F.-C. Dark septate endophyte Falciphora oryzae-assisted alleviation of cadmium in rice. J. Hazard Mater. 2021, 419, 126435. [Google Scholar] [CrossRef]
- Potisek, M.; Likar, M.; Vogel-Mikuš, K.; Arcon, I.; Grdadolnik, J.; Regvar, M. 1,8-dihydroxy naphthalene (DHN)-melanin confers tolerance to cadmium in isolates of melanised dark septate endophytes. Ecotoxicol Environ. Saf. 2021, 222, 112493. [Google Scholar] [CrossRef]
- Li, X.; He, X.; Hou, L.; Ren, Y.; Wang, S.; Su, F. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci. Rep. 2018, 8, 7896. [Google Scholar] [CrossRef]
- Xie, L.; He, X.; Wang, K.; Hou, L.; Sun, Q. Spatial dynamics of dark septate endophytes in the roots and rhizospheres of Hedysarum scoparium in northwest China and the influence of edaphic variables. Fungal Ecol. 2017, 26, 135–143. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Herrera, J.; Sinsabaugh, R.L.; Odenbach, K.J.; Lowrey, T.; Natvig, D.O. Novel root fungal consortium associated with a dominant desert grass. Appl. Environ. Microb. 2008, 74, 2805–2813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Y.; Su, F.; He, X.; Li, M. Colonization by dark septate endophytes improves the growth of Hedysarum scoparium under multiple inoculum levels. Symbiosis 2020, 82, 201–214. [Google Scholar] [CrossRef]
- Santos, S.G.; Silva, P.R.; Garcia, A.C.; Zilli, J.É.; Berbara, R.L. Dark septate endophyte decreases stress on rice plants. Braz. J. Microbiol. 2017, 48, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, M.J.; Zhang, X.T.; Zhang, H.B.; Sha, T.; Zhao, Z.W. Improved tolerance of maize (Zea mays L.) to heavy metals by colonization of a dark septate endophyte (DSE) Exophiala pisciphila. Sci. Total Environ. 2011, 409, 1069–1074. [Google Scholar] [CrossRef]
- Li, X.; He, X.L.; Zhou, Y.; Hou, Y.T.; Zuo, Y.L. Effects of dark septate endophytes on the performance of Hedysarum scoparium under water deficit stress. Front. Plant Sci. 2019, 10, 903. [Google Scholar] [CrossRef]
- Farias, G.C.; Nunes, K.G.; Soares, M.A.; de Siqueira, K.A.; Lima, W.C.; Neves, A.L.R.; de Lacerda, C.F.; Filho, E.G. Dark septate endophytic fungi mitigate the effects of salt stress on cowpea plants. Braz. J. Microbiol. 2020, 51, 243–253. [Google Scholar] [CrossRef]
- Li, Y.P.; He, X.L.; Zhao, L.L. Tempo-spatial dynamics of arbuscular mycorrhizal fungi under clonal plant Psammochloa villosa Trin. Bor in Mu Us sandland. Eur. J. Soil Biol. 2010, 46, 295–301. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, T.; Su, X.; Ren, Z. Complete chloroplast genome of Psammochloa villosa (Poaceae), a pioneer grass endemic to sand dunes in Northwest China. Cytol. Genet. 2020, 54, 582–587. [Google Scholar] [CrossRef]
- Hou, L. Species Diversity and Salt Tolerance of Dark Septate Endophytes in Three Desert Plants. Ph.D. Dissertation, Hebei University, Baoding, China, 2020. [Google Scholar]
- Zuo, Y.; Hu, Q.; Liu, J.; He, X.L. Relationship of root dark septate endophytes and soil factors to plant species and seasonal variation in extremely arid desert in Northwest China. Appl. Soil Ecol. 2022, 175, 104454. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Xu, M.; Ye, Q.; Gao, H.; He, X. Improved tolerance of Artemisia ordosica to drought stress via dark septate endophyte (DSE) symbiosis. J. Fungi 2022, 8, 730. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wei, X. Dark septate endophyte improves the drought-stress resistance of Ormosia hosiei seedlings by altering leaf morphology and photosynthetic characteristics. Plant Ecol. 2021, 222, 761–771. [Google Scholar] [CrossRef]
- Li, X.; He, C.; He, X.; Su, F.; Hou, L.; Ren, Y.; Hou, Y. Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil 2019, 439, 259–272. [Google Scholar] [CrossRef]
- Zuo, Y.; Hu, Q.; Zhang, K.; He, X. Host and tissue affiliations of culturable endophytic fungi associated with xerophytic plants in the desert region of Northwest China. Agronomy 2022, 12, 727. [Google Scholar] [CrossRef]
- Elavarthi, S.; Martin, B. Spectrophotometric assays for antioxidant enzymes in plants. Methods Mol. Biol. 2010, 639, 273–280. [Google Scholar]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Method Enzymol. 1985, 113, 548–555. [Google Scholar]
- Peever, T.L.; Higgins, V.J. Electrolyte leakage, lipoxygenase, and lipid peroxidation induced in tomato leaf tissue by specific and nonspecific elicitors from Cladosporium fulvum. Plant Physiol. 1989, 90, 867–875. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Christos, D.G.; Konstantinos, G.; George, Z. Mechanism of Coomassie brilliant blue G-250 binding to proteins: A hydrophobic assay for nanogram quantities of proteins. Anal. Bioanal. Chem. 2008, 3912, 391–403. [Google Scholar]
- Hou, L.; Li, X.; He, X.; Zuo, Y.; Zhang, D.; Zhao, L. Effect of dark septate endophytes on plant performance of Artemisia ordosica and associated soil microbial functional group abundance under salt stress. Appl. Soil Ecol. 2021, 165, 103998. [Google Scholar] [CrossRef]
- Khaksar, G.; Treesubsuntorn, C.; Thiravetyan, P. Effect of endophytic Bacillus cereus ERBP inoculation into non-native host: Potentials and challenges for airborne formaldehyde removal. Plant Physiol. Biochem. 2016, 107, 326–336. [Google Scholar] [CrossRef]
- Irizarry, I.; White, J.F. Application of bacteria from non-cultivated plants to promote growth, alter root architecture and alleviate salt stress of cotton. J. Appl. Microbiol. 2017, 122, 1110–1120. [Google Scholar] [CrossRef]
- Ban, Y.; Xu, Z.; Yang, Y.; Zhang, H.; Chen, H.; Tang, M. Effect of dark septate endophytic fungus Gaeumannomyces cylindrosporus on plant growth, photosynthesis and pb tolerance of maize (Zea mays L.). Pedosphere 2017, 27, 283–292. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowakhuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Song, X.; Halifu, S.; Yu, W.; Song, R. Effects of dark septate endophytes strain A024 on damping-off biocontrol, plant growth and the rhizosphere soil environment of Pinus sylvestris var. mongolica annual seedlings. Plants 2020, 9, 913. [Google Scholar]
- Arunyanark, A.; Jogloy, S.; Akkasaeng, C.; Vorasoot, N.; Patanothai, A. Chlorophyll stability is an indicator of drought tolerance in peanut. J. Agron. Crop Sci. 2010, 194, 113–125. [Google Scholar] [CrossRef]
- Ghobadi, M.; Taherabadi, S.; Ghobadi, M.E.; Mohammadi, G.R.; Jalali-Honarmand, S. ntioxidant capacity, photosynthetic characteristics and water relations of sunflower (Helianthus annuus L.) cultivars in response to drought stress. Ind. Crops Prod. 2013, 50, 29–38. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, Y.; Zhang, K.; Tian, C.; Guo, J. Arbuscular mycorrhizal fungi improve plant growth of Ricinus communis by altering photosynthetic properties and increasing pigments under drought and salt stress. Ind Crops Prod. 2018, 117, 13–19. [Google Scholar] [CrossRef]
- Flexas, J.; Bota, J.; Loreto, F.; Cornic, G.; Sharkey, T.D. Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol. 2004, 6, 269–279. [Google Scholar] [CrossRef]
- Lubna, A.S.; Hamayun, M.; Khan, A.L.; Waqas, M.; Khan, M.A.; Jan, R.; In-Jung, L.; Hussain, A. Salt tolerance of Glycine max. L induced by endophytic fungus Aspergillus flavus CSH1, via regulating its endogenous hormones and antioxidative system. Plant Physiol. Biochem. 2018, 128, 13–23. [Google Scholar] [CrossRef]
- Santner, A.; Calderon-Villalobos, L.I.A.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Bücker-Neto, L.; Paiva, A.L.S.; Machado, R.D.; Arenhart, R.A.; Margis-Pinheiro, M. Interactions between plant hormones and heavy metals responses. Genet. Mol. Biol. 2017, 40, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Chen, L.; Ye, T.; Liu, X.; Ding, K.; Chan, Z. Modulation of auxin content in Arabidopsis confers improved drought stress resistance. Plant Physiol. Biochem. 2014, 82, 209–217. [Google Scholar] [CrossRef]
- Xu, L.; Wu, C.; Oelmueller, R.; Zhang, W. Role of phytohormones in Piriformospora indica-induced growth promotion and stress tolerance in plants: More questions than answers. Front. Microbiol. 2018, 9, 1646. [Google Scholar] [CrossRef]
- Khan, A.R.; Ullah, I.; Waqas, M.; Shahzad, R.; Hong, S.; Park, G.; Jung, B.K.; Lee, I.; Shin, J. Plant growth-promoting potential of endophytic fungi isolated from Solanum nigrum leaves. World J. Microbiol. Biotechnol. 2015, 31, 1461–1466. [Google Scholar] [CrossRef] [PubMed]
- Impa, S.M.; Nadaradjan, S.; Jagadish, S.V.K. Drought stress induced reactive oxygen species and anti-oxidants in plants. In Abiotic Stress Responses in Plants; Springer: New York, NY, USA, 2012; pp. 131–147. [Google Scholar]
- Dastogeer, K.M.G. Influence of fungal endophytes on plant physiology is more pronounced under stress than well-watered conditions: A meta-analysis. Planta 2018, 248, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Guler, N.S.; Pehlivan, N.; Karaoglu, S.A.; Guzel, S.; Bozdeveci, A. Trichoderma atroviride ID20G inoculation ameliorates drought stress-induced damages by improving antioxidant defense in maize seedlings. Acta Physiol. Plant 2016, 38, 132. [Google Scholar] [CrossRef]
- Tyagi, J.; Varma, A.; Pudake, R.N. Evaluation of comparative effects of arbuscular mycorrhiza (Rhizophagus intraradices) and endophyte (Piriformospora indica) association with finger millet (Eleusine coracana) under drought stress. Eur. J. Soil Biol. 2017, 81, 1–10. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Khan, A.L.; Kang, S.M.; Lee, I. A comparative study of phosphate solubilization and the host plant growth promotion ability of Fusarium verticillioides RK01 and Humicola sp. KNU01 under salt stress. Ann. Microbiol. 2015, 65, 585–593. [Google Scholar] [CrossRef]
- Wang, C.; Wang, X.; Tian, Y.; Xue, Y.; Xu, X.; Sui, Y.; Yu, H. Oxidative stress and potential biomarkers in tomato seedlings subjected to soil lead contamination. Ecotox. Environ. Safe 2008, 71, 685–691. [Google Scholar] [CrossRef]
- He, C.; Wang, W.; Hou, J. Plant growth and soil microbial impacts of enhancing licorice with inoculating dark septate endophytes under drought stress. Front. Microbiol. 2019, 10, 2277. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Wang, Y.; Xia, D.; Gao, C.; Chao, C. Overexpression of a GST gene (ThGSTZ1) from Tamarix hispida improves drought and salinity tolerance by enhancing the ability to scavenge reactive oxygen species. Plant Cell Tissue Org. 2014, 117, 99–112. [Google Scholar] [CrossRef]
- Sadeghi, F.; Samsampour, D.; Seyahooei, M.A.; Bagheri, A.; Soltani, J. Fungal endophytes alleviate drought-induced oxidative stress in mandarin (Citrus reticulata L.): Toward regulating the ascorbate-glutathione cycle. Sci. Hortic. 2020, 261, 108991. [Google Scholar] [CrossRef]
- Sandhya, V.; Ali, S.Z.; Grover, M.; Reddy, G.; Venkateswarlu, B. Effect of plant growth promoting Pseudomonas spp. on compatible solutes, antioxidant status and plant growth of maize under drought stress. Plant Growth Regul. 2010, 62, 21–30. [Google Scholar] [CrossRef]
- Chen, L.; Liu, L.; Lu, B.; Ma, T.; Li, C. Exogenous melatonin promotes seed germination and osmotic regulation under salt stress in cotton (Gossypium hirsutum L.). PLoS ONE 2020, 15, e0228241. [Google Scholar]
- Pang, Z.; Zhao, Y.; Xu, P.; Yu, D. Microbial diversity of upland rice roots and their influence on rice growth and drought tolerance. Microorganisms 2020, 8, 1329. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Environ. Sci. Bio/Technol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Alibolandi, M.; Mirzahoseini, H. Chemical assistance in refolding of bacterial inclusion bodies. Biochem. Res. Int. 2011, 2011, 631607. [Google Scholar]
- Moghaddam, M.S.H.; Safaie, N.; Soltani, J.; Hagh-Doust, N. Desert-adapted fungal endophytes induce salinity and drought stress resistance in model crops. Plant Physiol. Biochem. 2021, 160, 225–238. [Google Scholar] [CrossRef]
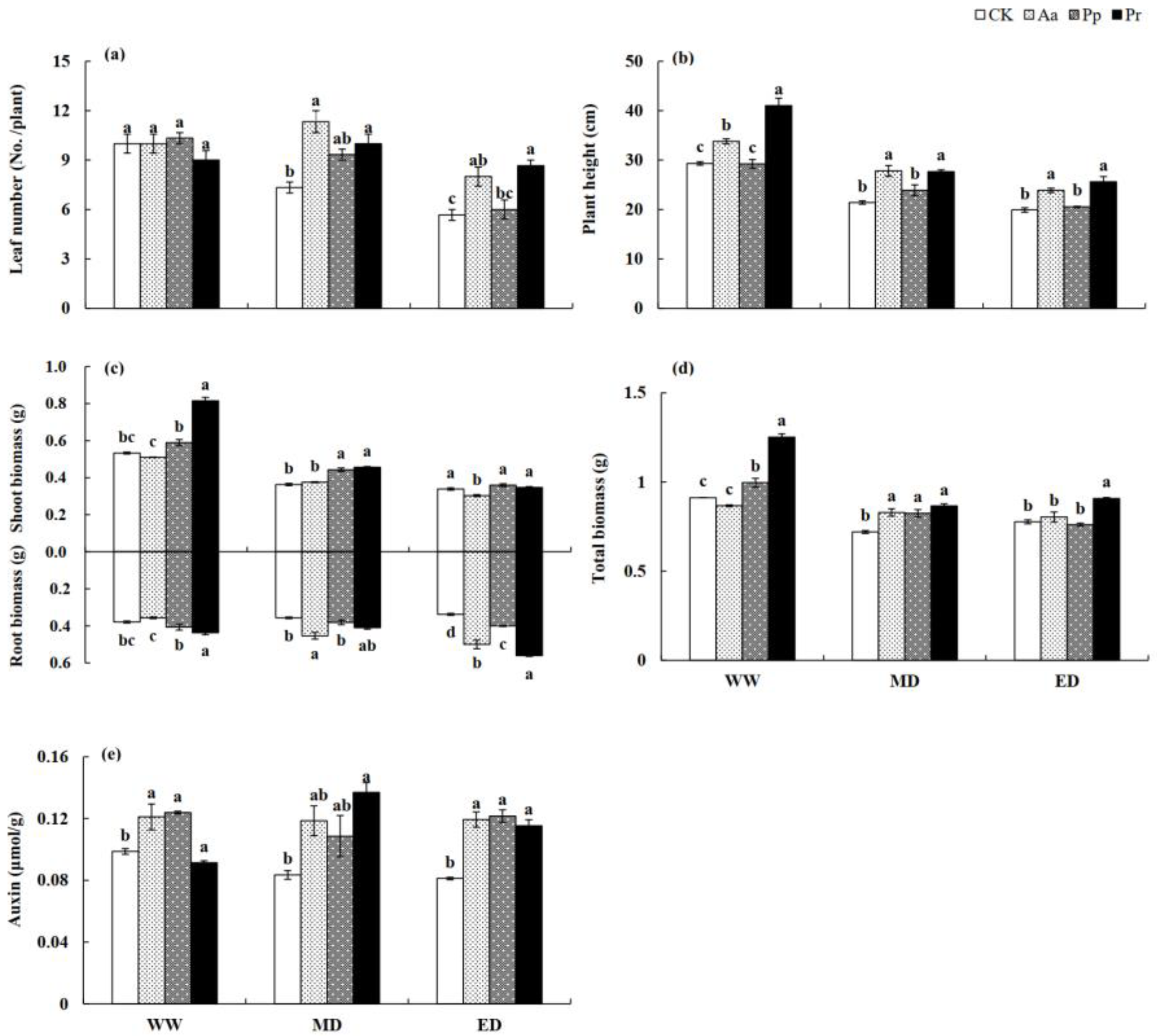



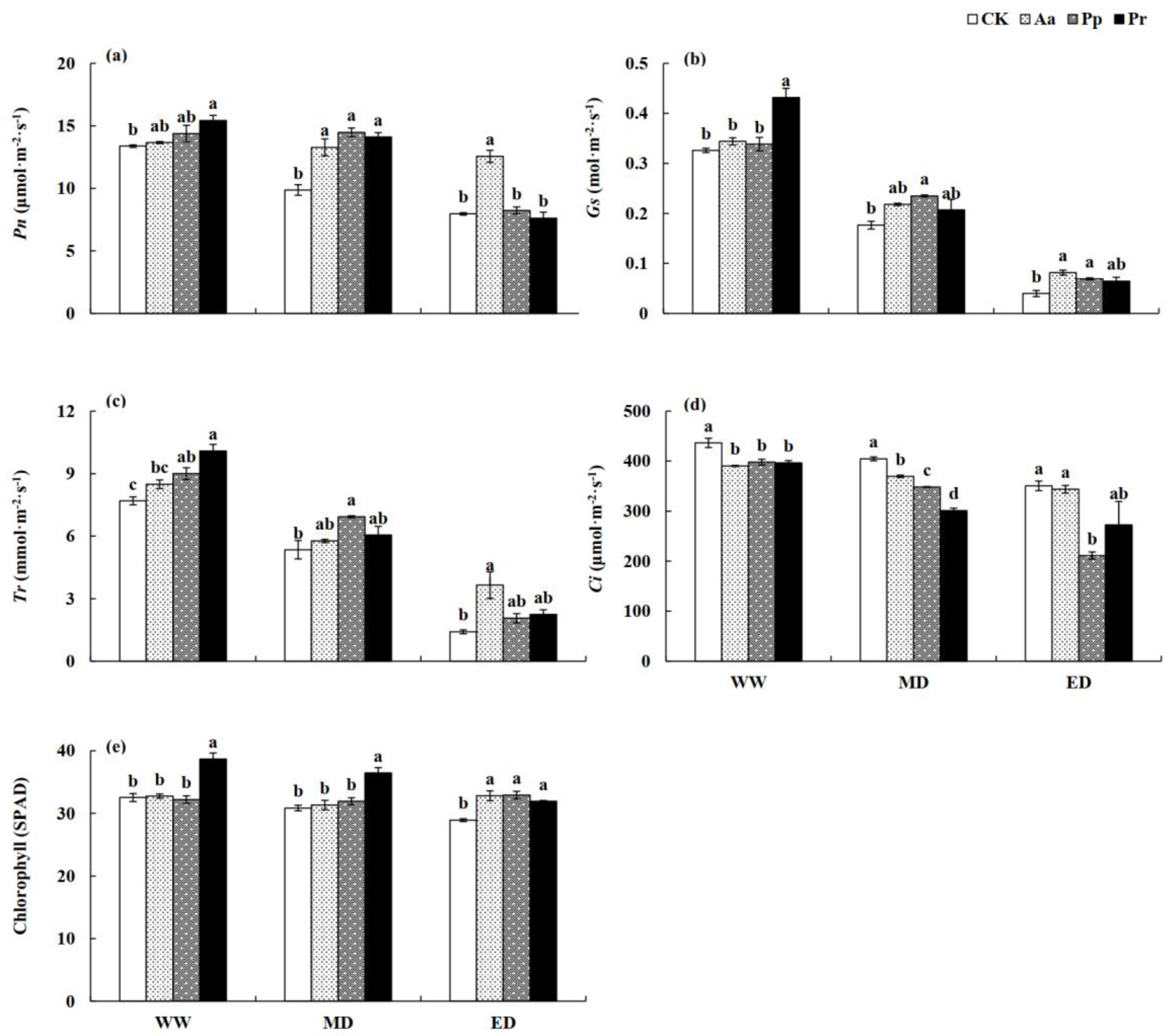
| Leaf Number | Plant Height | Shoot Biomass | Root Biomass | Total Biomass | Auxin Content | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| DSE | 9.91 | <0.001 | 62.04 | 0.000 | 159.50 | <0.001 | 53.19 | <0.001 | 98.98 | <0.001 | 17.76 | <0.001 |
| Water | 36.04 | <0.001 | 199.08 | 0.000 | 1033.79 | <0.001 | 27.76 | <0.001 | 202.50 | <0.001 | 0.30 | 0.747 |
| DSE × Water | 5.62 | 0.001 | 6.16 | 0.001 | 62.01 | <0.001 | 22.20 | <0.001 | 25.70 | <0.001 | 5.89 | 0.001 |
| GSH | SOD | MDA | Soluble Protein | Soluble Sugar | Proline | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | F | p | |
| DSE | 5.62 | 0.005 | 55.76 | <0.001 | 16.78 | 0.000 | 16.85 | <0.001 | 109.74 | <0.001 | 107.81 | <0.001 |
| Water | 376.29 | <0.001 | 265.38 | <0.001 | 7.84 | 0.002 | 21.41 | <0.001 | 320.68 | <0.001 | 299.35 | <0.001 |
| DSE × Water | 7.68 | <0.001 | 23.11 | <0.001 | 15.36 | 0.000 | 3.92 | 0.007 | 25.07 | <0.001 | 19.32 | <0.001 |
| Photosynthetic Rate | Stomatal Conductance | Transpiration Rate | Intercellular CO2 Concentration | Chlorophyll | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | F | p | F | p | F | p | F | p | |
| DSE | 24.52 | <0.001 | 14.50 | <0.001 | 14.43 | <0.001 | 19.54 | <0.001 | 33.53 | <0.001 |
| Water | 167.07 | <0.001 | 856.99 | <0.001 | 406.11 | <0.001 | 57.48 | <0.001 | 14.88 | <0.001 |
| DSE × Water | 19.11 | <0.001 | 8.57 | <0.001 | 5.34 | 0.001 | 5.74 | 0.001 | 8.80 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liu, Y.; Ye, Q.; Xu, M.; He, X. Application of Desert DSEs to Nonhost Plants: Potential to Promote Growth and Alleviate Drought Stress of Wheat Seedlings. Agriculture 2022, 12, 1539. https://doi.org/10.3390/agriculture12101539
Li X, Liu Y, Ye Q, Xu M, He X. Application of Desert DSEs to Nonhost Plants: Potential to Promote Growth and Alleviate Drought Stress of Wheat Seedlings. Agriculture. 2022; 12(10):1539. https://doi.org/10.3390/agriculture12101539
Chicago/Turabian StyleLi, Xia, Yanxia Liu, Qiannan Ye, Minghui Xu, and Xueli He. 2022. "Application of Desert DSEs to Nonhost Plants: Potential to Promote Growth and Alleviate Drought Stress of Wheat Seedlings" Agriculture 12, no. 10: 1539. https://doi.org/10.3390/agriculture12101539




