Mitigation of High Solar Irradiance and Heat Stress in Kiwifruit during Summer via the Use of Alleviating Products with Different Modes of Action—Part 1 Effects on Leaf Physiology and Biochemistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Site Location—Plant Material
2.2. Treatments and Measurements
2.3. Leaf Sclerophylly Indexes
2.4. Phenolic Compound Concentration and Antioxidant Capacity Determination
2.5. Soluble Sugars Determination
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lashof, D.A.; Ahuja, D.R. Relative contributions of greenhouse gas emissions to global warming. Nature 1990, 344, 529–531. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological responses to drought, salinity, and heat stress in plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Cline, W.R. Global Warming and Agriculture: End-of-Century Estimates by Country; Peterson Institute for International Economics: Washington, DC, USA, 2007. [Google Scholar]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Barbanti, L.; Aamer, M.; Iqbal, M.M.; Nawaz, M.; Mahmood, A.; Ali, A.J.P.B. Heat stress in cultivated plants: Nature, impact, mechanisms, and mitigation strategies—A review. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2021, 155, 211–234. [Google Scholar] [CrossRef]
- Atkinson, C.J. Effects of climate change on the behaviour of woody perennials. In Environmental Pollution and Plant Responses; Agrawal, S.B., Agrawal, M., Eds.; Lewis Publishers: Boca Raton, FL, USA, 2000; pp. 19–32. [Google Scholar]
- Wang, X.; Fang, Z.; Zhao, D.; Tao, J. Effects of high-temperature stress on photosynthetic characteristics and antioxidant enzyme, system of Paeonia ostii. Phyton 2022, 91, 599–615. [Google Scholar] [CrossRef]
- Ul Hassan, M.; Rasool, T.; Iqbal, C.; Arshad, A.; Abrar, M.; Abrar, M.M.; Habib-ur-Rahman, M.; Noor, M.A.; Sher, A.; Fahad, S. Linking plants functioning to adaptive responses under heat stress conditions: A mechanistic review. J. Plant Growth Regul. 2021, 41, 2596–2613. [Google Scholar] [CrossRef]
- Sharma, S.; Manjeet, M. Heat stress effects in fruit crops. Agric. Rev. 2020, 41, 73–78. [Google Scholar] [CrossRef]
- Abbate, A.P.; Campbell, J.W.; Vinson, E.L.; Williams, G.R. The pollination and fruit quality of two kiwifruit cultivars (Actinidia chinensis var. chinensis ‘AU Golden Sunshine’and ‘AU Gulf Coast Gold’)(Ericales: Actinidiaceae) grown in the Southeastern United States. J. Econ. Entomol. 2021, 114, 1234–1241. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; Garcıa, P.C.; Lopez-Lefebre, L.R.; Sánchez, E.; Romero, L.J. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Wahid, A.; Gelani, S.; Ashraf, M.; Foolad, M.R.J. Heat tolerance in plants: An overview. Environ. Exp. Bot. 2007, 61, 199–223. [Google Scholar] [CrossRef]
- Calderón-Orellana, A.; Silva, D.I.; Bastías, R.M.; Bambach, N.; Aburto, F.J. Late-season plastic covering delays the occurrence of severe water stress and improves intrinsic water use efficiency and fruit quality in kiwifruit vines. Agric. Water Manag. 2021, 249, 106795. [Google Scholar] [CrossRef]
- Gucci, R.; Massai, R.; Xiloyannis, C.; Flore, J.A. The Effect of drought and vapour pressure deficit on gas exchange of young kiwifruit (Actinidia deliciosa var. deliciosa) vines. Ann. Bot. 1996, 77, 605–613. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Xiloyannis, C.J. Shade mitigates photoinhibition and enhances water use efficiency in kiwifruit under drought. Photosynthetica 2009, 47, 363–371. [Google Scholar] [CrossRef]
- Smith, G.S.; Walton, E.F. Kiwifruit. In Temperate Fruit Crops in Warm Climates; Erez, A., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; pp. 367–379. [Google Scholar]
- Greaves, A.; Buwalda, J.J. Observations of diurnal decline of photosynthetic gas exchange in kiwifruit and the effect of external CO2 concentration. N. Z. J. Crop Hortic. Sci. 1996, 24, 361–369. [Google Scholar] [CrossRef] [Green Version]
- Chartzoulakis, K.; Noitsakis, B.; Therios, I. Photosynthesis, plant growth and dry matter distribution in kiwifruit as influenced by water deficits. Irrig. Sci. 1993, 14, 1–5. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Yuan, F.-R.; He, K.-J.; Bu, F.-W. Effects of overhead shading on yield and fruit quality of kiwifruit in regions with high temperatures in summer. Acta Hortic. 2007, 753, 399–407. [Google Scholar] [CrossRef]
- Morgan, D.; Warrington, I.; Halligan, E.J. Effect of temperature and photosynthetic photon flux density on vegetative growth of kiwifruit (Actinidia chinensis). N. Z. J. Agric. Res. 1985, 28, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Buwalda, J.; Green, T.; Meekings, J.; Coneybear, D. Measurement of canopy gas exchange of kiwifruit vines using a suite of whole-canopy cuvettes. Environ. Exp. Bot. 1992, 32, 425–438. [Google Scholar] [CrossRef]
- Karaat, F.E.; Denizhan, H. The effects of different particle film applications on almond trees. Ciência Rural 2023, 53, e20210757. [Google Scholar] [CrossRef]
- Sotiropoulos, T.; Petridis, A.; Koukourikou-Petridou, M.; Koundouras, S. Evaluation of ‘Sun Protect’in protecting apples (Malus × domestica Borkh.) against sunburn. Hortic. Sci. 2016, 43, 175–180. [Google Scholar] [CrossRef]
- Roussos, P.; Ntanos, E.; Tsafouros, A.; Denaxa, N.-K. Strawberry physiological and biochemical responses to chilling and freezing stress and application of alleviating factors as countermeasures. J. Berry Res. 2020, 10, 437–457. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Roussos, P.A.; Denaxa, N.-K.; Damvakaris, T.; Stournaras, V.; Argyrokastritis, I. Effect of alleviating products with different mode of action on physiology and yield of olive under drought. Sci. Hortic. 2010, 125, 700–711. [Google Scholar] [CrossRef]
- Denaxa, N.-K.; Roussos, P.A.; Damvakaris, T.; Stournaras, V. Comparative effects of exogenous glycine betaine, kaolin clay particles and Ambiol on photosynthesis, leaf sclerophylly indexes and heat load of olive cv. Chondrolia Chalkidikis under drought. Sci. Hortic. 2012, 137, 87–94. [Google Scholar] [CrossRef]
- Khedr, R.A.; Sorour, S.G.R.; Aboukhadrah, S.H.; El Shafey, N.M.; Abd Elsalam, H.E.; El-Sharnouby, M.E.; El-Tahan, A.M. Alleviation of salinity stress effects on agro-physiological traits of wheat by auxin, glycine betaine, and soil additives. Saudi J. Biol. Sci. 2022, 29, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Shemi, R.; Wang, R.; Gheith, E.-S.; Hussain, H.A.; Hussain, S.; Irfan, M.; Cholidah, L.; Zhang, K.; Zhang, S.; Wang, L. Effects of salicylic acid, zinc and glycine betaine on morpho-physiological growth and yield of maize under drought stress. Sci. Rep. 2021, 11, 3195. [Google Scholar] [CrossRef]
- Islam, S.; Parrey, Z.A.; Shah, S.H.; Mohammad, F. Glycine betaine mediated changes in growth, photosynthetic efficiency, antioxidant system, yield and quality of mustard. Sci. Hortic. 2021, 285, 110170. [Google Scholar] [CrossRef]
- Salinger, M.; Kenny, G.; Morley-Bunker, M. Climate and kiwifruit cv. Hayward 1. Influences on development and growth. N. Z. J. Crop Hortic. Sci. 1993, 21, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; YavaŞ, İ.; Alhammad, B.A.; Shami, A.; Hasanuzzaman, M.; Kalderis, D. Glycine betaine accumulation, significance and interests for heavy metal tolerance in plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef]
- Sorwong, A.; Sakhonwasee, S. Foliar application of glycine betaine mitigates the effect of heat stress in three marigold (Tagetes erecta) cultivars. Hortic. J. 2015, 48, 161–171. [Google Scholar] [CrossRef]
- Ahmed, N.; Zhang, Y.; Li, K.; Zhou, Y.; Zhang, M.; Li, Z. Exogenous application of glycine betaine improved water use efficiency in winter wheat (Triticum aestivum L.) via modulating photosynthetic efficiency and antioxidative capacity under conventional and limited irrigation conditions. Crop J. 2019, 7, 635–650. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Ashraf, M.; Siddique, K.H. Role of glycine betaine in the thermotolerance of plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]
- Patanè, C.; Pellegrino, A.; Di Silvestro, I. Effects of calcium carbonate application on physiology, yield and quality of field-grown tomatoes in a semi-arid Mediterranean climate. Crop Pasture Sci. 2018, 69, 411–418. [Google Scholar] [CrossRef]
- Tsai, M.; Lee, T.; Chang, P. Comparison of paper bags, calcium carbonate, and shade nets for sunscald protection in ‘Murcott’ Tangor fruit. HortTechnology 2013, 23, 659–667. [Google Scholar] [CrossRef]
- Attia, F.; Martinez, L.; Lamaze, T. Foliar application of processed calcite particles improves leaf photosynthesis of potted Vitis vinifera L. (var.‘Cot’) grown under water deficit. OENO One 2014, 48, 237–245. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, H.L.; Di Bella, C.M.; Colavita, G.M.; Oricchio, P.; Straschnoy, J. Comparative effects of kaolin and calcium carbonate on apple fruit surface temperature and leaf net CO2 assimilation. J. Appl. Hortic. 2015, 17, 176–180. [Google Scholar] [CrossRef]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Smith, G.S.; Buwalda, J.G. Temperate Crops: Kiwifruit. In Handbook of Environmental Physiology of Fruit Crops; Schaffer, B., Andersen, P.C., Eds.; CRC Press: Boca Raton, FL, USA, 1994; pp. 135–163. [Google Scholar]
- Tzortzakis, N.; Chrysargyris, A.; Aziz, A. Adaptive response of a native mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 2020, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Linić, I.; Mlinarić, S.; Brkljačić, L.; Pavlović, I.; Smolko, A.; Salopek-Sondi, B. Ferulic acid and Salicylic acid foliar treatments reduce short-term salt stress in Chinese cabbage by increasing phenolic compounds accumulation and photosynthetic performance. Plants 2021, 10, 2346. [Google Scholar] [CrossRef]
- Lalarukh, I.; Shahbaz, M. Response of antioxidants and lipid peroxidation to exogenous application of alpha-tocopherol in sunflower (Helianthus annuus L.) under salt stress. Pak. J. Bot. 2020, 52, 75–83. [Google Scholar] [CrossRef]
- Nardozza, S.; Boldingh, H.L.; Osorio, S.; Höhne, M.; Wohlers, M.; Gleave, A.P.; MacRae, E.A.; Richardson, A.C.; Atkinson, R.G.; Sulpice, R. Metabolic analysis of kiwifruit (Actinidia deliciosa) berries from extreme genotypes reveals hallmarks for fruit starch metabolism. J. Exp. Bot. 2013, 64, 5049–5063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Zorita, M.; Fernandez-Canigia, M.; Grosso, G.A. Applications of foliar fertilizers containing glycinebetaine improve wheat yields. J. Agron. Crop Sci. 2001, 186, 209–215. [Google Scholar] [CrossRef]
- Gupta, N.; Thind, S. Foliar application of glycine betaine alters sugar metabolism of wheat leaves under prolonged field drought stress. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 877–884. [Google Scholar] [CrossRef]
- Lievre, D.L.; Anderson, R.; Boldingh, H.; Cooney, J.; Seelye, R.; Gould, N.; Hunter, D.; Jensen, D.; Pereira, T.; Wohlers, M. Modifying carbohydrate supply to fruit during development changes the composition and flavour of Actinidia chinensis var. chinensis ‘Zesy002’ Kiwifruit. Plants 2021, 10, 1328. [Google Scholar] [CrossRef]
- Boldingh, H.; Smith, G.; Klages, K. Seasonal concentrations of non-structural carbohydrates of five Actinidia species in fruit, leaf and fine root tissue. Ann. Bot. 2000, 85, 469–476. [Google Scholar] [CrossRef]
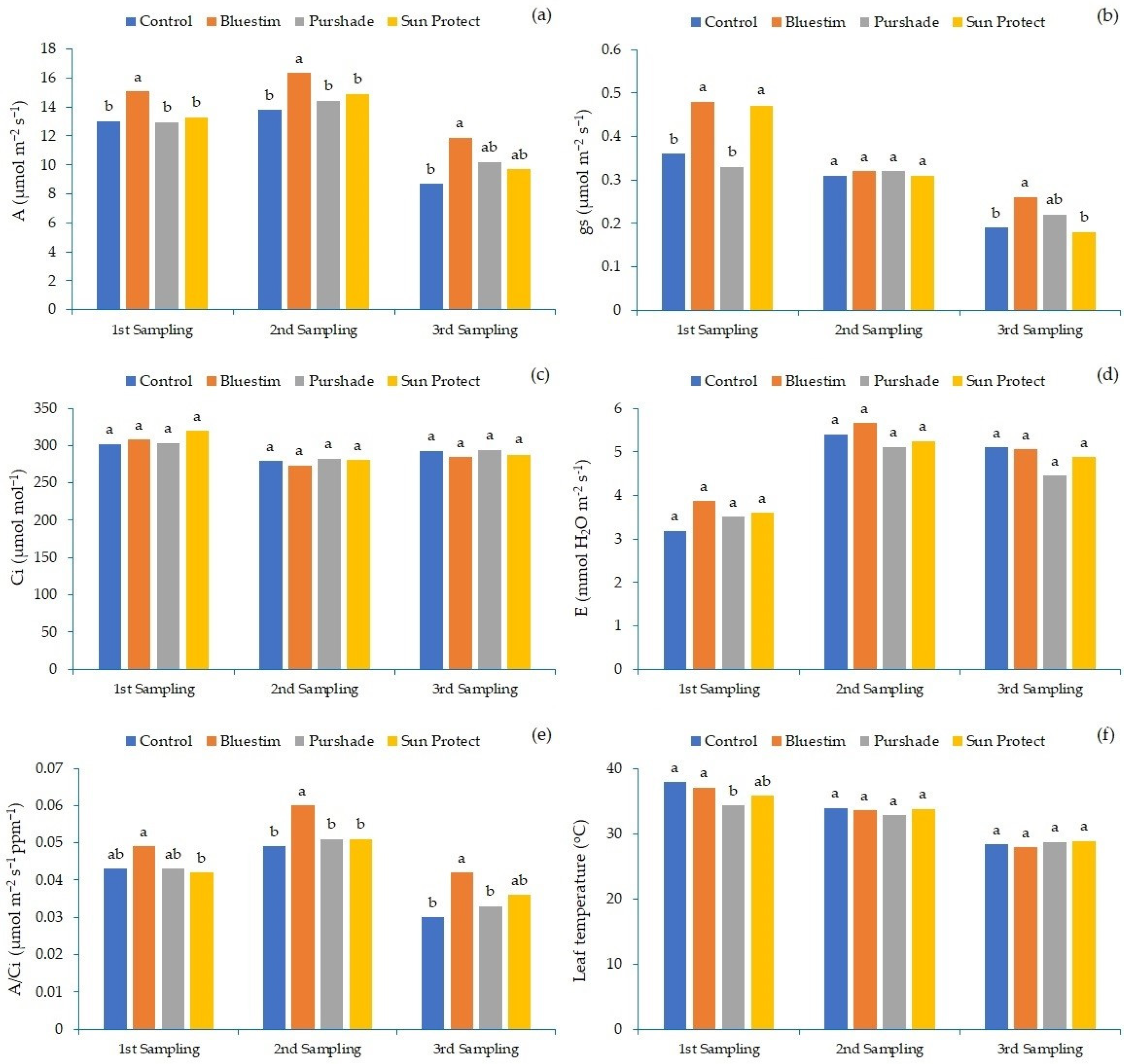

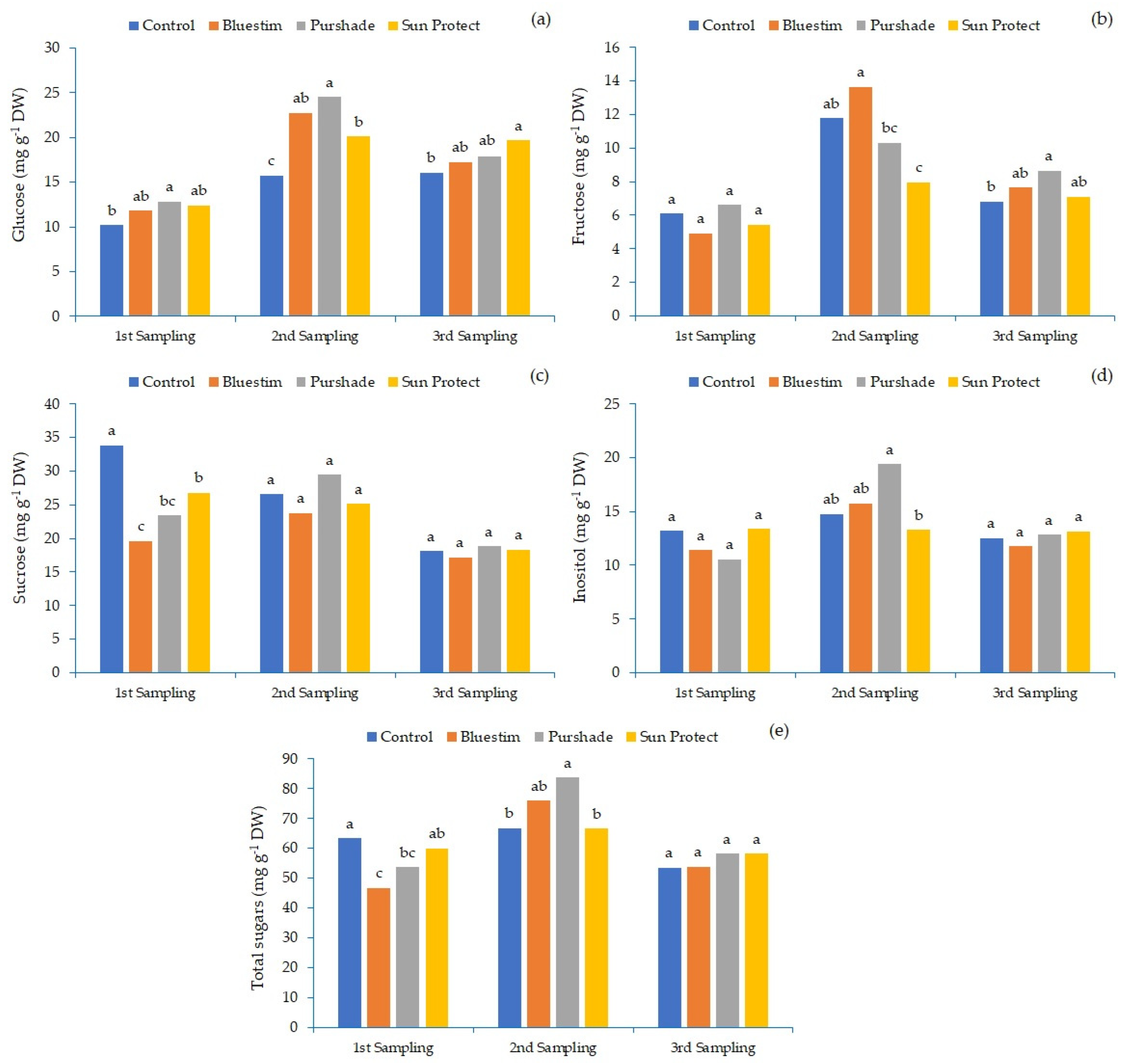
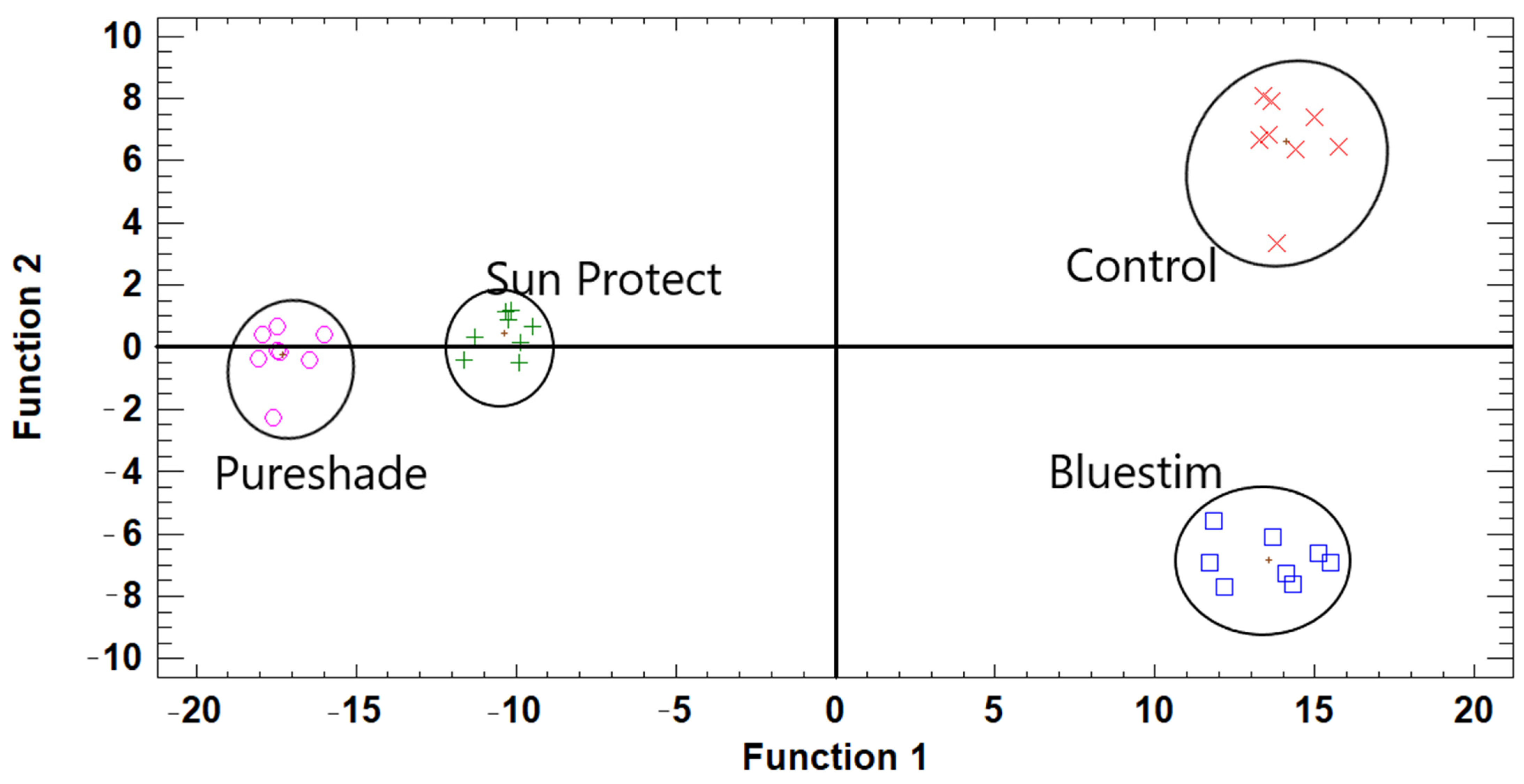


| Treatment | WC | RWC | LTD | WSD | WCS | SLA FW | SUC |
|---|---|---|---|---|---|---|---|
| ns | *** | ns | *** | ns | ns | *** | |
| Control | 70.61 a | 67.94 ab | 29.39 a | 32.06 ab | 3.57 a | 21.25 a | 0.034 ab |
| BlueStim | 72.87 a | 73.37 a | 27.13 a | 26.63 b | 3.79 a | 19.68 a | 0.038 a |
| Pureshade | 71.76 a | 66.30 b | 28.24 a | 33.69 a | 3.93 a | 21.63 a | 0.033 b |
| Sun Protect | 71.48 a | 66.14 b | 28.52 a | 33.87 a | 3.87 a | 21.65 a | 0.033 b |
| Treatment | WC | RWC | LTD | WSD | WCS | SLA FW | SUC |
|---|---|---|---|---|---|---|---|
| ns | ns | ns | ns | ns | ns | ns | |
| Control | 69.73 a | 79.53 a | 30.27 a | 20.46 a | 3.02 a | 26.79 a | 0.027 a |
| BlueStim | 67.01 a | 78.56 a | 32.99 a | 21.44 a | 2.67 a | 26.17 a | 0.026 a |
| Pureshade | 66.39 a | 75.50 a | 33.61 a | 24.50 a | 2.61 a | 26.92 a | 0.025 a |
| Sun Protect | 68.82 a | 79.86 a | 31.18 a | 20.14 a | 2.83 a | 24.14 a | 0.029 a |
| Treatment | WC | RWC | LTD | WSD | WCS | SLA FW | SUC |
|---|---|---|---|---|---|---|---|
| ns | ns | ns | ns | ns | ns | ns | |
| Control | 66.93 a | 64.98 a | 33.07 a | 35.02 a | 3.15 a | 28.79 a | 0.024 a |
| BlueStim | 68.69 a | 66.91 a | 31.32 a | 33.09 a | 3.32 a | 24.89 a | 0.028 a |
| Pureshade | 67.43 a | 65.05 a | 32.57 a | 34.95 a | 3.23 a | 26.14 a | 0.026 a |
| Sun Protect | 66.25 a | 64.63 a | 33.75 a | 35.37 a | 3.08 a | 25.77 a | 0.025 a |
| Treatment | 1st Sampling (August 7th) | 2nd Sampling (August 20th) | 3rd Sampling (September 21st) | |||
|---|---|---|---|---|---|---|
| SPAD | Fv/Fm | SPAD | Fv/Fm | SPAD | Fv/Fm | |
| ns | ns | ns | ns | ns | ns | |
| Control | 60.03 a | 0.775 a | 62.98 a | 0.774 a | 62.09 a | 0.754 a |
| BlueStim | 61.00 a | 0.776 a | 59.13 a | 0.756 a | 60.72 a | 0.784 a |
| Pureshade | 62.06 a | 0.741 a | 63.29 a | 0.755 a | 67.09 a | 0.769 a |
| Sun Protect | 58.86 a | 0.758 a | 62.04 a | 0.769 a | 60.81 a | 0.758 a |
| 1st Sampling (August 7th) | 2nd Sampling (August 20th) | 3rd Sampling (September 21st) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | DPPH | ABTS |
| ns | *** | ** | ns | ns | ns | ns | ns | ns | |
| Control | 313.21 a | 56.54 a | 439.17 a | 298.67 a | 45.20 a | 370.12 a | 358.66 a | 57.76 a | 416.41 a |
| BlueStim | 274.91 a | 35.08 bc | 405.74 ab | 336.91 a | 58.27 a | 394.74 a | 331.45 a | 33.23 a | 386.63 a |
| Pureshade | 281.05 a | 23.99 c | 381.87 b | 289.71 a | 44.91 a | 351.32 a | 313.48 a | 31.25 a | 378.28 a |
| Sun Protect | 344.32 a | 54.07 ab | 442.38 a | 290.54 a | 46.68 a | 371.98 a | 346.37 a | 41.81 a | 336.10 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntanos, E.; Tsafouros, A.; Denaxa, N.-K.; Kosta, A.; Bouchagier, P.; Roussos, P.A. Mitigation of High Solar Irradiance and Heat Stress in Kiwifruit during Summer via the Use of Alleviating Products with Different Modes of Action—Part 1 Effects on Leaf Physiology and Biochemistry. Agriculture 2022, 12, 2121. https://doi.org/10.3390/agriculture12122121
Ntanos E, Tsafouros A, Denaxa N-K, Kosta A, Bouchagier P, Roussos PA. Mitigation of High Solar Irradiance and Heat Stress in Kiwifruit during Summer via the Use of Alleviating Products with Different Modes of Action—Part 1 Effects on Leaf Physiology and Biochemistry. Agriculture. 2022; 12(12):2121. https://doi.org/10.3390/agriculture12122121
Chicago/Turabian StyleNtanos, Efstathios, Athanassios Tsafouros, Nikoleta-Kleio Denaxa, Anna Kosta, Pavlos Bouchagier, and Peter Anargyrou Roussos. 2022. "Mitigation of High Solar Irradiance and Heat Stress in Kiwifruit during Summer via the Use of Alleviating Products with Different Modes of Action—Part 1 Effects on Leaf Physiology and Biochemistry" Agriculture 12, no. 12: 2121. https://doi.org/10.3390/agriculture12122121





