The Quantity and Quality of Humic Substances following Different Land Uses in Karst Peak-Cluster Depression in Guangxi, China
Abstract
:1. Introduction
2. Materials and Methods
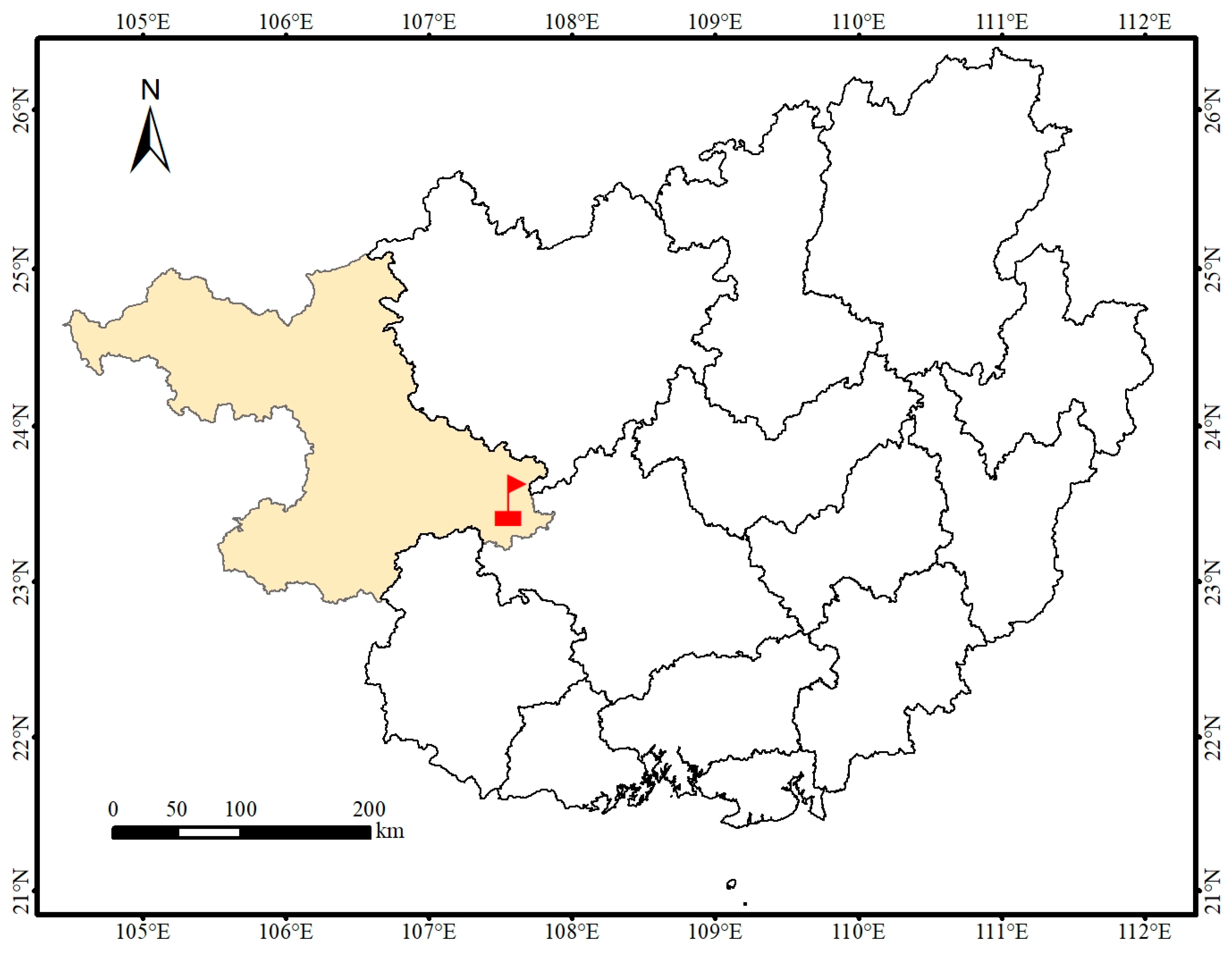
2.1. Site Description and Soil Collection
2.2. Quantitative Analysis and Extraction of Humic Substances
2.3. Elemental Compositions
2.4. The Solid State 13C NMR Analysis
2.5. Statistical Analysis
3. Results and Discussion
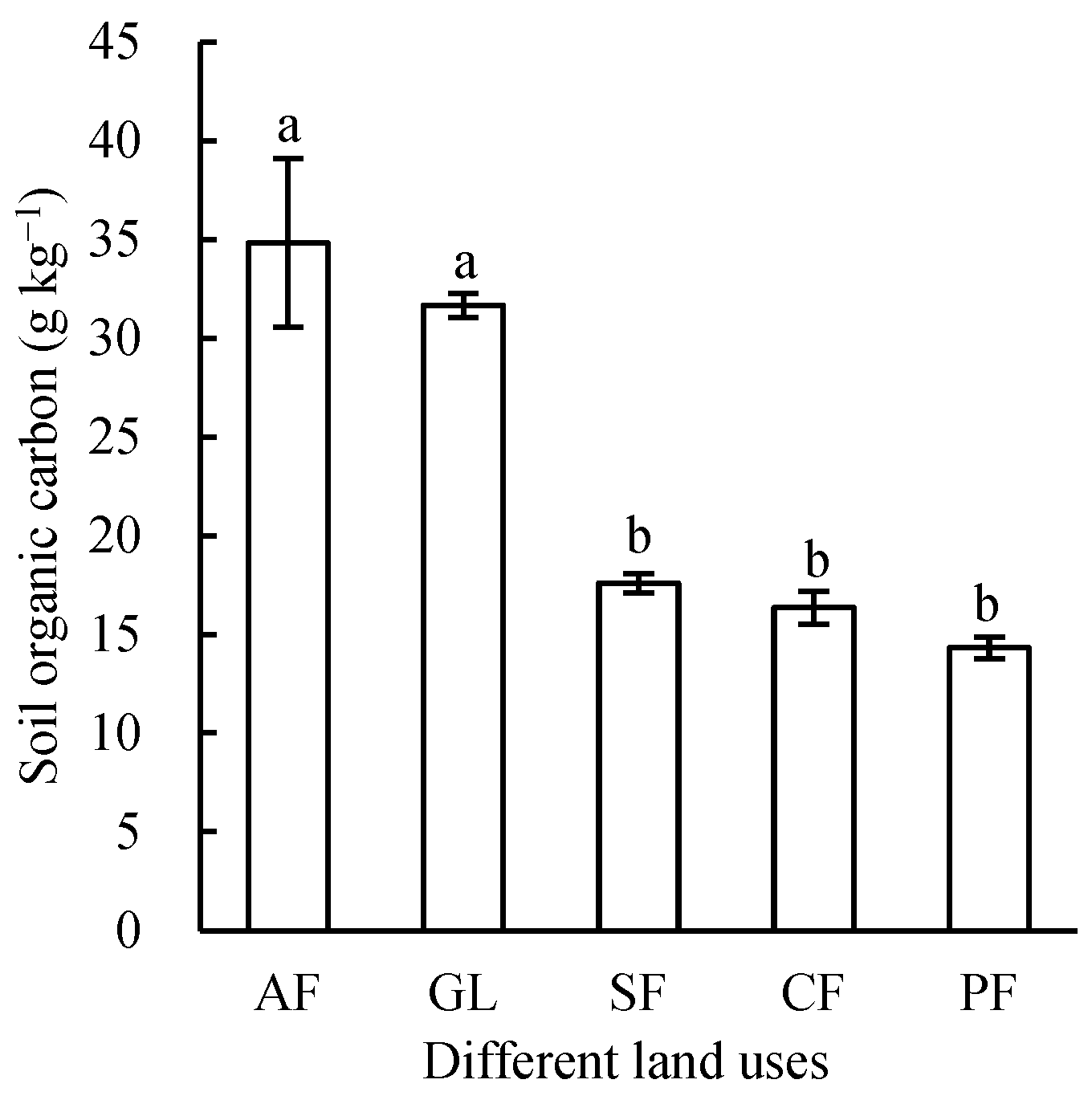
3.1. Effects of Different Land Uses on Soil Organic Carbon
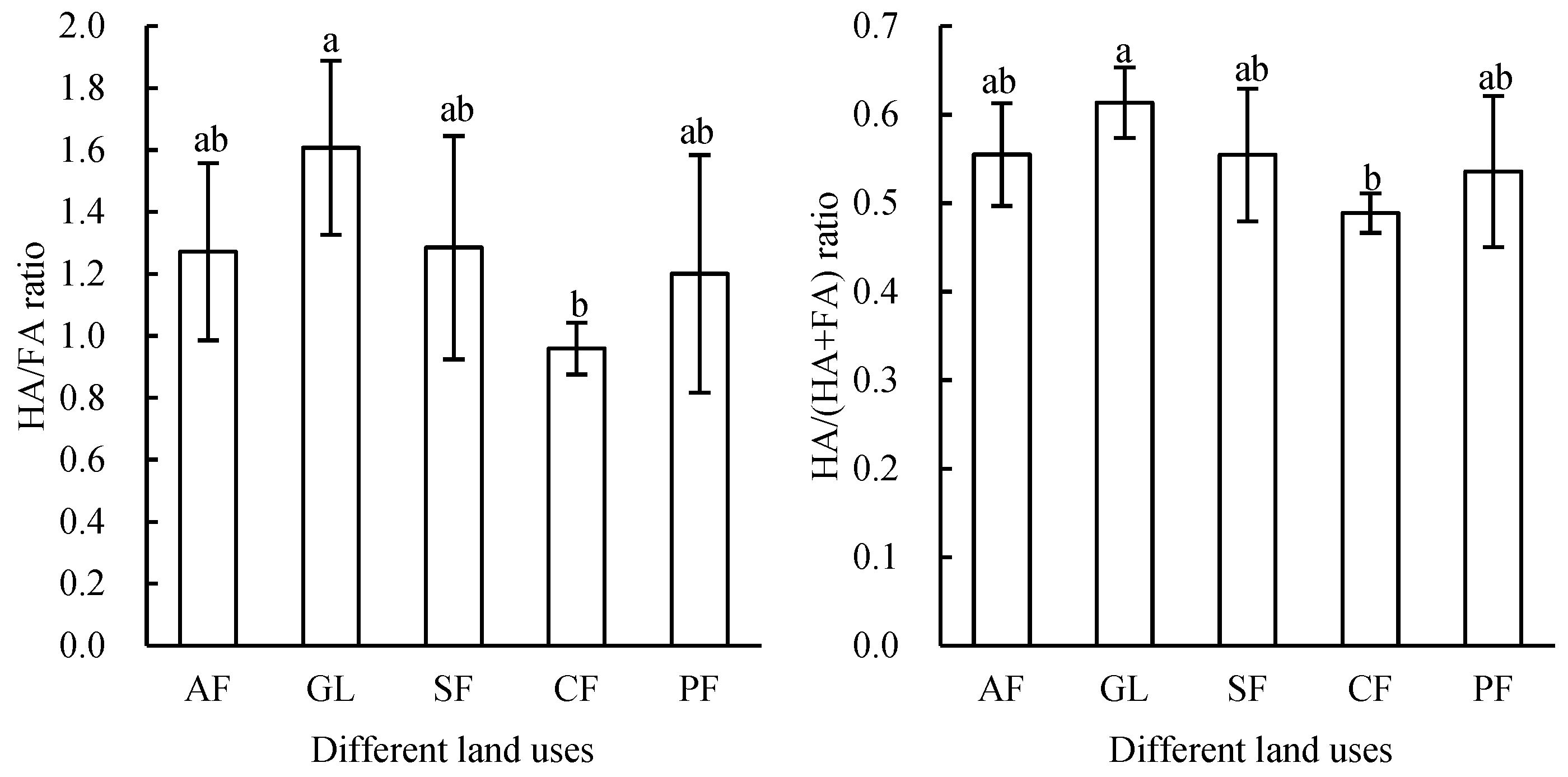
3.2. Effects of Different Land Uses on Humic Fractions
3.3. Effects of Different Land Uses on Elemental Compositions of Humic Fractions
3.4. Structural Diversity Analysis of Humic Substances Observed by 13C NMR
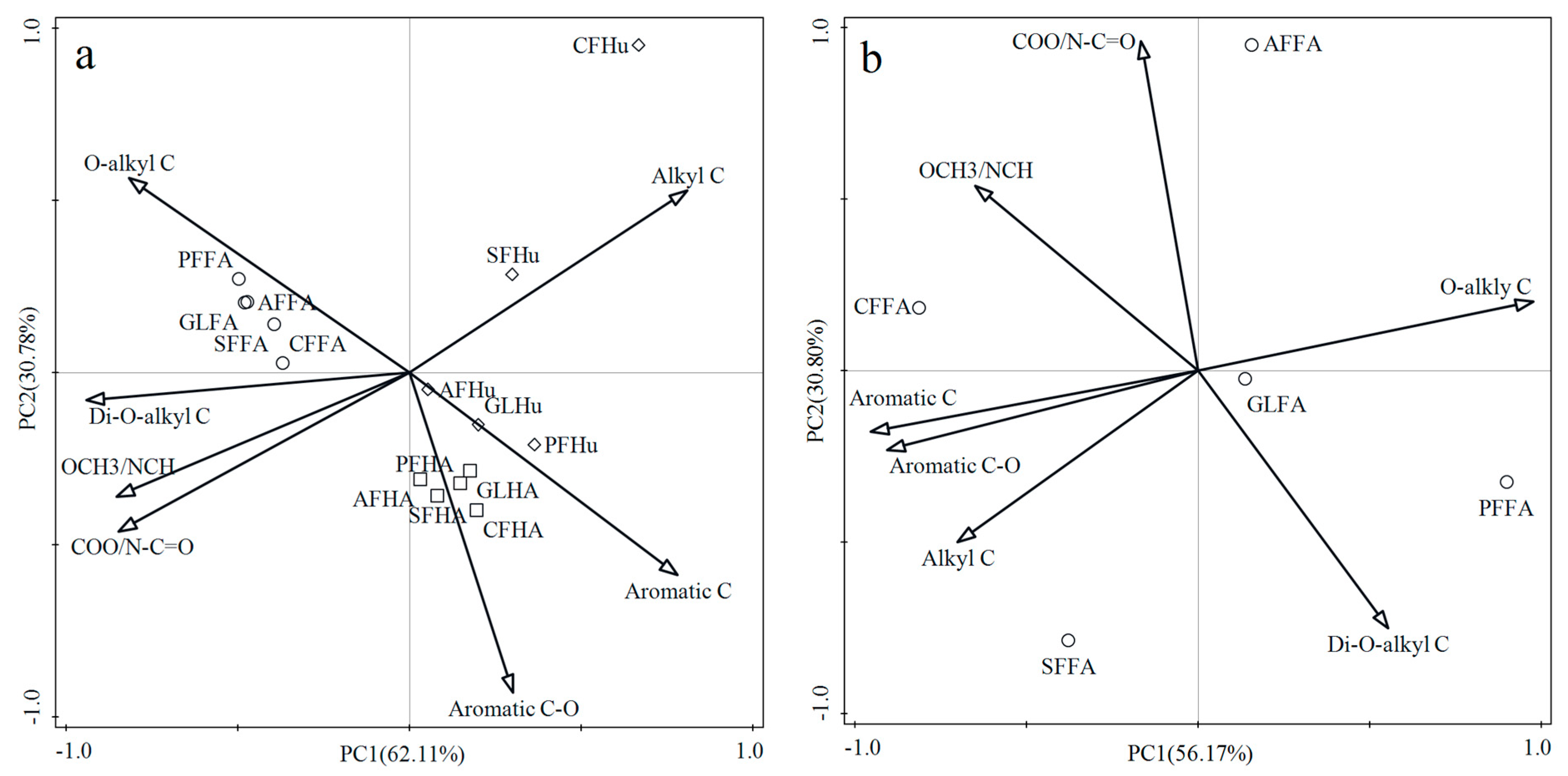
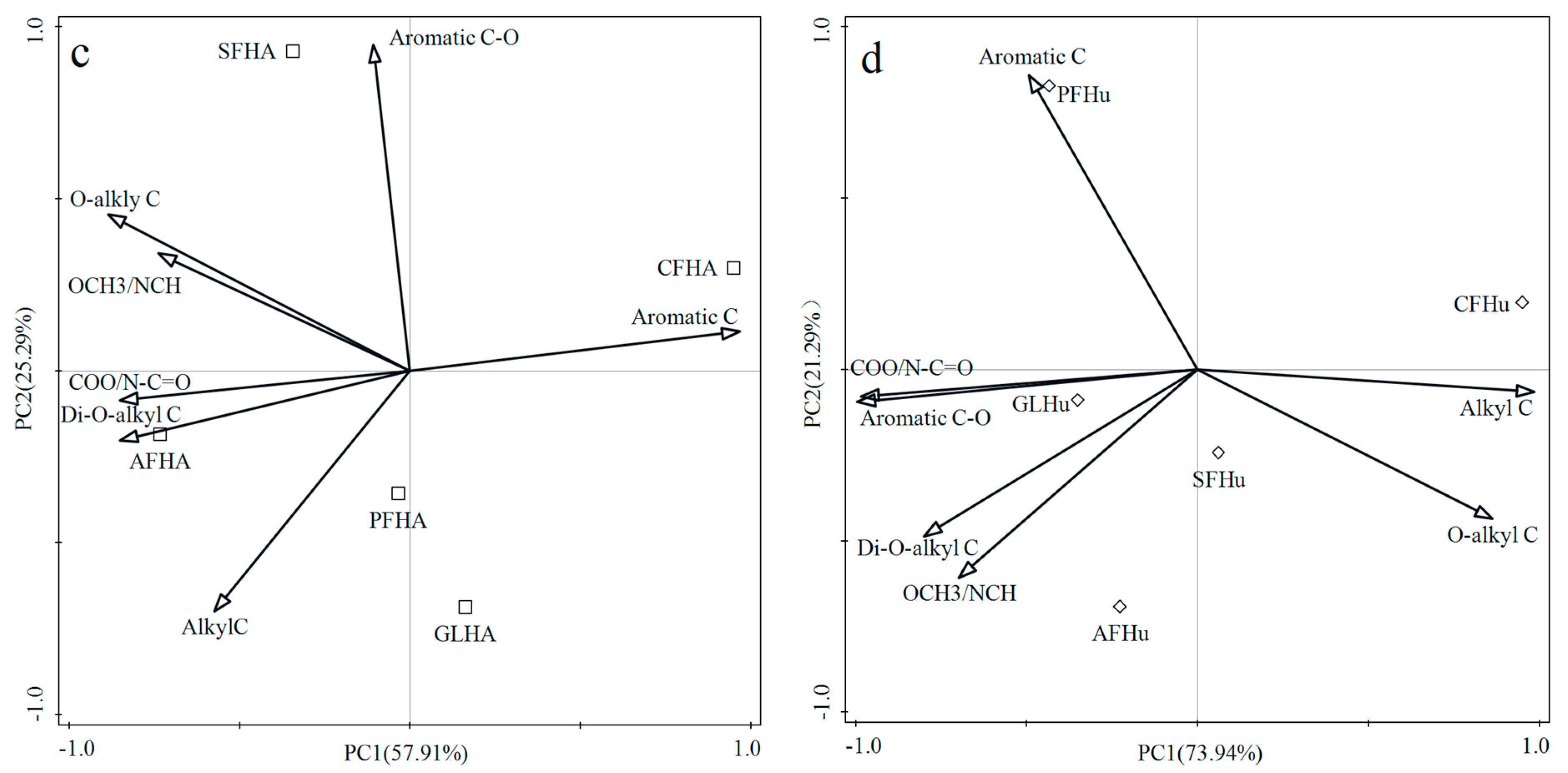
3.5. The Principal Component Analysis (PCA) for Humic Substances Based on the Organic Carbon Functional Groups as Revealed by 13C NMR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chowdhury, S.; Bolan, N.; Farrell, M.; Sarkar, B.; Sarker, J.R.; Kirkham, M.B.; Hossain, M.Z.; Kim, G.-H. Chapter Two—Role of cultural and nutrient management practices in carbon sequestration in agricultural soil. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: New York, NY, USA, 2021; Volume 166, pp. 131–196. [Google Scholar]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Tinoco, P.; Almendros, G.; González-Vila, F.J.; Sanz, J.; González-Pérez, J.A. Revisiting molecular characteristics responsive for the aromaticity of soil humic acids. J. Soils Sediments 2015, 15, 781–791. [Google Scholar] [CrossRef]
- De Nobili, M.; Bravo, C.; Chen, Y. The spontaneous secondary synthesis of soil organic matter components: A critical examination of the soil continuum model theory. Appl. Soil. Ecol. 2020, 154, 103655. [Google Scholar] [CrossRef]
- Dou, S.; Shan, J.; Song, X.; Cao, R.; Wu, M.; Li, C.; Guan, S. Are humic substances soil microbial residues or unique synthesized compounds? A perspective on their distinctiveness. Pedosphere 2020, 30, 159–167. [Google Scholar] [CrossRef]
- Berg, B.; Mcclaugherty, C. Plant Litter, Decomposition, Humus Formation, Carbon Sequestration, 4th ed.; Springer Nature Switzerland AG: Cham, Switzerland, 2020. [Google Scholar]
- Ding, Y.; Nie, Y.; Chen, H.; Wang, K.; Querejeta, J.I. Water uptake depth is coordinated with leaf water potential, water-use efficiency and drought vulnerability in karst vegetation. New Phytol. 2021, 229, 1339–1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, C.; Chen, H.; Yue, Y.; Zhang, W.; Zhang, M.; Qi, X.; Fu, Z. Karst landscapes of China: Patterns, ecosystem processes and services. Landsc. Ecol. 2019, 34, 2743–2763. [Google Scholar] [CrossRef]
- Jiang, Z.; Lian, Y.; Qin, X. Rocky desertification in Southwest China: Impacts, causes, and restoration. Earth Sci. Rev. 2014, 132, 1–12. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Xu, X.; Kuang, W.; Zhou, W.; Zhang, S.; Li, R.; Yan, C.; Yu, D.; Wu, S.; et al. Spatial patterns and driving forces of land use change in China during the early 21st century. J. Geogr. Sci. 2010, 20, 483–494. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Swift, R.S. Vindication of humic substances as a key component of organic matter in soil and water. In Advances in Agronomy; Academic Press: New York, NY, USA, 2020; Volume 163, pp. 1–37. [Google Scholar]
- Wang, Z.; Yue, F.; Xue, L.; Wang, Y.; Qin, C.; Zeng, J.; Ding, H.; Fu, Y.; Li, S. Soil nitrogen transformation in different land use and implications for karst soil nitrogen loss controlling. Catena 2023, 225, 107026. [Google Scholar] [CrossRef]
- Gupta, M.K.; Sharma, S.D.; Kumar, M. Sequestered organic carbon stock in the soils under different land uses in western region of Haryana. Indian. For. 2015, 141, 718–725. [Google Scholar]
- Martí-Roura, M.; Hagedorn, F.; Rovira, P.; Romanyà, J. Effect of land use and carbonates on organic matter stabilization and microbial communities in Mediterranean soils. Geoderma 2019, 351, 103–115. [Google Scholar] [CrossRef]
- Poeplau, C.; Don, A.; Vesterdal, L.; Leifeld, J.; Van Wesemael, B.; Schumacher, J.; Gensior, A. Temporal dynamics of soil organic carbon after land-use change in the temperate zone–carbon response functions as a model approach. Glob. Chang. Biol. 2011, 17, 2415–2427. [Google Scholar] [CrossRef]
- Han, X.; Zhao, F.; Tong, X.; Deng, J.; Yang, G.; Chen, L.; Kang, D. Understanding soil carbon sequestration following the afforestation of former arable land by physical fractionation. Catena 2017, 150, 317–327. [Google Scholar] [CrossRef]
- Zhong, Z.; Han, X.; Xu, Y.; Zhang, W.; Fu, S.; Liu, W.; Ren, C.; Yang, G.; Ren, G. Effects of land use change on organic carbon dynamics associated with soil aggregate fractions on the Loess Plateau, China. Land. Degrad. Dev. 2019, 30, 1070–1082. [Google Scholar] [CrossRef]
- Volikov, A.B.; Kholodov, V.A.; Kulikova, N.A.; Philippova, O.I.; Ponomarenko, S.A.; Lasareva, E.V.; Parfyonova, A.M.; Hatfield, K.; Perminova, I.V. Silanized humic substances act as hydrophobic modifiers of soil separates inducing formation of water-stable aggregates in soils. Catena 2016, 137, 229–236. [Google Scholar] [CrossRef]
- Deng, S.; Zheng, X.; Chen, X.; Zheng, S.; He, X.; Ge, T.; Kuzyakov, Y.; Wu, J.; Su, Y.; Hu, Y. Divergent mineralization of hydrophilic and hydrophobic organic substrates and their priming effect in soils depending on their preferential utilization by bacteria and fungi. Biol. Fert. Soils 2021, 57, 65–76. [Google Scholar] [CrossRef]
- Ma, L.; Xiao, B.; Di, X.; Huang, W.; Wang, S. Characteristics and distributions of humic acids in two soil profiles of the southwest China Karst area. Acta Geochim. 2016, 35, 85–94. [Google Scholar] [CrossRef]
- Dou, S. Soil Organic Matter; Science Press: Beijing, China, 2010. [Google Scholar]
- Cui, T.; Li, Z.; Wang, S. Effects of in-situ straw decomposition on composition of humus and structure of humic acid at different soil depths. J. Soils Sediments 2017, 17, 2391–2399. [Google Scholar] [CrossRef]
- Kuwatsuka, S.; Watanabe, A.; Itoh, K.; Arai, S. Comparison of two methods of preparation of humic and fulvic acids, IHSS method and NAGOYA method. Soil. Sci. Plant Nutr. 1992, 38, 23–30. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Wang, X.; Huang, S.; Sun, K.; Xia, X. Differences in structure and composition of soil humic substances and their binding for polycyclic aromatic hydrocarbons in different climatic zones. Environ. Pollut. 2023, 322, 121121. [Google Scholar] [CrossRef]
- dos Santos, J.V.; Fregolente, L.G.; Mounier, S.; Hajjoul, H.; Ferreira, O.P.; Moreira, A.B.; Bisinoti, M.C. Fulvic acids from Amazonian anthropogenic soils: Insight into the molecular composition and copper binding properties using fluorescence techniques. Ecotoxicol. Environ. Safe 2020, 205, 111173. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Han, J.; Xue, J.; Hatten, J.A.; Wang, M.; Guo, Y.; Li, P. Soil organic carbon pool and chemical composition under different types of land use in wetland: Implication for carbon sequestration in wetlands. Sci. Total Environ. 2020, 716, 136996. [Google Scholar] [CrossRef] [PubMed]
- de Aguiar, T.C.; de Oliveira Torchia, D.F.; van Tol de Castro, T.A.; Tavares, O.C.H.; de Abreu Lopes, S.; de Souza da Silva, L.; Castro, R.N.; Berbara, R.L.L.; Pereira, M.G.; García, A.C. Spectroscopic–chemometric modeling of 80 humic acids confirms the structural pattern identity of humified organic matter despite different formation environments. Sci. Total Environ. 2022, 833, 155133. [Google Scholar] [CrossRef]
- Gogoi, A.; Sahoo, U.K.; Saikia, H. Vegetation and ecosystem carbon recovery following shifting cultivation in Mizoram-Manipur-Kachin rainforest eco-region, Southern Asia. Ecol. Process 2020, 9, 21. [Google Scholar] [CrossRef]
- Ahirwal, J.; Gogoi, A.; Sahoo, U.K. Stability of soil organic carbon pools affected by land use and land cover changes in forests of eastern Himalayan region, India. Catena 2022, 215, 106308. [Google Scholar] [CrossRef]
- Bai, X.; Guo, Z.; Huang, Y.; An, S. Root cellulose drives soil fulvic acid carbon sequestration in the grassland restoration process. Catena 2020, 191, 104575. [Google Scholar] [CrossRef]
- Baer, S.G.; Kitchen, D.J.; Blair, J.M.; Rice, C.W. Changes in Ecosystem Structure and Function along a Chronosequence of Restored Grasslands. Ecol. Appl. 2002, 12, 1688–1701. [Google Scholar] [CrossRef]
- Lal, R.; Negassa, W.; Lorenz, K. Carbon sequestration in soil. Curr. Opin. Environ. Sustain. 2015, 15, 79–86. [Google Scholar] [CrossRef]
- Mishra, G.; Sarkar, A. Studying the relationship between total organic carbon and soil carbon pools under different land management systems of Garo hills, Meghalaya. J. Environ. Manag. 2020, 257, 110002. [Google Scholar] [CrossRef]
- Lungmuana; Singh, S.B.; Vanthawmliana; Saha, S.; Dutta, S.K.; Rambuatsaiha; Singh, A.R.; Boopathi, T. Impact of secondary forest fallow period on soil microbial biomass carbon and enzyme activity dynamics under shifting cultivation in North Eastern Hill region, India. Catena 2017, 156, 10–17. [Google Scholar] [CrossRef]
- Chu, L.; Grafton, R.Q.; Nguyen, H. A global analysis of the break-even prices to reduce atmospheric carbon dioxide via forest plantation and avoided deforestation. Policy Econ. 2022, 135, 102666. [Google Scholar] [CrossRef]
- Guimarães, D.V.; Gonzaga, M.I.S.; da Silva, T.O.; da Silva, T.L.; da Silva Dias, N.; Matias, M.I.S. Soil organic matter pools and carbon fractions in soil under different land uses. Soil. Tillage Res. 2013, 126, 177–182. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Wang, Y.; Zheng, N.; Wu, X.; Liu, Q. Effect of long-term cultivation on soil organic carbon fractions and metal distribution in humic and fulvic acid in black soil, Northeast China. Soil. Res. 2012, 50, 562–569. [Google Scholar] [CrossRef]
- Loke, P.F.; Kotzé, E.; Preez, C.C.; Twigge, L. Dynamics of Soil Carbon Concentrations and Quality Induced by Agricultural Land Use in Central South Africa. Soil. Sci. Soc. Am. J. 2019, 83, 366–379. [Google Scholar] [CrossRef]
- Kotzé, E.; Loke, P.F.; Akhosi-Setaka, M.C.; Du Preez, C.C. Land use change affecting soil humic substances in three semi-arid agro-ecosystems in South Africa. Agric. Ecosyst. Environ. 2016, 216, 194–202. [Google Scholar] [CrossRef]
- Caravaca, F.; Lax, A.; Albaladejo, J. Aggregate stability and carbon characteristics of particle-size fractions in cultivated and forested soils of semiarid Spain. Soil. Tillage Res. 2004, 78, 83–90. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Raiesi, F. The quantity and quality of soil organic matter and humic substances following dry-farming and subsequent restoration in an upland pasture. Catena 2021, 202, 105249. [Google Scholar] [CrossRef]
- Godlewska, P.; Schmidt, H.P.; Ok, Y.S.; Oleszczuk, P. Biochar for composting improvement and contaminants reduction. A review. Bioresour. Technol. 2017, 246, 193–202. [Google Scholar] [CrossRef]
- Yu, Z.; Liu, X.; Chen, C.; Liao, H.; Chen, Z.; Zhou, S. Molecular insights into the transformation of dissolved organic matter during hyperthermophilic composting using ESI FT-ICR MS. Bioresour. Technol. 2019, 292, 22007. [Google Scholar] [CrossRef]
- Abakumov, E.V.; Cajthaml, T.; Brus, J.; Frouz, J. Humus accumulation, humification, and humic acid composition in soils of two post-mining chronosequences after coal mining. J. Soils Sediments 2013, 13, 491–500. [Google Scholar] [CrossRef]
- He, S.; Zheng, Z.; Zhu, R. Long-term tea plantation effects on composition and stabilization of soil organic matter in Southwest China. Catena 2021, 199, 105132. [Google Scholar] [CrossRef]
- Polyakov, V.; Loiko, S.; Istigechev, G.; Lapidus, A.; Abakumov, E. Elemental and molecular composition of humic acids isolated from soils of tallgrass temperate rainforests (Chernevaya taiga) by1H-13C HECTCOR NMR spectroscopy. Agronomy 2021, 11, 1998. [Google Scholar] [CrossRef]
- Mielnik, L.; Hewelke, E.; Weber, J.; Oktaba, L.; Jonczak, J.; Podlasiński, M. Changes in the soil hydrophobicity and structure of humic substances in sandy soil taken out of cultivation. Agric. Ecosyst. Environ. 2021, 319, 107554. [Google Scholar] [CrossRef]
- Jindo, K.; Hernández, T.; García, C.; Sánchez-Monedero, M.A. Influence of Stability and Origin of Organic Amendments on Humification in Semiarid Soils. Soil. Sci. Soc. Am. J. 2011, 75, 2178–2187. [Google Scholar] [CrossRef]
- Tan, K.H. Humic Matter in Soil and the Environment: Principles and Controversies, 2nd ed; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Tadini, A.M.; Bernardi, A.C.C.; Milori, D.M.B.P.; Oliveira, P.P.A.; Pezzopane, J.R.M.; Martin-Neto, L. Spectroscopic characteristics of humic acids extracted from soils under different integrated agricultural production systems in tropical regions. Geoderma Reg. 2022, 28, e00476. [Google Scholar] [CrossRef]
- Tadini, A.M.; Xavier, A.A.P.; Milori, D.M.B.P.; Oliveira, P.P.A.; Pezzopane, J.R.; Bernardi, A.C.C.; Martin-Neto, L. Evaluation of soil organic matter from integrated production systems using laser-induced fluorescence spectroscopy. Soil. Till Res. 2021, 211, 105001. [Google Scholar] [CrossRef]
- Tadini, A.M.; Goranov, A.I.; Martin-Neto, L.; Bernardi, A.C.C.; Oliveira, P.P.A.; Pezzopane, J.R.M.; Hatcher, P.G. Structural characterization using 2D NMR spectroscopy and TMAH-GC × GC-MS: Application to humic acids from soils of an integrated agricultural system and an Atlantic native forest. Sci. Total Environ. 2022, 815, 152605. [Google Scholar] [CrossRef] [PubMed]
- Dou, S.; Chen, E.; Xu, X.; Tan, S.; Zhang, J. Effect of improving soil fertility by organic material application (ISFOMA) on structural characteristics of humic acids in soil. Acta Pedol. Sin. 1992, 29, 199–207. [Google Scholar]
- Aranda, V.; Macci, C.; Peruzzi, E.; Masciandaro, G. Biochemical activity and chemical-structural properties of soil organic matter after 17 years of amendments with olive-mill pomace co-compost. J. Environ. Manag. 2015, 147, 278–285. [Google Scholar] [CrossRef]
- Spaccini, R.; Piccolo, A. Molecular characteristics of humic acids extracted from compost at increasing maturity stages. Soil. Biol. Biochem. 2009, 41, 1164–1172. [Google Scholar] [CrossRef]
- Vinci, G.; Cangemi, S.; Bridoux, M.; Spaccini, R.; Piccolo, A. Molecular properties of the Humeome of two calcareous grassland soils as revealed by GC/qTOF-MS and NMR spectroscopy. Chemosphere 2021, 279, 130518. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, B.; Li, Z.; Chu, W.; Mao, J.; Olk, D.C.; Zhang, J.; Xin, X.; Wei, W. Demonstration of chemical distinction among soil humic fractions using quantitative solid-state 13C NMR. J. Agric. Food Chem. 2019, 67, 8107–8118. [Google Scholar] [CrossRef]
- Ferrari, E.; Francioso, O.; Nardi, S.; Saladini, M.; Ferro, N.D.; Morari, F. DRIFT and HR MAS NMR characterization of humic substances from a soil treated with different organic and mineral fertilizers. J. Mol. Struct. 2011, 998, 216–224. [Google Scholar] [CrossRef]
- Guo, X.; Liu, H.; Wu, S. Humic substances developed during organic waste composting: Formation mechanisms, structural properties, and agronomic functions. Sci. Total Environ. 2019, 662, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, B.; Li, S.; Han, Y.; Zhang, X.; Gong, D.; Ma, M.; Liang, G.; Wu, H.; Wu, X.; et al. Effects of different long-term tillage systems on the composition of organic matter by 13C CP/TOSS NMR in physical fractions in the Loess Plateau of China. Soil. Tillage Res. 2019, 194, 104321. [Google Scholar] [CrossRef]
- Mylotte, R.; Verheyen, V.; Reynolds, A.; Dalton, C.; Patti, A.F.; Chang, R.R.; Burdon, J.; Hayes, M.H.B. Isolation and characterisation of recalcitrant organic components from an estuarine sediment core. J. Soils Sediments 2015, 15, 211–224. [Google Scholar] [CrossRef]
- Zhang, J.; Dou, S.; Song, X. Effect of long-term combined nitrogen and phosphorus fertilizer application on 13C CPMAS NMR spectra of humin in a typic hapludoll of northeast China. Eur. J. Soil. Sci. 2009, 60, 966–973. [Google Scholar] [CrossRef]
- Preston, C.M.; Newman, R.H. A long-term effect of N fertilization on the 13C CPMAS NMR of de-ashed soil humin in a second-growth Douglas-fir stand of coastal British Columbia. Geoderma 1995, 68, 229–241. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, N.; Cao, X.; Chua, T.; Mao, J.; Mandel, R.D.; Bettis, E.A.; Thompson, M.L. Composition of clay-fraction organic matter in Holocene paleosols revealed by advanced solid-state NMR spectroscopy. Geoderma 2014, 223–225, 54–61. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Mylotte, R.; Swift, R.S. Humin: Its Composition and Importance in Soil Organic Matter. In Advances in Agronomy; Academic Press: New York, NY, USA, 2017; Volume 143, pp. 47–138. [Google Scholar]
- Sun, H.; Jiang, J.; Cui, L.; Feng, W.; Wang, Y.; Zhang, J. Soil organic carbon stabilization mechanisms in a subtropical mangrove and salt marsh ecosystems. Sci. Total Environ. 2019, 673, 502–510. [Google Scholar] [CrossRef]


| C (%) | H (%) | N (%) | O (%) | C/N | H/C | O/C | |
|---|---|---|---|---|---|---|---|
| GL-Hu | 56.24 | 5.25 | 4.01 | 34.5 | 16.37 | 1.12 | 0.46 |
| AF-Hu | 55.27 | 5.49 | 3.54 | 35.7 | 18.24 | 1.19 | 0.48 |
| SF-Hu | 45.19 | 6.58 | 2.04 | 46.2 | 25.85 | 1.75 | 0.77 |
| CF-Hu | 41.51 | 6.33 | 2.34 | 49.8 | 20.70 | 1.83 | 0.90 |
| PF-Hu | 36.99 | 3.32 | 1.90 | 57.8 | 22.74 | 1.08 | 1.17 |
| GL-HA | 53.73 | 4.71 | 4.42 | 37.1 | 14.18 | 1.05 | 0.52 |
| AF-HA | 52.82 | 4.83 | 4.67 | 37.7 | 13.18 | 1.10 | 0.54 |
| SF-HA | 51.80 | 4.62 | 4.07 | 39.5 | 14.86 | 1.07 | 0.57 |
| CF-HA | 52.56 | 4.59 | 3.99 | 38.9 | 15.36 | 1.05 | 0.55 |
| PF-HA | 53.27 | 4.57 | 4.03 | 38.1 | 15.41 | 1.03 | 0.54 |
| GL-FA | 37.78 | 5.51 | 2.18 | 54.5 | 20.26 | 1.75 | 1.08 |
| AF-FA | 35.72 | 5.16 | 1.32 | 57.8 | 31.50 | 1.73 | 1.21 |
| SF-FA | 54.35 | 6.28 | 2.21 | 37.2 | 28.65 | 1.39 | 0.51 |
| CF-FA | 47.38 | 5.32 | 2.50 | 44.8 | 22.11 | 1.35 | 0.71 |
| PF-FA | 36.57 | 5.02 | 1.97 | 56.4 | 21.71 | 1.65 | 1.16 |
| Alkyl C | OCH3/NCH | O-Alkyl C | Di-O-Alkyl C | Aromatic C | Aromatic C-O | COO/N-C=O | Alkyl C/O-Alkyl C | Aliphatic C/Aromatic C | Hydrophobic C/Hydrophilic C | Aromaticity | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–45 | 45–60 | 60–90 | 90–110 | 110–145 | 145–160 | 160–190 | |||||
| Fulvic acid | |||||||||||
| CD | 13.57 | 8.20 | 30.29 | 10.10 | 15.93 | 3.79 | 19.25 | 0.28 | 3.15 | 0.49 | 4.21 |
| LD | 12.34 | 8.27 | 31.60 | 8.49 | 15.40 | 3.74 | 20.16 | 0.26 | 3.17 | 0.46 | 4.12 |
| GZ | 14.55 | 8.12 | 27.58 | 9.81 | 17.60 | 4.57 | 17.77 | 0.32 | 2.71 | 0.58 | 3.85 |
| HL | 11.76 | 7.81 | 33.46 | 10.22 | 14.75 | 3.66 | 18.32 | 0.23 | 3.43 | 0.43 | 4.03 |
| YM | 13.29 | 8.19 | 26.04 | 8.59 | 19.19 | 5.10 | 19.60 | 0.31 | 2.31 | 0.60 | 3.76 |
| Humic acid | |||||||||||
| CD | 20.56 | 6.39 | 9.78 | 5.39 | 33.73 | 7.78 | 16.37 | 0.95 | 1.01 | 1.64 | 4.33 |
| LD | 19.83 | 8.17 | 11.48 | 5.91 | 30.09 | 7.83 | 16.52 | 0.78 | 1.20 | 1.37 | 3.84 |
| GZ | 17.48 | 7.39 | 11.93 | 5.55 | 32.77 | 8.24 | 16.64 | 0.70 | 1.03 | 1.41 | 3.98 |
| HL | 18.12 | 6.34 | 10.51 | 5.80 | 34.96 | 7.79 | 16.67 | 0.80 | 0.95 | 1.55 | 4.49 |
| YM | 16.02 | 6.58 | 9.27 | 5.23 | 39.12 | 7.93 | 15.68 | 0.76 | 0.79 | 1.72 | 4.94 |
| Humin | |||||||||||
| CD | 26.25 | 7.09 | 11.02 | 4.99 | 29.40 | 7.87 | 13.65 | 1.14 | 1.32 | 1.73 | 3.73 |
| LD | 25.00 | 7.73 | 15.21 | 7.47 | 25.52 | 7.47 | 11.60 | 0.82 | 1.68 | 1.38 | 3.41 |
| GZ | 31.66 | 4.70 | 15.05 | 6.27 | 26.96 | 5.64 | 9.72 | 1.22 | 1.77 | 1.80 | 4.78 |
| HL | 20.21 | 4.79 | 10.21 | 5.00 | 39.58 | 7.71 | 12.29 | 1.01 | 0.85 | 2.09 | 5.14 |
| YM | 46.45 | 3.32 | 18.01 | 0.47 | 26.54 | 1.42 | 3.79 | 2.13 | 2.44 | 2.91 | 18.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, T.; Zhang, J.; Luo, W. The Quantity and Quality of Humic Substances following Different Land Uses in Karst Peak-Cluster Depression in Guangxi, China. Agriculture 2023, 13, 2246. https://doi.org/10.3390/agriculture13122246
Cui T, Zhang J, Luo W. The Quantity and Quality of Humic Substances following Different Land Uses in Karst Peak-Cluster Depression in Guangxi, China. Agriculture. 2023; 13(12):2246. https://doi.org/10.3390/agriculture13122246
Chicago/Turabian StyleCui, Tingting, Jianbing Zhang, and Weiqun Luo. 2023. "The Quantity and Quality of Humic Substances following Different Land Uses in Karst Peak-Cluster Depression in Guangxi, China" Agriculture 13, no. 12: 2246. https://doi.org/10.3390/agriculture13122246





