Mitigation of High Solar Irradiance and Heat Stress in Kiwifruit during Summer via the Use of Alleviating Products with Different Modes of Action—Part 2 Effects on Fruit Quality, Organoleptic, and Phytochemical Properties at Harvest and after Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Treatments
2.3. Sampling and Physiological Properties Determination
2.4. Determination of Organoleptic Characteristics
2.5. Soluble Sugars Determination
2.6. Organic Acids Determination
2.7. Phenolic Compounds and Antioxidant Capacity Determination
2.8. Statistical Analysis
3. Results
3.1. Effect of Alleviating Products on Fruit Physiological, Quality, and Organoleptic Characteristics
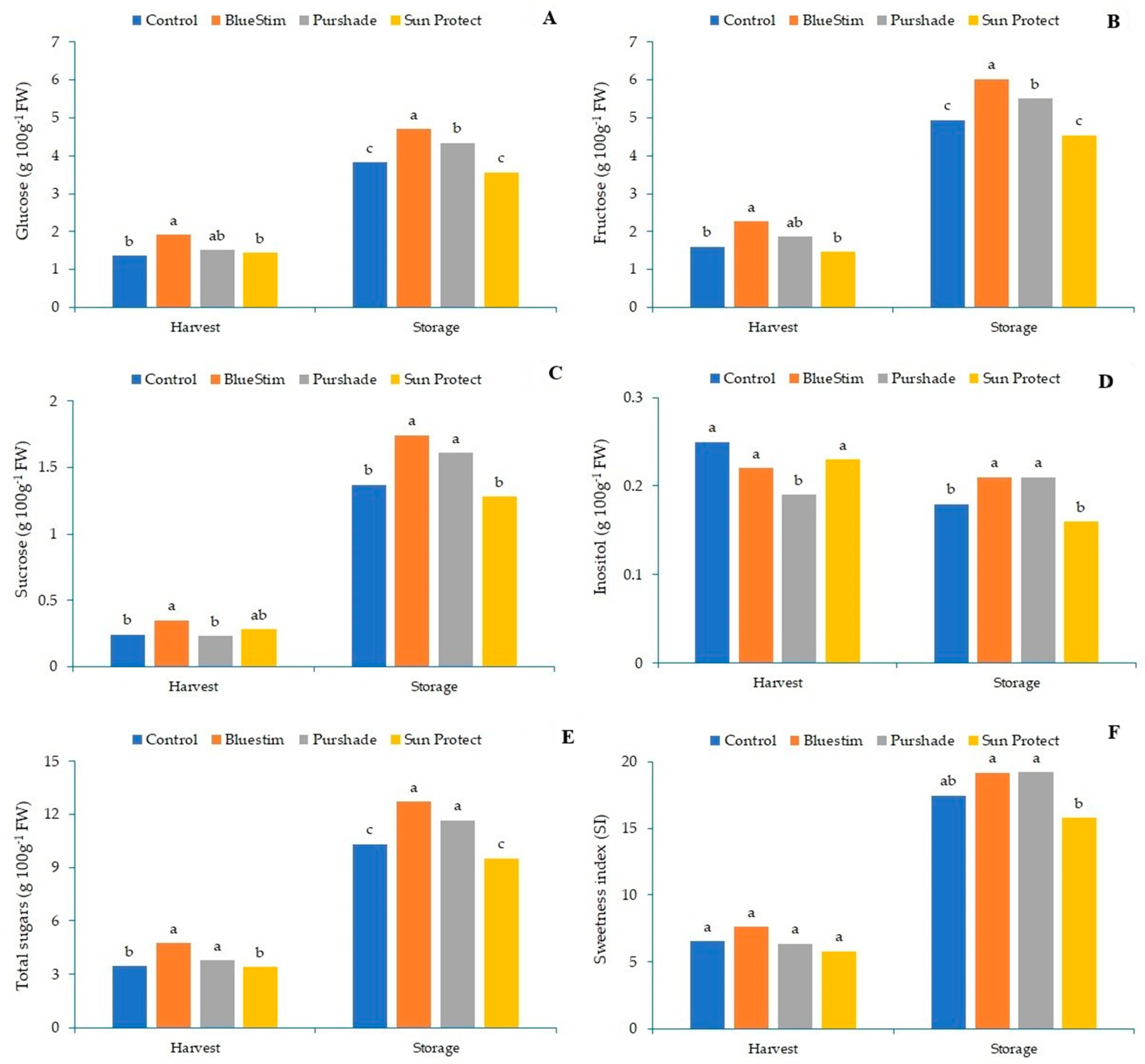
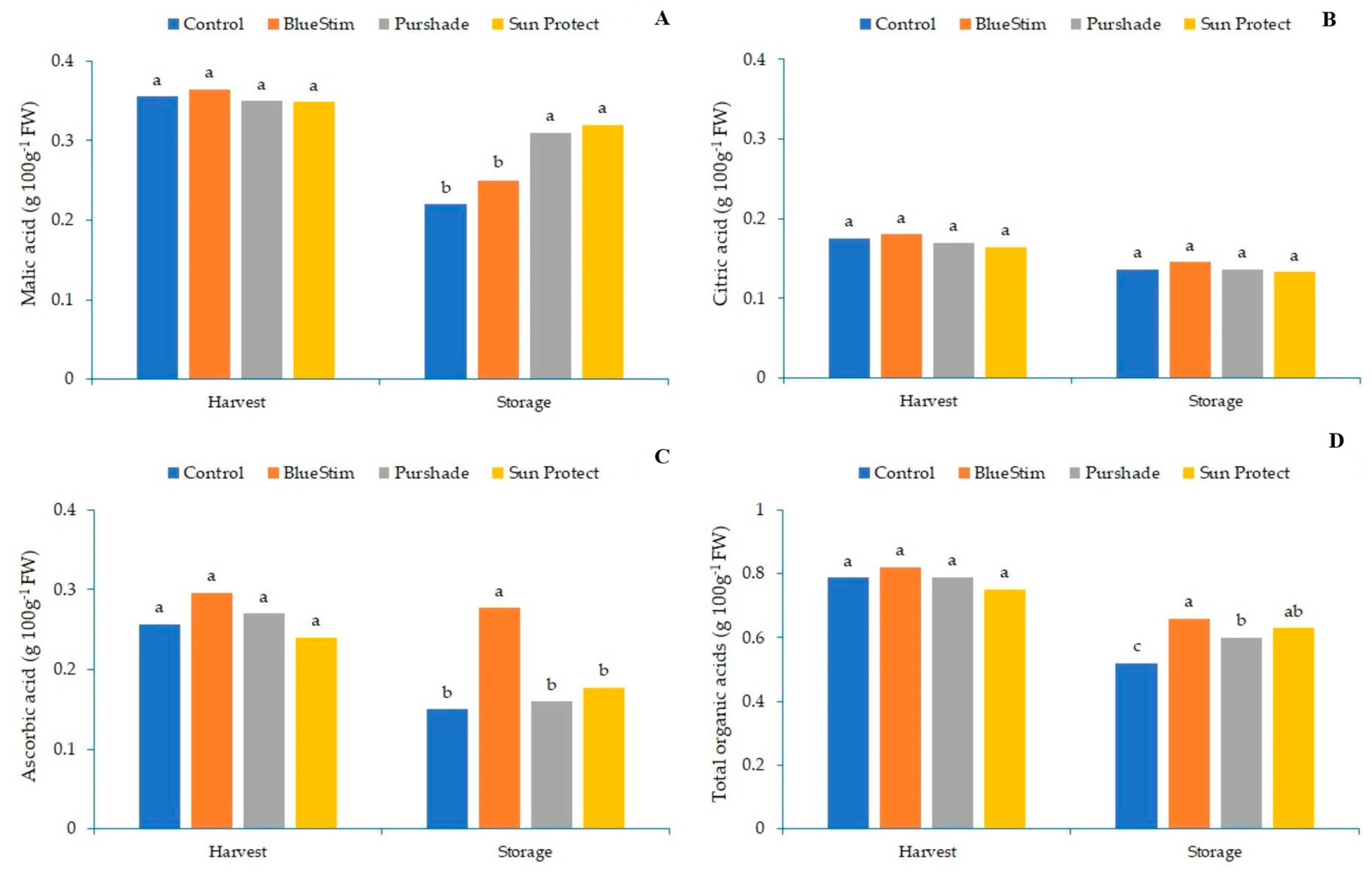
3.2. Effect of Alleviating Products on Kiwifruit Soluble Sugars and Organic Acids
3.3. Effect of Alleviating Products on Kiwifruit Phenolic Compounds and Antioxidant Capacity
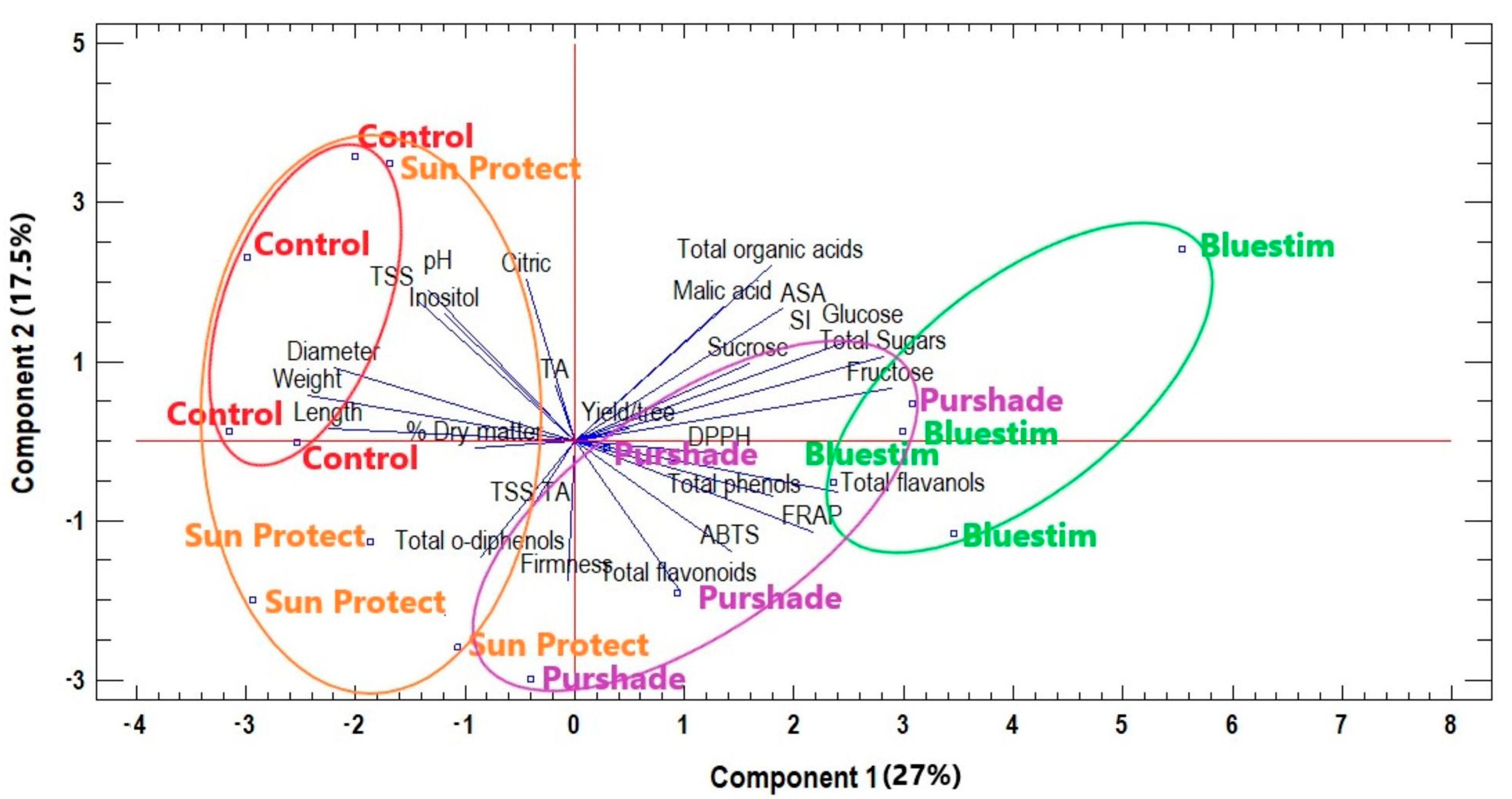
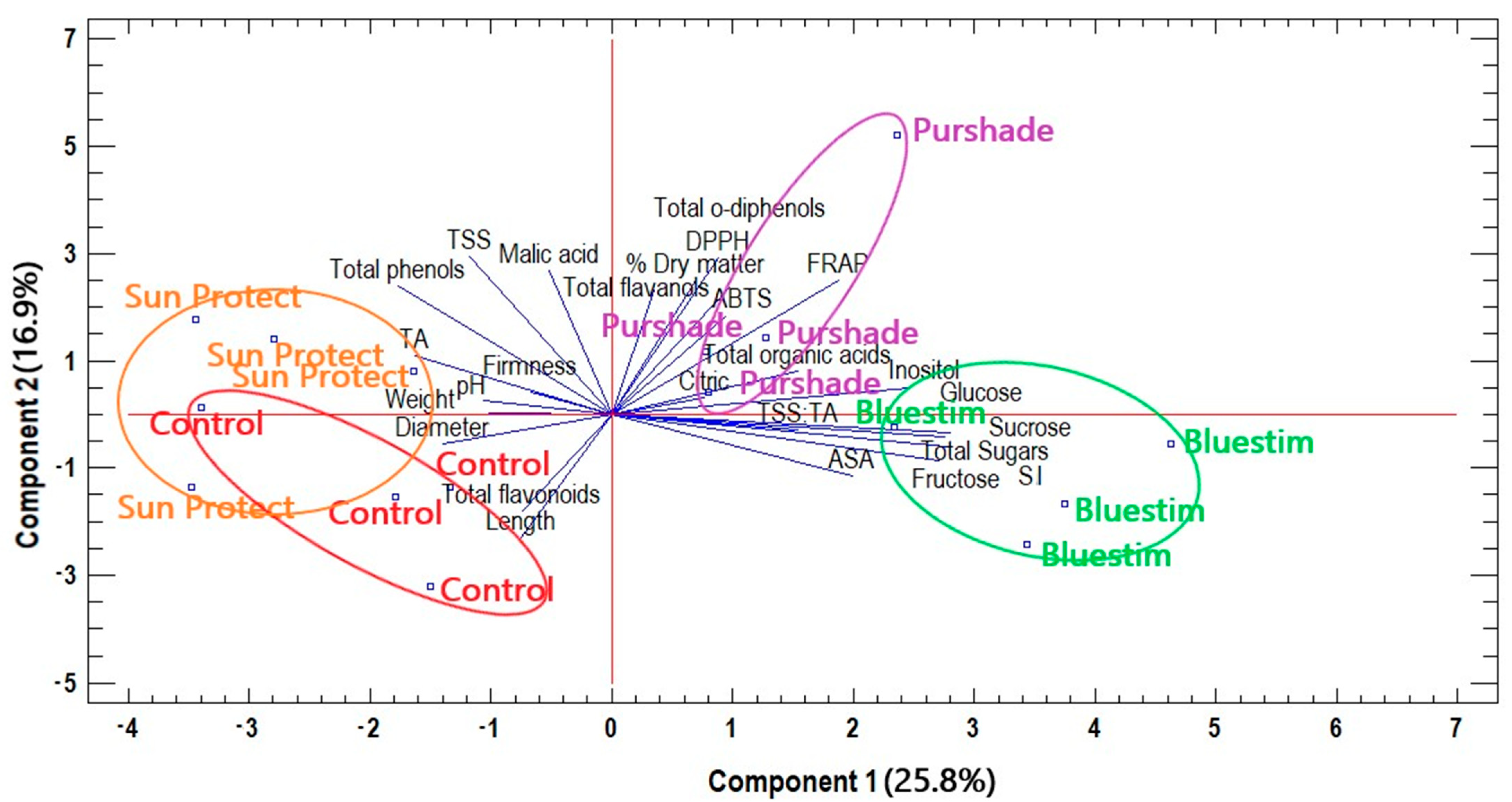
3.4. Overall Effects of Alleviating Products Treatments on Kiwifruit Quality Characteristics and Biochemical Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderón-Orellana, A.; Silva, D.I.; Bastías, R.M.; Bambach, N.; Aburto, F.J. Late-season plastic covering delays the occurrence of severe water stress and improves intrinsic water use efficiency and fruit quality in kiwifruit vines. Agric. Water Manag. 2021, 249, 106795. [Google Scholar] [CrossRef]
- Gucci, R.; Massai, R.; Xiloyannis, C.; Flore, J.A. The effect of drought and vapour pressure deficit on gas exchange of young kiwifruit (Actinidia deliciosa var. deliciosa) vines. Ann. Bot. 1996, 77, 605–613. [Google Scholar] [CrossRef]
- Diaz-Espejo, A.; Nicolas, E.; Fernandez, J.E. Seasonal evolution of diffusional limitations and photosynthetic capacity in olive under drought. Plant Cell Environ. 2007, 30, 922–933. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Yuan, F.-R.; He, K.-J.; Bu, F.-W. Effects of overhead shading on yield and fruit quality of kiwifruit in regions with high temperatures in summer. Acta Hortic. 2007, 753, 399–407. [Google Scholar] [CrossRef]
- Morgan, D.; Warrington, I.; Halligan, E.J. Effect of temperature and photosynthetic photon flux density on vegetative growth of kiwifruit (Actinidia chinensis). N. Z. J. Agric. Res. 1985, 28, 109–116. [Google Scholar] [CrossRef] [Green Version]
- Greer, D.H.; Laing, W.A. Photoinhibition of photosynthesis in intact kiwifruit (Actinidia deliciosa) leaves: Effect of growth temperature on photoinhibition and recovery. Planta 1989, 180, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Hopkirk, G.; Snelgar, W.P.; Horne, S.F.; Manson, P.J. Effect of increased preharvest temperature on fruit quality of kiwifruit (Actinidia deliciosa). J. Hortic. Sci. 1989, 64, 227–237. [Google Scholar] [CrossRef]
- Seager, N.G.; Warrington, I.J.; Hewett, E.W. Maturation of kiwifruit grown at different temperatures in controlled environments. J. Hortic. Sci. 1996, 71, 639–652. [Google Scholar] [CrossRef]
- Richardson, A.C.; Marsh, K.B.; Boldingh, H.L.; Pickering, A.H.; Bulley, S.M.; Frearson, N.J.; Ferguson, A.R.; Thornber, S.E.; Bolitho, K.M.; Macrae, E.A. High growing temperatures reduce fruit carbohydrate and vitamin C in kiwifruit. Plant Cell Environ. 2004, 27, 423–435. [Google Scholar] [CrossRef]
- Greer, D.H.; Weedon, M.M.; Weston, C. Reductions in biomass accumulation, photosynthesis in situ and net carbon balance are the costs of protecting Vitis vinifera ‘Semillon’ grapevines from heat stress with shade covering. AoB Plants 2011, 2011, plr023. [Google Scholar] [CrossRef]
- Roussos, P.A.; Tsafouros, A.; Ntanos, E.; Denaxa, N.-K.; Kosta, A.; Bouchagier, P. Could black anti-hail net have an extra role as an amelioration agent against heat stress in kiwifruit? J. Berry Res. 2022, 12, 131–147. [Google Scholar] [CrossRef]
- Lybbert, T.; Sumner, D. Agricultural technologies for climate change mitigation and adaptation in developing countries: Policy options for innovation and technology diffusion. In ICTSD–IPC Platform on Climate Change, Agriculture and Trade, Issue Brief No.6; International Centre for Trade and Sustainable Development: Geneva, Switzerland; International Food & Agricultural Trade Policy Council: Washington, DC, USA, 2010. [Google Scholar]
- Karavolias, N.G.; Horner, W.; Abugu, M.N.; Evanega, S.N. Application of gene editing for climate change in agriculture. Front. Sustain. Food Syst. 2021, 5, 685801. [Google Scholar] [CrossRef]
- Key, S.; Ma, J.K.; Drake, P.M. Genetically modified plants and human health. J. R. Soc. Med. 2008, 101, 290–298. [Google Scholar] [CrossRef] [Green Version]
- Ul Hassan, M.; Rasool, T.; Iqbal, C.; Arshad, A.; Abrar, M.; Abrar, M.M.; Habib-ur-Rahman, M.; Noor, M.A.; Sher, A.; Fahad, S. Linking plants functioning to adaptive responses under heat stress conditions: A mechanistic review. J. Plant Growth Regul. 2021, 41, 2596–2613. [Google Scholar] [CrossRef]
- Denaxa, N.-K.; Tsafouros, A.; Ntanos, E.; Roussos, P.A. Role of glycine betaine in the protection of plants against environmental stresses. In Plant Stress Mitigators: Types, Techniques and Functions; Ghorbanpour, A., Shahid, M.A., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 127–158. [Google Scholar]
- Surabhi, G.; Rout, A. Glycine betaine and crop abiotic stress tolerance: An update. In Protective Chemical Agents in the Amelioration of Plant Abiotic Stress: Biochemical and Molecular Perspectives; Roychoudhury, A., Tripathi, D.K., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2020; pp. 24–52. [Google Scholar]
- Mäkelä, P.; Jokinen, K.; Kontturi, M.; Peltonen-Sainio, P.; Pehu, E.; Somersalo, S. Foliar application of glycinebetaine—A novel product from sugar beet—As an approach to increase tomato yield. Ind. Crop. Prod. 1998, 7, 139–148. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, S.; Zhang, H.; Zhao, Y.; Zhao, H.; Liu, H. Glycine betaine application in grain filling wheat plants alleviates heat and high light-induced photoinhibition by enhancing the psbA transcription and stomatal conductance. Acta Physiol. Plant. 2014, 36, 2195–2202. [Google Scholar] [CrossRef]
- Moussa, H.R.; Jaleel, C.A. Physiological effects of glycinebetaine on gamma-irradiated stressed fenugreek plants. Acta Physiol. Plant. 2011, 33, 1135–1140. [Google Scholar] [CrossRef]
- Mohammed, A.R.; Tarpley, L. Effects of enhanced ultraviolet-B (UV-B) radiation and antioxidative-type plant growth regulators on rice (Oryza sativa L.) leaf photosynthetic rate, photochemistry and physiology. J. Agric. Sci. 2013, 5, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Glenn, D.M. Effect of highly processed calcined kaolin residues on apple water use efficiency. Sci. Hortic. 2016, 205, 127–132. [Google Scholar] [CrossRef]
- Gharaghani, A.; Javarzari, A.M.; Vahdati, K. Kaolin particle film alleviates adverse effects of light and heat stresses and improves nut and kernel quality in Persian walnut. Sci. Hortic. 2018, 239, 35–40. [Google Scholar] [CrossRef]
- Da Silva, P.S.O.; Oliveira, L.F.G.; Silva Gonzaga, M.I.; de Oliveira Alves Sena, E.; dos Santos Maciel, L.B.; Pinheiro Fiaes, M.; de Mattos, E.C.; Gutierrez Carnelossi, M.A. Effects of calcium particle films and natural shading on ecophysiological parameters of conilon coffee. Sci. Hortic. 2019, 245, 171–177. [Google Scholar] [CrossRef]
- Da Silva, P.S.O.; de Oliveira Alves Sena, E.; Silva Gonzaga, M.I.; Ganassali de Oliveira, L.F.; dos Santos Maciel, L.B.; Pinheiro Fiaes dos Santos, M.; de Mattos, E.C.; Lima Dias, K.L.; Botelho Carneiro, R.; Gutierrez Carnelossi, M.A. Calcium carbonate particle films and water regimes affect the acclimatization, ecophysiology and reproduction of tomato. Environ. Exp. Bot. 2019, 165, 19–29. [Google Scholar] [CrossRef]
- Zaman, L.; Shafqat, W.; Qureshi, A.; Sharif, N.; Raza, K.; Ud Din, S.; Ikram, S.; Jaskani, M.J.; Muhammad, K. Effect of foliar spray of zinc sulphate and calcium carbonate on fruit quality of Kinnow mandarin (Citrus reticulata Blanco). J. Glob. Innov. Agric. Soc. Sci. 2019, 7, 157–161. [Google Scholar] [CrossRef]
- Sadak, M.S.; Talaat, I.M. Attenuation of negative effects of saline stress in wheat plant by chitosan and calcium carbonate. Bull. Natl. Res. Cent. 2021, 45, 136. [Google Scholar] [CrossRef]
- Cvetkovska, M.; Rampitsch, C.; Bykova, N.; Xing, T. Genomic analysis of MAP kinase cascades in Arabidopsis defense responses. Plant. Mol. Biol. Rep. 2005, 23, 331–343. [Google Scholar] [CrossRef]
- Havaux, M.; Eymery, F.; Porfirova, S.; Rey, P.; Dörmann, P. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell 2005, 17, 3451–3469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munné-Bosch, S. The role of α-tocopherol in plant stress tolerance. J. Plant Physiol. 2005, 162, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Semchuk, N.M.; Vasylyk, Y.V.; Kubrak, O.I.; Lushchak, V.I. Effect of sodium nitroprusside and S-nitrosoglutathione on pigment content and antioxidant system of tocopherol-deficient plants of Arabidopsis thaliana. Ukr. Biokhim. Zh. 2011, 83, 69–79. [Google Scholar]
- Macrae, E.; Quick, W.P.; Benker, C.; Stitt, M. Carbohydrate metabolism during postharvest ripening in kiwifruit. Planta 1992, 188, 314–323. [Google Scholar] [CrossRef]
- Roussos, P.; Ntanos, E.; Tsafouros, A.; Denaxa, N.-K. Strawberry physiological and biochemical responses to chilling and freezing stress and application of alleviating factors as countermeasures. J. Berry Res. 2020, 10, 437–457. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Shahid, M.A. Plant Stress Mitigators: Types, Techniques and Functions, 1st ed.; Academic Press: Cambridge, MA, USA, 2023; p. 540. [Google Scholar]
- Sotiropoulos, T.; Petridis, A.; Koukourikou-Petridou, M.; Koundouras, S. Evaluation of “Sun Protect” in protecting apples (Malus × domestica Borkh.) against sunburn. Hortic. Sci. 2016, 43, 175–180. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dutta, T.; Neelapu, N.R.R.; Wani, S.H.; Surekha, C. Role and regulation of osmolytes as signaling molecules to abiotic stress tolerance. In Plant Signaling Molecules; Elsevier: Amsterdam, The Netherlands, 2019; pp. 459–477. [Google Scholar] [CrossRef]
- Delfani, K.; Asadi, M.; Golein, B.; Babakhani, B.; Jadid, R.R. Foliar application of glycine betaine affects morpho-physiological, biochemical and fruit quality traits of Thomson Navel orange under deficit irrigation. J. Plant Growth Regul. 2022, 1–17. [Google Scholar] [CrossRef]
- Khalid, M.; Rehman, H.M.; Ahmed, N.; Nawaz, S.; Saleem, F.; Ahmad, S.; Uzair, M.; Rana, I.A.; Atif, R.M.; Zaman, Q.U.; et al. Using Exogenous Melatonin, Glutathione, Proline, and Glycine Betaine Treatments to Combat Abiotic Stresses in Crops. Int. J. Mol. Sci. 2022, 23, 12913. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, M.; Aït Barka, E.; Clément, C.; Vaillant-Gaveau, N.; Jacquard, C. Cross-talk between environmental stresses and plant metabolism during reproductive organ abscission. J. Exp. Bot. 2015, 66, 1707–17019. [Google Scholar] [CrossRef] [Green Version]
- Basile, B.; Giaccone, M.; Cirillo, C.; Ritieni, A.; Graziani, G.; Shahak, Y.; Forlani, M. Photo-selective hail nets affect fruit size and quality in Hayward kiwifruit. Sci. Hortic. 2012, 141, 91–97. [Google Scholar] [CrossRef]
- Xu, F.; An, H.; Zhang, J.; Xu, Z.; Jiang, F. Effects of fruit load on sugar/acid quality and puffiness of delayed-harvest citrus. Horticulturae 2021, 7, 189. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Mujumdar, A.S.; Jin, X.; Liu, Z.-L.; Zhang, Y.; Xiao, H.-W. Effects of postharvest ripening on physicochemical properties, microstructure, cell wall polysaccharides contents (pectin, hemicellulose, cellulose) and nanostructure of kiwifruit (Actinidia deliciosa). Food Hydrocoll. 2021, 118, 106808. [Google Scholar] [CrossRef]
- Do Amarante, C.V.T.; Stanger, M.C.; Coldebella, M.C.; Vilvert, J.C.; dos Santos, A.; Steffens, C.A. Fruit quality and yield of ‘Imperial Gala’ apple trees protected by anti-hail nets of different colorations in southern Brazil. Acta Hortic. 2018, 1205, 897–904. [Google Scholar] [CrossRef]
- Vukovic, M.; Brkljaca, M.; Rumora, J.; Fruk, M.; Jatoi, M.A.; Jemric, T. Vegetative and reproductive traits of young peaches and nectarines grown under red photoselective net. Agric. Conspec. Sci. 2016, 81, 181–185. [Google Scholar]
- Mditshwa, A.; Magwaza, L.S.; Tesfay, S.Z. Shade netting on subtropical fruit: Effect on environmental conditions, tree physiology and fruit quality. Sci. Hortic. 2019, 256, 108556. [Google Scholar] [CrossRef]
- Ntanos, E.; Tsafouros, A.; Denaxa, N.-K.; Kosta, A.; Bouchagier, P.; Roussos, P.A. Mitigation of high solar irradiance and heat stress in kiwifruit during summer via the use of alleviating products with different modes of action—Part 1 Effects on leaf physiology and biochemistry. Agriculture 2022, 12, 2121. [Google Scholar] [CrossRef]
- Gullo, G.; Dattola, A.; Liguori, G.; Vonella, V.; Zappia, R.; Inglese, P. Evaluation of fruit quality and antioxidant activity of kiwifruit during ripening and after storage. J. Berry Res. 2016, 6, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Rivera-López, J.; Vázquez-Ortiz, F.A.; Ayala-Zavala, J.F.; Sotelo-Mundo, R.R.; González-Aguilar, G.A. Cutting shape and storage temperature affect overall quality of fresh-cut papaya cv.‘Maradol’. J. Food Sci. 2005, 70, 482–489. [Google Scholar] [CrossRef]
- Tavarini, S.; Degl’Innocenti, E.; Remorini, D.; Massai, R.; Guidi, L. Antioxidant capacity, ascorbic acid, total phenols and carotenoids changes during harvest and after storage of Hayward kiwifruit. Food Chem. 2008, 107, 282–288. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Razavi, F.; Rabiei, V.; Gohari, G.; Palou, L. Application of Glycine betaine coated chitosan nanoparticles alleviate chilling injury and maintain quality of plum (Prunus domestica L.) fruit. Int. J. Biol. Macromol. 2022, 207, 965–977. [Google Scholar] [CrossRef]
- Salinger, M.; Kenny, G.; Morley-Bunker, M. Climate and kiwifruit cv. Hayward 1. Influences on development and growth. N. Z. J. Crop Hortic. Sci. 1993, 21, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Thind, S. Foliar application of glycine betaine alters sugar metabolism of wheat leaves under prolonged field drought stress. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2019, 89, 877–884. [Google Scholar] [CrossRef]
- Walton, E.F.; de Jong, T.M. Estimating the bioenergetic cost of a developing kiwifruit berry and its growth and maintenance respiration components. Ann. Bot. 1990, 66, 417–424. [Google Scholar] [CrossRef]
- Macrae, E.A.; Lallu, N.; Searle, A.N.; Bowen, J.H. Changes in the softening and composition of kiwifruit (Actinidia deliciosa) affected by maturity at harvest and postharvest treatments. J. Sci. Food Agric. 1989, 49, 413–430. [Google Scholar] [CrossRef]
- Habibi, F.; Valero, D.; Serrano, M.; Guillén, F. Exogenous application of glycine betaine maintains bioactive compounds, antioxidant activity, and physicochemical attributes of blood orange fruit during prolonged cold storage. Front. Nutr. 2022, 9, 873915. [Google Scholar] [CrossRef] [PubMed]
- Batista-Silva, W.; Nascimento, V.L.; Medeiros, D.B.; Nunes-Nesi, A.; Ribeiro, D.M.; Zsögön, A.; Araújo, W.L. Modifications in organic acid profiles during fruit development and ripening: Correlation or causation? Front. Plant Sci. 2018, 9, 1689. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef] [Green Version]
- Aghdam, M.S.; Bodbodak, S. Postharvest Heat Treatment for Mitigation of Chilling Injury In Fruits and Vegetables. Food Bioprocess Technol. 2014, 7, 37–53. [Google Scholar] [CrossRef]
- Smith, G.S.; Buwalda, J.G. Kiwifruit. In Handbook of Environmental Physiology of Fruit Crops; CRC Press: Boca Raton, FL, USA, 2018; pp. 135–163. [Google Scholar]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Impact of temperature on phenolic and osmolyte contents in in vitro cultures and micropropagated plants of two mediterranean plant species, Lavandula viridis and Thymus lotocephalus. Plants 2022, 11, 3516. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A.; Aziz, A. Adaptive response of a native Mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 2020, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The role of polyphenols in abiotic stress response: The influence of molecular structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Hosseini, M.S.; Abadia, J.; Marjani, M. Melatonin foliar sprays elicit salinity stress tolerance and enhance fruit yield and quality in strawberry (Fragaria ananassa Duch.). Plant Physiol. Biochem. 2020, 149, 313–323. [Google Scholar] [CrossRef]
- Wang, L.; Shan, T.; Xie, B.; Ling, C.; Shao, S.; Jin, P.; Zheng, Y. Glycine betaine reduces chilling injury in peach fruit by enhancing phenolic and sugar metabolisms. Food Chem. 2019, 272, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, H.L.; Di Bella, C.M.; Colavita, G.M.; Oricchio, P.; Straschnoy, J. Comparative effects of kaolin and calcium carbonate on apple fruit surface temperature and leaf net CO2 assimilation. J. Appl. Hortic. 2015, 17, 176–180. [Google Scholar] [CrossRef]
- Kalt, W. Effects of production and processing factors on major fruit and vegetable antioxidants. J. Food Sci. 2005, 70, 11–19. [Google Scholar] [CrossRef]
- Blankenship, S.M.; Richardson, D.G. Changes in phenolic acids and internal ethylene during long-term cold storage of pear. J. Am. Soc. Hortic. Sci. 1985, 110, 33. [Google Scholar] [CrossRef]
- Connor, A.M.; Luby, J.J.; Hancock, J.F.; Berkheimer, S.; Hanson, E.J. Changes in fruit antioxidant activity among blueberry cultivars during cold-temperature storage. J. Agric. Food Chem. 2002, 50, 893–898. [Google Scholar] [CrossRef]
- Van der Sluis, A.A.; Dekker, M.; de Jager, A.; Jongen, W.M.F. 2001. Activity and concentration of polyphenolic antioxidants in apple: Effect of cultivar, harvest year, and storage conditions. J. Agric. Food Chem. 2001, 49, 3606–3613. [Google Scholar] [CrossRef] [PubMed]
- Kevers, C.; Falkowski, M.; Tabart, J.; Defraigne, J.O.; Dommes, J.; Pincemail, J. Evolution of antioxidant capacity during storage of selected fruit and vegetables. J. Agric. Food Chem. 2007, 55, 8596–8603. [Google Scholar] [CrossRef]
- Kim, A.-N.; Kim, H.-J.; Chun, J.; Heo, H.J.; Kerr, W.L.; Choi, S.-G. Degradation kinetics of phenolic content and antioxidant activity of hardy kiwifruit (Actinidia arguta) puree at different storage temperatures. LWT 2018, 89, 535–541. [Google Scholar] [CrossRef]
- Krupa, T.; Latocha, P.; Liwińska, A. Changes of physicochemical quality, phenolics and vitamin C content in hardy kiwifruit (Actinidia arguta and its hybrid) during storage. Sci. Hortic. 2011, 130, 410–417. [Google Scholar] [CrossRef]
- Bang, I.H.; Kim, Y.E.; Min, S.C. Preservation of mandarins using a microbial decontamination system integrating calcium oxide solution washing, modified atmosphere packaging, and dielectric barrier discharge cold plasma treatment. Food Packag. Shelf Life 2021, 29, 100682. [Google Scholar] [CrossRef]
- Supapvanich, S.; Arkajak, R.; Yalai, K. Maintenance of postharvest quality and bioactive compounds of fresh-cut sweet leaf bush (Sauropus androgynus L. Merr.) through hot CaCl2 dips. Int. J. Food Sci. 2012, 47, 2662–2670. [Google Scholar] [CrossRef]
- Ziogas, V.; Bravos, N.; Hussain, S.B. Preharvest foliar application of Si–Ca-based biostimulant affects postharvest quality and shelf-life of clementine mandarin (Citrus clementina Hort. Ex Tan). Horticulturae 2022, 8, 996. [Google Scholar] [CrossRef]
- Cid-López, M.L.; Soriano-Melgar, L.D.A.A.; García-González, A.; Cortéz-Mazatán, G.; Mendoza-Mendoza, E.; Rivera-Cabrera, F.; Peralta-Rodríguez, R.D. The benefits of adding calcium oxide nanoparticles to biocompatible polymeric coatings during cucumber fruits postharvest storage. Sci. Hortic. 2021, 287, 110285. [Google Scholar] [CrossRef]

| Treatment | Weight | Diameter | Length | Firmness | Dry Matter | Total Yield/Vine |
|---|---|---|---|---|---|---|
| *** | *** | *** | ns | ns | *** | |
| Control | 131.47 ab | 57.66 a | 75.96 a | 27.94 a | 16.67 a | 39.69 b |
| BlueStim | 117.91 c | 54.97 b | 72.69 b | 28.94 a | 16.19 a | 46.38 a |
| Purshade | 124.86 bc | 56.95 a | 73.68 b | 26.52 a | 16.23 a | 42.81 ab |
| Sun Protect | 137.78 a | 57.93 a | 76.91 a | 30.15 a | 16.28 a | 43.38 ab |
| Treatment | Weight | Diameter | Length | Firmness | Dry Matter |
|---|---|---|---|---|---|
| ns | ns | ns | ** | ns | |
| Control | 124.13 a | 55.55 a | 74.20 a | 11.81 b | 16.95 a |
| BlueStim | 112.90 a | 54.08 a | 72.71 a | 11.86 b | 17.28 a |
| Purshade | 118.98 a | 55.84 a | 72.37 a | 13.01 ab | 17.26 a |
| Sun Protect | 135.18 a | 56.20 a | 72.23 a | 15.71 a | 17.08 a |
| Treatment | pH | TSS | TA | TSS:TA |
|---|---|---|---|---|
| ns | ** | ns | ns | |
| Control | 3.48 a | 7.16 a | 2.40 a | 3.09 a |
| BlueStim | 3.39 a | 6.55 b | 2.22 a | 2.96 a |
| Purshade | 3.42 a | 6.85 ab | 2.60 a | 2.72 a |
| Sun Protect | 3.53 a | 6.75 ab | 2.21 a | 3.06 a |
| Treatment | pH | TSS | TA | TSS:TA |
|---|---|---|---|---|
| ns | ns | ns | ns | |
| Control | 3.39 a | 13.98 a | 1.90 a | 7.47 a |
| BlueStim | 3.24 a | 13.60 a | 1.71 a | 7.95 a |
| Purshade | 3.33 a | 14.08 a | 1.82 a | 7.77 a |
| Sun Protect | 3.26 a | 14.10 a | 1.87 a | 7.58 a |
| Treatment | Harvest | Storage | ||||
|---|---|---|---|---|---|---|
| FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | |
| *** | *** | *** | * | * | * | |
| Control | 4.40 c | 2.90 b | 6.43 b | 1.69 b | 1.44 b | 3.32 b |
| BlueStim | 5.32 a | 3.01 b | 6.75 b | 1.99 ab | 1.64 ab | 3.53 ab |
| Purshade | 5.61 a | 3.31 a | 7.70 a | 2.10 a | 1.96 a | 3.75 a |
| Sun Protect | 4.88 b | 2.89 b | 6.40 b | 1.72 b | 1.57 b | 3.41 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denaxa, N.-K.; Tsafouros, A.; Ntanos, E.; Kosta, A.; Roussos, P.A. Mitigation of High Solar Irradiance and Heat Stress in Kiwifruit during Summer via the Use of Alleviating Products with Different Modes of Action—Part 2 Effects on Fruit Quality, Organoleptic, and Phytochemical Properties at Harvest and after Storage. Agriculture 2023, 13, 701. https://doi.org/10.3390/agriculture13030701
Denaxa N-K, Tsafouros A, Ntanos E, Kosta A, Roussos PA. Mitigation of High Solar Irradiance and Heat Stress in Kiwifruit during Summer via the Use of Alleviating Products with Different Modes of Action—Part 2 Effects on Fruit Quality, Organoleptic, and Phytochemical Properties at Harvest and after Storage. Agriculture. 2023; 13(3):701. https://doi.org/10.3390/agriculture13030701
Chicago/Turabian StyleDenaxa, Nikoleta-Kleio, Athanassios Tsafouros, Efstathios Ntanos, Anna Kosta, and Peter Anargyrou Roussos. 2023. "Mitigation of High Solar Irradiance and Heat Stress in Kiwifruit during Summer via the Use of Alleviating Products with Different Modes of Action—Part 2 Effects on Fruit Quality, Organoleptic, and Phytochemical Properties at Harvest and after Storage" Agriculture 13, no. 3: 701. https://doi.org/10.3390/agriculture13030701





