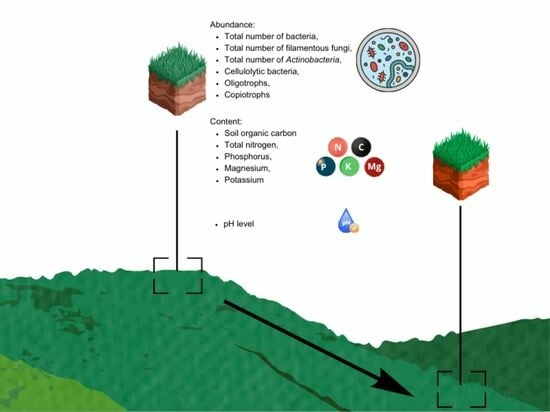
Evaluation of Microbiological and Chemical Properties of Soils as a Result of Anthropogenic Denudation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Microbiological Analyses
- Actinobacteria (Ac),
- Total bacterial count (B),
- Total fungal count (Ff),
- Cellulolytic bacteria (Ce),
- Oligotrophs (Ol),
- Copiotrophs (Co).
2.3. Soil Sample Chemical Analyses
2.4. Statistical Analyses
3. Results
3.1. Microbiological Analysis
3.2. Chemical Analysis
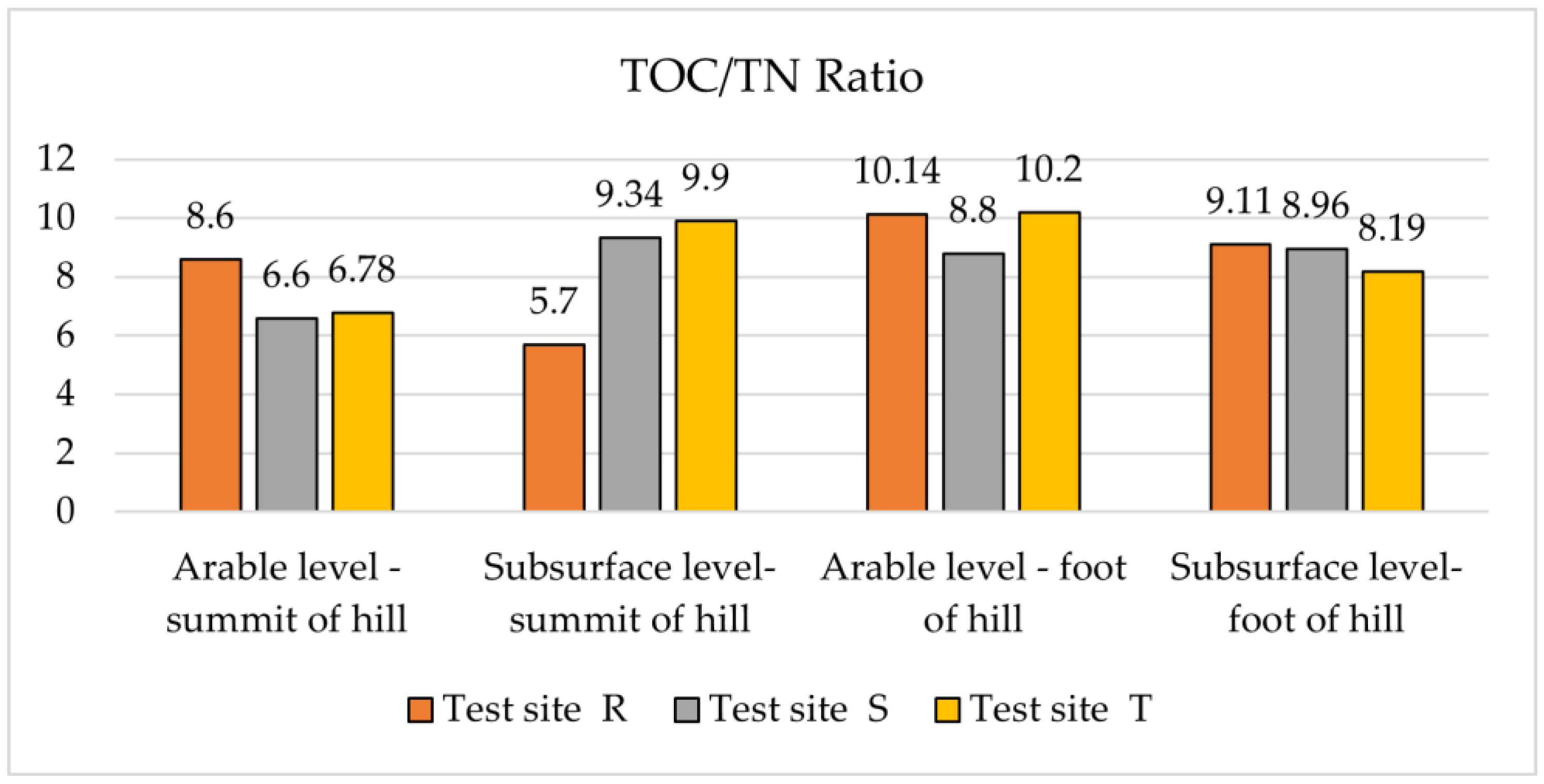
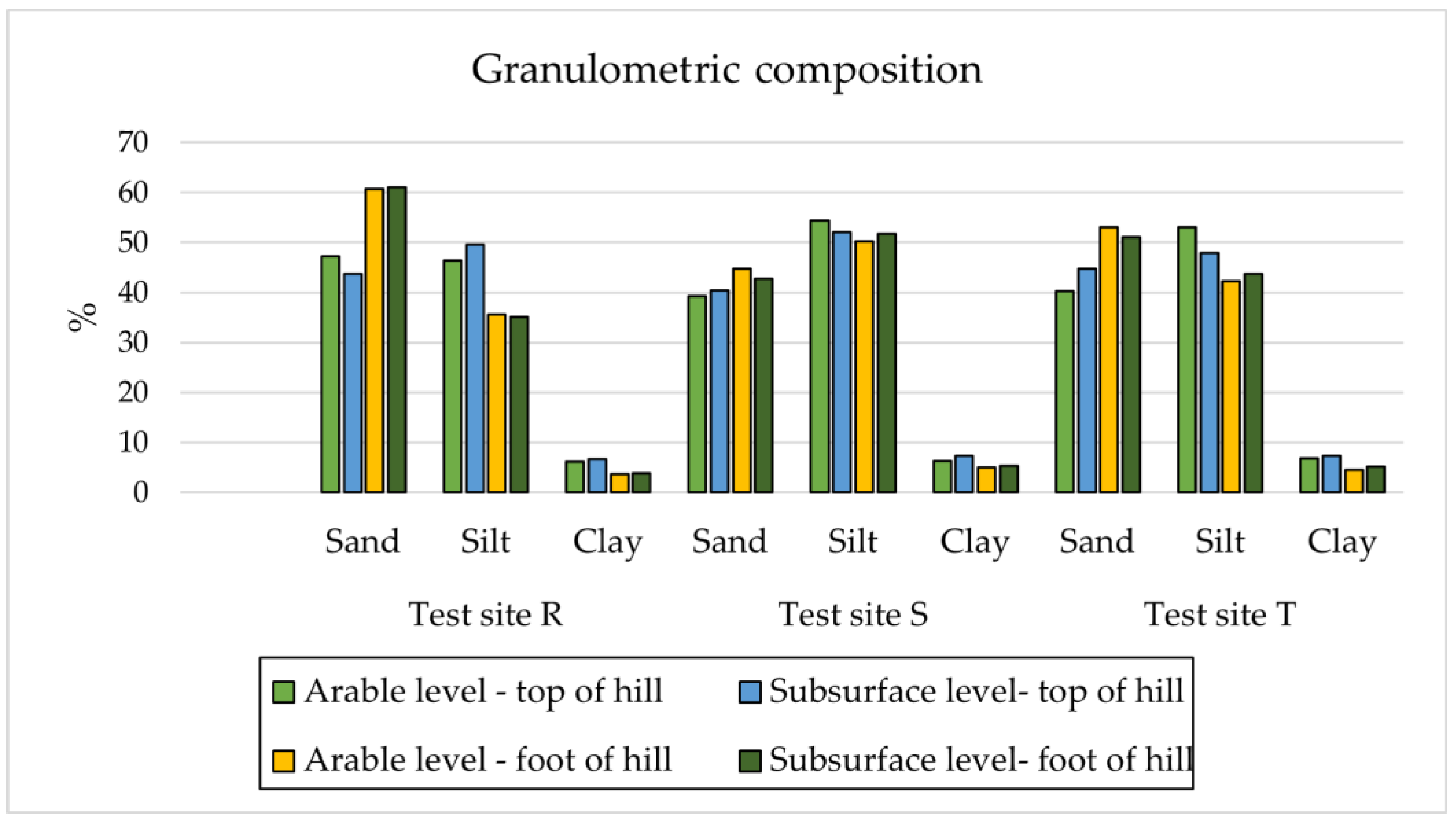
3.3. TOC:TN Ratio and Granulometric Composition
3.4. Soil Electrical Conductivity
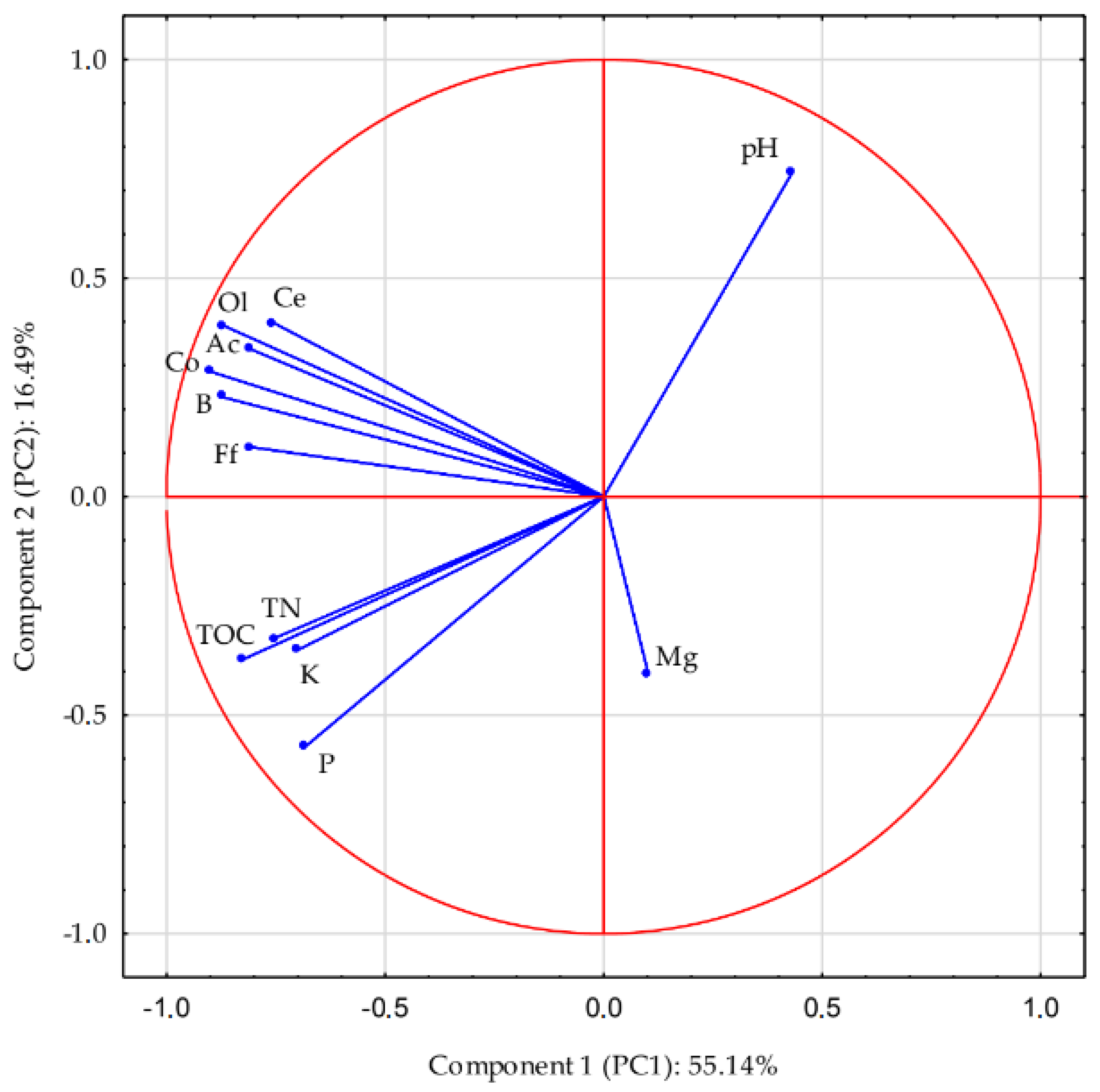
3.5. Principal Component Analysis—PCA
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Alewell, C.; Egli, M.; Meusburger, K. An Attempt to Estimate Tolerable Soil Erosion Rates by Matching Soil Formation with Denudation in Alpine Grasslands. J. Soils Sediments 2015, 15, 1383–1399. [Google Scholar] [CrossRef]
- Conforti, M.; Buttafuoco, G. Assessing Space–Time Variations of Denudation Processes and Related Soil Loss from 1955 to 2016 in Southern Italy (Calabria Region). Environ. Earth Sci. 2017, 76, 457. [Google Scholar] [CrossRef]
- Przewoźna, B. Changes of Bulk Density, Air-Water Properties and Morphology of Soils in Basins without Outlets as an Effect of Erosion and Anthropogenic Denudation (a Study from Northwestern Poland). Soil Sci. Plant Nutr. 2014, 60, 30–37. [Google Scholar] [CrossRef]
- Kabała, C.; Musztyfaga, E. Clay-Illuvial Soils in the Polish and International Soil Classifications. Soil Sci. Annu. 2015, 66, 204–213. [Google Scholar] [CrossRef]
- Gray, J.M.; Leys, J.F.; Yang, X.; Zhang, M. Monitoring of Sustainable Land Management Using Remotely Sensed Vegetation Cover and Variable Tolerable Soil Erosion Targets across New South Wales, Australia. Soil Use Manag. 2023, 39, 849–866. [Google Scholar] [CrossRef]
- Świtoniak, M.; Bednarek, R. Denudacja Antropogeniczna. In Antropogeniczne Przekształcenia Pokrywy Glebowej Brodnickiego Parku Krajobrazowego; Wyd. Nauk.: Toruń, Poland, 2014; pp. 57–84. ISBN 978-83-231-3280-6. [Google Scholar]
- Tomlinson, I. Doubling Food Production to Feed the 9 Billion: A Critical Perspective on a Key Discourse of Food Security in the UK. J. Rural Stud. 2013, 29, 81–90. [Google Scholar] [CrossRef]
- Al-Kaisi, M.M.; Elmore, R.W.; Guzman, J.G.; Hanna, H.M.; Hart, C.E.; Helmers, M.J.; Hodgson, E.W.; Lenssen, A.W.; Mallarino, A.P.; Robertson, A.E.; et al. Drought Impact on Crop Production and the Soil Environment: 2012 Experiences from Iowa. J. Soil Water Conserv. 2013, 68, 19A–24A. [Google Scholar] [CrossRef]
- Jin, F.; Yang, W.; Fu, J.; Li, Z. Effects of Vegetation and Climate on the Changes of Soil Erosion in the Loess Plateau of China. Sci. Total Environ. 2021, 773, 145514. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, Y.; Zhou, S.; Song, J.; Fu, B. Soil Moisture Determines the Recovery Time of Ecosystems from Drought. Glob. Change Biol. 2023, 29, 3562–3574. [Google Scholar] [CrossRef]
- Pandey, S.; Kumar, P.; Zlatic, M.; Nautiyal, R.; Panwar, V.P. Recent Advances in Assessment of Soil Erosion Vulnerability in a Watershed. Int. Soil Water Conserv. Res. 2021, 9, 305–318. [Google Scholar] [CrossRef]
- Boardman, J.; Poesen, J.; Evans, R. Socio-Economic Factors in Soil Erosion and Conservation. Environ. Sci. Policy 2003, 6, 1–6. [Google Scholar] [CrossRef]
- Montanarella, L.; Pennock, D.J.; McKenzie, N.; Badraoui, M.; Chude, V.; Baptista, I.; Mamo, T.; Yemefack, M.; Singh Aulakh, M.; Yagi, K.; et al. World’s Soils Are under Threat. Soil 2016, 2, 79–82. [Google Scholar] [CrossRef]
- Panagos, P.; Borrelli, P.; Robinson, D. FAO Calls for Actions to Reduce Global Soil Erosion. Mitig. Adapt. Strateg. Glob. Change 2020, 25, 789–790. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, Q.; Zhu, H.; Reich, P.B.; Banerjee, S.; van der Heijden, M.G.A.; Sadowsky, M.J.; Ishii, S.; Jia, X.; Shao, M.; et al. Erosion Reduces Soil Microbial Diversity, Network Complexity and Multifunctionality. ISME J. 2021, 15, 2474–2489. [Google Scholar] [CrossRef]
- Abdul Rahman, N.S.N.; Abdul Hamid, N.W.; Nadarajah, K. Effects of Abiotic Stress on Soil Microbiome. Int. J. Mol. Sci. 2021, 22, 9036. [Google Scholar] [CrossRef]
- Lehman, R.M.; Cambardella, C.A.; Stott, D.E.; Acosta-Martinez, V.; Manter, D.K.; Buyer, J.S.; Maul, J.E.; Smith, J.L.; Collins, H.P.; Halvorson, J.J.; et al. Understanding and Enhancing Soil Biological Health: The Solution for Reversing Soil Degradation. Sustainability 2015, 7, 988–1027. [Google Scholar] [CrossRef]
- Khmelevtsova, L.E.; Sazykin, I.S.; Azhogina, T.N.; Sazykina, M.A. Influence of Agricultural Practices on Bacterial Community of Cultivated Soils. Agriculture 2022, 12, 371. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Kobierski, M.; Długosz, J. Enzymatic Activity and Physicochemical Properties of Soil Profiles of Luvisols. Materials 2021, 14, 6364. [Google Scholar] [CrossRef]
- Loba, A.; Zhang, J.; Tsukamoto, S.; Kasprzak, M.; Beata Kowalska, J.; Frechen, M.; Waroszewski, J. Multiproxy Approach to the Reconstruction of Soil Denudation Events and the Disappearance of Luvisols in the Loess Landscape of South-Western Poland. Catena 2023, 220, 106724. [Google Scholar] [CrossRef]
- Radziuk, H.; Świtoniak, M. The Effect of Erosional Transformation of Soil Cover on the Stability of Soil Aggregates within Young Hummocky Moraine Landscapes in Northern Poland. Agronomy 2022, 12, 2595. [Google Scholar] [CrossRef]
- Kobierski, M. Morphology, Properties and Mineralogical Composition of Eroded Luvisols in Selected Morainic Areas of the Kujavian and Pomeranian Province. Univ. Technol. Life Sci. Bydg. 2013, Monograph No. 166, 1–121. [Google Scholar]
- Świtoniak, M. Use of Soil Profile Truncation to Estimate Influence of Accelerated Erosion on Soil Cover Transformation in Young Morainic Landscapes, North-Eastern Poland. Catena 2014, 116, 173–184. [Google Scholar] [CrossRef]
- Świtoniak, M.; Mroczek, P.; Bednarek, R. Luvisols or Cambisols? Micromorphological Study of Soil Truncation in Young Morainic Landscapes—Case Study: Brodnica and Chełmno Lake Districts (North Poland). Catena 2016, 137, 583–595. [Google Scholar] [CrossRef]
- Pindral, S.; Świtoniak, M. The Usefulness of Soil-Agricultural Maps to Identify Classes of Soil Truncation. Soil Sci. Annu. 2017, 68, 2–10. [Google Scholar] [CrossRef]
- Solon, J.; Borzyszkowski, J.; Bidłasik, M.; Richling, A.; Badora, K.; Balon, J.; Brzezińska-Wójcik, T.; Chabudziński, Ł.; Dobrowolski, R.; Grzegorczyk, I.; et al. Physico-Geographical Mesoregions of Poland: Verification and Adjustment of Boundaries on the Basis of Contemporary Spatial Data. Geogr. Pol. 2018, 91, 143–170. [Google Scholar] [CrossRef]
- Kostrzewski, A.; Zwoliński, Z.; Andrzejewski, L.; Florek, W.; Mazurek, M.; Niewiarowski, W.; Podgórski, Z.; Rachlewicz, G.; Smolska, E.; Stach, A.; et al. Współczesna ewolucja rzeźby młodoglacjalnej Niżu Polskiego. In Współczesne Przemiany Rzeźby Polski; Bogucki Wydawnictwo Naukowe: Kraków, Poland, 2021; pp. 483–571. ISBN 978-83-7986-382-2. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps, 4th ed.; International Union of Soil Sciences (IUSS): Vienna, Austria, 2022. [Google Scholar]
- Crawford, D.L.; Lynch, J.M.; Whipps, J.M.; Ousley, M.A. Isolation and Characterization of Actinomycetes Antagonists of a Fungal Root Pathogen. Appl. Environ. Microbiol. 1996, 59, 3899–3905. [Google Scholar] [CrossRef]
- Atlas, R.M. Handbook of Microbiological Media; CRC Press: Boca Raton, FL, USA, 2010; ISBN 978-0-429-13049-6. [Google Scholar]
- Gupta, V.; Gulati, P.; Bhagat, N.; Dhar, M.S.; Virdi, J. Detection of Yersinia Enterocolitica in Food: An Overview. European. J. Clin. Microbiol. Infect. Dis. 2015, 34, 641–650. [Google Scholar] [CrossRef]
- Hattori, R.; Hattori, T. Sensitivity to Salts and Organic Coumpounds of Soil Bacteria Isolated on Diluted Media. J. Gen. Appied Microbiol. 1980, 26, 1–14. [Google Scholar] [CrossRef]
- ISO 10390:2005; Soil, Treated Biowaste and Sludge—Determination of PH. Polish Committee for Standardizations: Warsaw, Poland, 2013.
- PN-R-04020; Agricultural Analysis. Determination of the Content Available Magnesium. Polish Standards Committee: Warszawa, Poland, 1994.
- PN-R-04023; Agricultural Analysis—Determination of the Content of Available Phosphorus in Mineral Soils. Polish Standards Committee: Warszawa, Poland, 1996.
- PN-R-04022; Agricultural Analysis—Determination of the Content Available Potassium in Mineral Soils. Polish Standards Committee: Warszawa, Poland, 1996.
- Ward, J.H. Hierarchical Grouping to Optimize an Objective Function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Statistica. Data Analysis Software System, Version 12; TIBCO Software Inc.: Palo Alto, CA, USA, 2019. Available online: https://www.Tibco.Com/Products/Data-Science(accessed on 15 October 2023).
- Cendrero, A.; Remondo, J.; Beylich, A.A.; Cienciala, P.; Forte, L.M.; Golosov, V.N.; Gusarov, A.V.; Kijowska-Strugała, M.; Laute, K.; Li, D.; et al. Denudation and Geomorphic Change in the Anthropocene; a Global Overview. Earth Sci. Rev. 2022, 233, 104186. [Google Scholar] [CrossRef]
- Zalasiewicz, J.; Waters, C.N.; Williams, M.; Barnosky, A.D.; Cearreta, A.; Crutzen, P.; Ellis, E.; Ellis, M.A.; Fairchild, I.J.; Grinevald, J.; et al. When Did the Anthropocene Begin? A Mid-Twentieth Century Boundary Level Is Stratigraphically Optimal. Quat. Int. 2015, 383, 196–203. [Google Scholar] [CrossRef]
- Mabuhay, J.A.; Nakagoshi, N.; Isagi, Y. Influence of Erosion on Soil Microbial Biomass, Abundance and Community Diversity. Land Degrad. Dev. 2004, 15, 183–195. [Google Scholar] [CrossRef]
- Gapeshin, D.I.; Ivanova, A.E.; Gracheva, T.A.; Pozdnyakov, L.A.; Demidov, V.V. Microbiological Indicators of Erosion Processes in Arable Alluvial Soils. Mosc. Univ. Soil Sci. Bull. 2022, 77, 374–383. [Google Scholar] [CrossRef]
- Michel, H.M.; Williams, M.A. Soil Habitat and Horizon Properties Impact Bacterial Diversity and Composition. Soil Sci. Soc. Am. J. 2011, 75, 1440–1448. [Google Scholar] [CrossRef]
- Alaoui, A.; Diserens, E. Mapping Soil Compaction—A Review. Curr. Opin. Environ. Sci. Health 2018, 5, 60–66. [Google Scholar] [CrossRef]
- Sul, W.J.; Asuming-Brempong, S.; Wang, Q.; Tourlousse, D.M.; Penton, C.R.; Deng, Y.; Rodrigues, J.L.M.; Adiku, S.G.K.; Jones, J.W.; Zhou, J.; et al. Tropical Agricultural Land Management Influences on Soil Microbial Communities through Its Effect on Soil Organic Carbon. Soil Biol. Biochem. 2013, 65, 33–38. [Google Scholar] [CrossRef]
- Denef, K.; Six, J.; Paustian, K.; Merckx, R. Importance of Macroaggregate Dynamics in Controlling Soil Carbon Stabilization: Short-Term Effects of Physical Disturbance Induced by Dry–Wet Cycles. Soil Biol. Biochem. 2001, 33, 2145–2153. [Google Scholar] [CrossRef]
- Jamshidi, A.H.; Sun, L.; Niu, Y.; Liu, X.; Fan, Z.; Yi, W.; Zhang, S. Slope Gradient Altered Microbial Community Composition in the Sloping Cropland in Black Soil. Catena 2023, 232, 107416. [Google Scholar] [CrossRef]
- Naylor, D.; McClure, R.; Jansson, J. Trends in Microbial Community Composition and Function by Soil Depth. Microorganisms 2022, 10, 540. [Google Scholar] [CrossRef]
- Huang, Y.-M.; Liu, D.; An, S.-S. Effects of Slope Aspect on Soil Nitrogen and Microbial Properties in the Chinese Loess Region. Catena 2015, 125, 135–145. [Google Scholar] [CrossRef]
- Selim, M.S.M.; Abdelhamid, S.A.; Mohamed, S.S. Secondary Metabolites and Biodiversity of Actinomycetes. J. Genet. Eng. Biotechnol. 2021, 19, 72. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Liu, J.; Zhang, J.; Li, D.; Xu, C.; Kuzyakov, Y. Divergence in Fungal Abundance and Community Structure between Soils under Long-Term Mineral and Organic Fertilization. Soil Tillage Res. 2020, 196, 104491. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G.; Knight, R.; Fierer, N. Soil Bacterial and Fungal Communities across a PH Gradient in an Arable Soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Hayat, Z.; Ramzan, M.; Shah, Z.; Hanif, M. Effect of Slope Position on Physico-Chemical Properties of Eroded Soil. Soil Environ. 2013, 31, 22–28. [Google Scholar]
- Matecka, P.; Świtoniak, M. Delineation, Characteristic and Classification of Soils Containing Carbonates in Plow Horizons within Young Moraine Areas. Soil Sci. Ann. 2020, 71, 23–36. [Google Scholar] [CrossRef]
- Jia, S.; He, X.; Wei, F. Soil Organic Carbon Loss under Different Slope Gradients in Loess Hilly Region. Wuhan Univ. J. Nat. Sci. 2007, 12, 695–698. [Google Scholar] [CrossRef]
- Dungait, J.A.J.; Ghee, C.; Rowan, J.S.; McKenzie, B.M.; Hawes, C.; Dixon, E.R.; Paterson, E.; Hopkins, D.W. Microbial Responses to the Erosional Redistribution of Soil Organic Carbon in Arable Fields. Soil Biol. Biochem. 2013, 60, 195–201. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wang, Y.; Li, F.C.; Zhang, J.H.; Wang, Y.; Li, F.C. Soil Organic Carbon and Nitrogen Losses Due to Soil Erosion and Cropping in a Sloping Terrace Landscape. Soil Res. 2015, 53, 87–96. [Google Scholar] [CrossRef]
- Nie, X.-J.; Zhang, H.-B.; Su, Y.-Y. Soil Carbon and Nitrogen Fraction Dynamics Affected by Tillage Erosion. Sci. Rep. 2019, 9, 16601. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, Q.; Xu, G.; Liang, G.; Kim, D.-G.; Carmona, C.R.; Yang, M.; Xue, J.; Xiang, Y.; et al. Enhancing Soil Carbon and Nitrogen through Grassland Conversion from Degraded Croplands in China: Assessing Magnitudes and Identifying Key Drivers of Phosphorus Reduction. Soil Tillage Res. 2024, 236, 105943. [Google Scholar] [CrossRef]
- Brubaker, S.C.; Jones, A.J.; Lewis, D.T.; Frank, K. Soil Properties Associated with Landscape Position. Soil Sci. Soc. Am. J. 1993, 57, 235–239. [Google Scholar] [CrossRef]
- Yarashynskaya, A.; Prus, P. Precision Agriculture Implementation Factors and Adoption Potential: The Case Study of Polish Agriculture. Agronomy 2022, 12, 2226. [Google Scholar] [CrossRef]
- Breza-Boruta, B.; Kotwica, K.; Bauza-Kaszewska, J. Effect of Tillage System and Organic Matter Management Interactions on Soil Chemical Properties and Biological Activity in a Spring Wheat Short-Time Cultivation. Energies 2021, 14, 7451. [Google Scholar] [CrossRef]
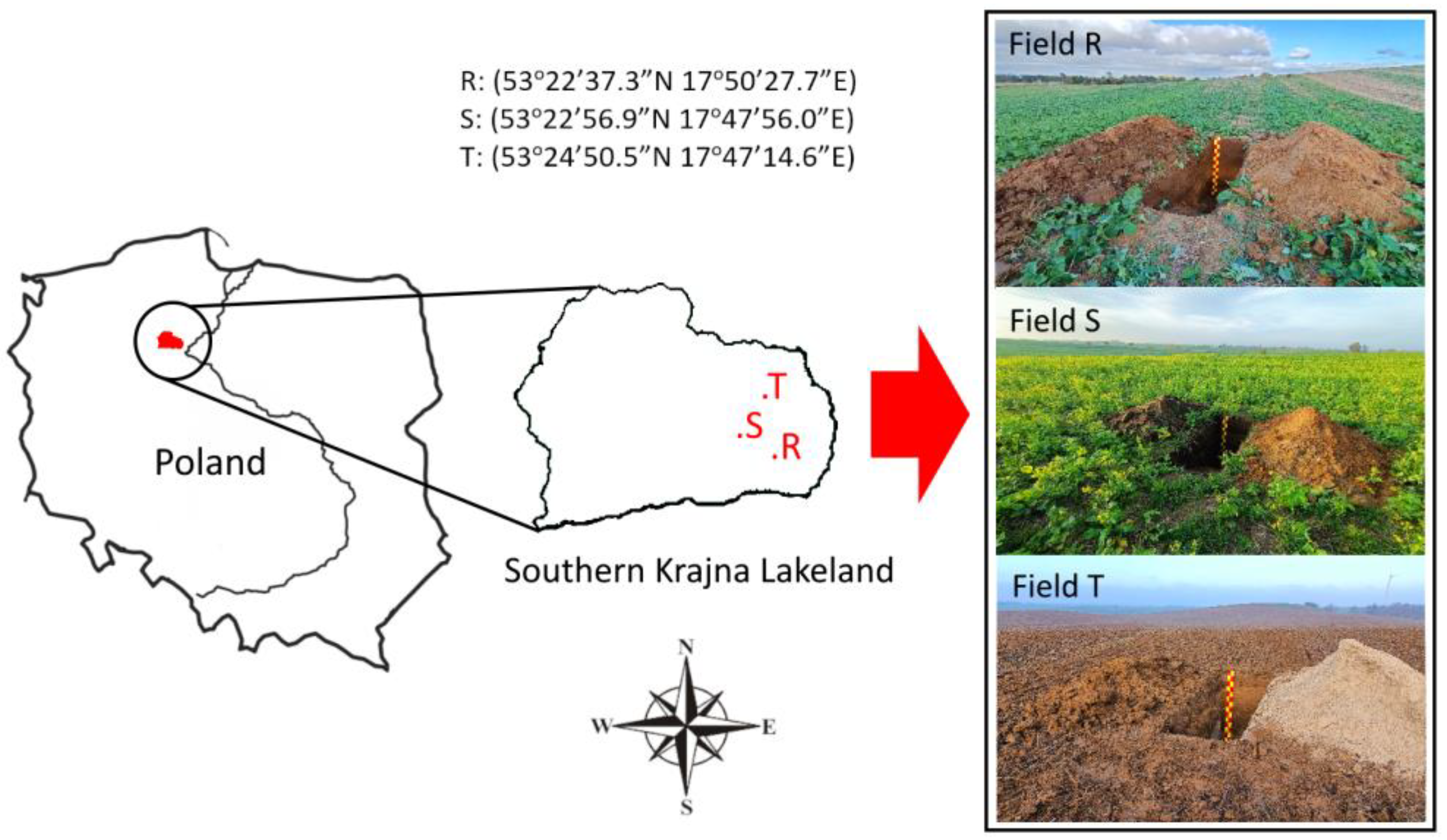


| Site | Profile | Soil Classification WRB 2022 [28] | Sampling |
|---|---|---|---|
| R | Ap-Bt-Ck1-Ck2 | Epicutanic Luvisol | A1—Summit: B1—Ap horizon; B2—Bt horizon |
| Ap-A2-Et-BC-C | Haplic Luvisol | A2—Footslope: B1—Ap horizon: B2—A2 horizon | |
| S | Ap-Ck1-Ck2-Ck3 | Calcaric, Eutric Regosol | A1—Summit: B1—Ap horizon; B2—Ck1 horizon |
| Ap-A2-Et-EB-Bt-C | Haplic Luvisol | A2—Footslope: B1—Ap horizon: B2—A2 horizon | |
| T | Ap-Ck1-Ck2-2Ck | Calcaric, Eutric Regosol | A1—Summit: B1—Ap horizon; B2—Ck1 horizon |
| Ap-A2-Et-EB-Bt1-Bt2-C | Haplic Luvisol | A2—Footslope: B1—Ap horizon: B2—A2 horizon |
| Tested Groups of Microorganisms | Applied Medium | Reference |
|---|---|---|
| Ac | Modified yeast extract-glucose medium (YGA) | [29] |
| B | Standard nutrient agar | [30] |
| Ff | Rose–Bengal agar with 30 µg mL−1 streptomycin | [30] |
| Ce | Agar containing 0.1% sodium carboxymethylcellulose | [31] |
| Ol | Modified medium with limited nutrients | [32] |
| Co | Modified medium with additional nutrients | [32] |
| Level of Factor B | Test Site R | Test Site S | Test Site T | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Level of Factor A * | |||||||||
| A1 | A2 | Mean | A1 | A2 | Mean | A1 | A2 | Mean | |
| Heterotrophic bacteria (B) (106 cfu g−1) | |||||||||
| B1 | 27.3 a | 23.0 a | 25.2 a | 18.7 a | 18.7 | 18.7 a | 3.1 a | 24.0 a | 13.6 a |
| B2 | 6.1 a | 5.2 a | 5.7 b | 2.3 b | 2.4 | 2.3 b | 2.5 a | 1.3 b | 1.9 b |
| Mean | 16.7 | 14.1 | 10.5 | 10.5 | 2.8 | 12.6 | |||
| LSD0.05 | A = n.s.; B = 5.499; A/B = n.s.; B/A = n.s. | A = n.s.; B = 3.991; A/B = n.s.; B/A = n.s. | A = 7.685; B = 7.685; A/B = 10.868; B/A = 10.868 | ||||||
| Actinobacteria (Ac) (105 cfu g−1) | |||||||||
| B1 | 28.7 a | 30.7 a | 29.7 a | 18.0 a | 28.7 a | 23.3 a | 27.0 a | 52.0 a | 3.9 b |
| B2 | 18.7 a | 26.3 a | 22.5 a | 8.6 b | 6.8 b | 7.6 b | 2.0 a | 10.8 a | 6.4 a |
| Mean | 23.7 | 28.5 | 18.6 | 12.3 | 14.0 | 31.0 | |||
| LSD0.05 | A = n.s.; B = n.s.; A/B = n.s.; B/A = n.s. | A = 0.305; B = 0.305; A/B = 0.431; B/A = 0.431 | A = 1.252; B = 1.252; A/B = n.s.; B/A = n.s. | ||||||
| Filamentous fungi (Ff) (105 cfu g−1) | |||||||||
| B1 | 5.0 a | 3.0 a | 4.0 a | 3.0 a | 4.0 a | 3.2 a | 2.0 a | 13.0 a | 7.5 a |
| B2 | 1.0 a | 1.0 a | 1.4 b | 1.0 a | 2.0 a | 1.2 b | 1.0 b | 1.0 b | 1.0 b |
| Mean | 3.1 | 2.3 | 1.6 | 2.8 | 1.30 | 0.7 | |||
| LSD0.05 | A = n.s.; B = 0.133; A/B = n.s.; B/A = n.s. | A = 0.108; B = 0.108; A/B = n.s.; B/A = n.s. | A = 0.436; B = 0.436; A/B = 0.616; B/A = 0.616 | ||||||
| Level of Factor B | Test Site R | Test Site S | Test Site T | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Level of Factor A | |||||||||
| A1 | A2 | Mean | A1 | A2 | Mean | A1 | A2 | Mean | |
| Cellulolytic microorganisms (Ce) (106 cfu g−1) | |||||||||
| B1 | 8.0 a | 5.3 a | 6.7 a | 6.3 a | 3.3 a | 4.8 a | 5.1 a | 4.8 a | 5.0 a |
| B2 | 1.0 a | 1.2 a | 1.1 b | 1.4 b | 3.3 a | 1.6 b | 0.5 a | 0.8 a | 0.6 b |
| Mean | 4.5 | 3.3 | 3.9 | 1.7 | 2.8 | 2.8 | |||
| LSD0.05 | A = n.s.; B = 2.255; A/B = n.s.; B/A = n.s. | A = 1.360; B = 1.360; A/B = 1.923; B/A = 1.923 | A = n.s; B = 4.218; A/B = n.s; B/A = n.s. | ||||||
| Copiotrophic microorganisms (Co) (106 cfu g−1) | |||||||||
| B1 | 14.3 a | 24.7 a | 19.5 a | 15.0 a | 19.3 a | 17.2 a | 11.3 a | 24.0 a | 15.5 a |
| B2 | 2.9 a | 6.0 a | 4.4 b | 2.6 a | 3.2 a | 3.0 b | 1.1 a | 1.3 a | 1.2 b |
| Mean | 8.6 | 15.3 | 8.8 | 11.3 | 4.0 | 12.7 | |||
| LSD0.05 | A = n.s.; B = 9.367; A/B = n.s.; B/A = n.s. | A = n.s.; B = 6.255; A/B = n.s.; B/A = n.s. | A = n.s.; B = 12.619; A/B= n.s.; B/A = n.s. | ||||||
| Oligotrophic microorganisms (Ol) (106 cfu g−1) | |||||||||
| B1 | 65.0 a | 40.0 a | 52.6 a | 51.0 a | 34.0 a | 42.5 a | 37.3 a | 64.0 a | 50.7 a |
| B2 | 13.9 a | 6.9 a | 10.4 b | 6.0 b | 14.2 b | 10.3 b | 2.2 a | 0.6 a | 1.4 b |
| Mean | 39.5 | 23.4 | 28.6 | 24.1 | 19.8 | 32.3 | |||
| LSD0.05 | A = 13.543; B = 13.543 A/B = n.s.; B/A = n.s. | A = n.s.; B = 9.224; A/B = 13.045; B/A = 13.045 | A = n.s.; B = 14.400; A/B = n.s.; B/A = n.s. | ||||||
| Level of Factor B | Test Site R | Test Site S | Test Site T | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Level of Factor A | |||||||||
| A1 | A2 | Mean | A1 | A2 | Mean | A1 | A2 | Mean | |
| pH value | |||||||||
| B1 | 6.33 a | 7.14 a | 6.73 a | 8.18 b | 6.48 a | 7.33 a | 8.36 b | 7.01 a | 7.66 a |
| B2 | 6.65 a | 6.94 a | 6.79 a | 8.52 a | 6.17 b | 7.34 a | 8.72 a | 6.67 b | 7.69 a |
| Mean | 6.49 | 7.04 | 8.35 | 6.33 | 8.54 | 6.82 | |||
| LSD0.05 | A = 0.313; B = n.s. A/B = n.s.; B/A = n.s. | A = 0.233; B = n.s. A/B = 0.330; B/A = 0.330 | A = 0.055; B = n.s. A/B = 0.077; B/A = 0.077 | ||||||
| Total Organic Carbon (TOC) (g·kg−1) | |||||||||
| B1 | 5.94 a | 7.30 a | 6.62 a | 6.92 a | 9.79 a | 8.33 a | 5.49 a | 9.21 a | 7.35 a |
| B2 | 2.85 b | 6.38 b | 4.62 b | 2.71 b | 8.25 b | 5.48 b | 3.94 b | 5.90 b | 4.92 b |
| Mean | 4.40 | 6.84 | 4.82 | 8.99 | 4.72 | 7.56 | |||
| LSD0.05 | A = 0.117; B = 0.117; A/B = 0.165; B/A = 0.165 | A = 1.055; B = 1.055; A/B = 1.492; B/A = 1.492 | A = 0.233; B = 0.233; A/B = 0.329.; B/A = 0.329 | ||||||
| Total Nitrogen (TN) (g·kg−1) | |||||||||
| B1 | 0.69 a | 0.71 a | 0.70 a | 1.05 a | 1.10 a | 1.08 a | 0.81 a | 0.90 a | 0.86 a |
| B2 | 0.50 a | 0.70 a | 0.60 a | 0.29 b | 0.92 b | 0.61 b | 0.40 b | 0.72 b | 0.56 b |
| Mean | 0.60 | 0.71 | 0.67 | 1.01 | 0.61 | 0.81 | |||
| LSD0.05 | A = n.s.; B = n.s.; A/B = n.s.; B/A = n.s. | A = 0.129; B = 0.129; A/B = 0.182; B/A = 0.182 | A = 0.091; B = 0.091; A/B = 0.129; B/A = 0.129 | ||||||
| Magnesium (Mg) (mg·kg−1) | |||||||||
| B1 | 30.7 a | 22.6 a | 26.65 a | 30.2 a | 27.6 a | 28.88 a | 17.5 a | 24.8 a | 21.13 a |
| B2 | 41.1 a | 19.8 a | 30.45 a | 21.00 a | 29.4 a | 25.18 a | 29.4 a | 30.2 a | 29.78 a |
| Mean | 35.90 | 21.20 | 25.55 | 29.40 | 23.45 | 27.45 | |||
| LSD0.05 | A = 11.851; B = n.s.; A/B = n.s.; B/A = n.s. | A = n.s.; B = n.s.; A/B = n.s.; B/A = n.s. | A = n.s; B = n.s.; A/B = n.s.; B/A = n.s. | ||||||
| Phosphorus (P) (mg·kg−1) | |||||||||
| B1 | 97.5 a | 119.1 a | 108.28 a | 32.5 a | 125.5 a | 79.00 a | 60.5 a | 122.7 a | 91.60 a |
| B2 | 51.7 b | 94.9 b | 73.28 b | 21.5 a | 98.9 a | 54.68 b | 31.1 b | 111.3 b | 71.20 b |
| Mean | 74.58 | 106.98 | 21.48 | 112.20 | 45.80 | 117.00 | |||
| LSD0.05 | A = 9.908; B = 9.908; A/B = 14.012; B/A = 14.012 | A = 10.175; B = 10.175; A/B = n.s.; B/A = n.s. | A = 6.276; B 6.276; A/B = 8.875; B/A = 8.875 | ||||||
| Potassium (K) (mg·kg−1) | |||||||||
| B1 | 128.4 a | 185.4 a | 156.9 a | 212.7 a | 251.0 a | 231.85 a | 125.8 a | 223.3 a | 174.55 a |
| B2 | 117.5 a | 101.2 b | 109.4 b | 116.3 a | 142.8 a | 129.55 b | 85.7 b | 221.1 a | 153.40 b |
| Mean | 122.93 | 143.30 | 164.50 | 196.90 | 105.75 | 222.20 | |||
| LSD0.05 | A = 13.381; B = 13.381; A/B = 18.923; B/A = 18.923 | A = 20.524; B = 20.524; A/B = n.s.; B/A = n.s. | A = 11.377; B = 11.377; A/B = 16.090; B/A = 16.090 | ||||||
| Location | Na | K | Mg | Ca | ECs |
| g·kg−1 | µS·cm−1 | ||||
| R A1B1 | 6.6 ± 3.39 | 44.3 ± 1.27 | 15.9 ± 5.80 | 79.0 ± 1.84 | 93.4 ± 8.20 |
| S A1B1 | 12.7 ± 4.17 | 77.9 ± 3.81 | 17.3 ± 1.27 | 201.0 ± 9.90 | 118.0 ± 3.68 |
| T A1B1 | 8.7 ± 2.05 | 36.7 ± 0.92 | 16.4 ± 4.24 | 210.5 ± 2.12 | 166.9 ± 3.46 |
| Mean | 9.33 | 53.0 | 16.3 | 163.5 | 126.1 |
| R A2B1 | 16.4 ± 4.38 | 38.3 ± 4.38 | 15.7 ± 1.63 | 40.8 ± 3.53 | 101.7 ± 1.34 |
| S A2B1 | 11.1 ± 3.32 | 49.0 ± 1.63 | 13.7 ± 3.53 | 47.7 ± 3.61 | 148.6 ± 1.27 |
| T A2B1 | 10.0 ± 0.35 | 21.2 ± 1.55 | 15.3 ± 0.56 | 33.4 ± 0.28 | 105.9 ± 0.78 |
| Mean | 12.5 | 36.2 | 14.0 | 40.6 | 118.7 |
| p = 0.30 | p = 0.32 | p = 0.022 * | p = 0.045 * | p = 0.79 | |
| Location | Na | K | Mg | Ca | ECs |
| g·kg−1 | µS·cm−1 | ||||
| R A1B2 | 9.5 ± 1.83 | 32.5 ± 1.55 | 11.6 ± 1.48 | 78.7 ± 4.10 | 70.2 ± 6.22 |
| S A1B2 | 10.7 ± 2.19 | 40.5 ± 3.60 | 17.3 ± 2.33 | 240.5 ± 3.53 | 68.6 ± 7.71 |
| T A1B2 | 10.6 ± 0,21 | 23.2 ± 0.99 | 13.6 ± 4.31 | 233.0 ± 2.12 | 159.3 ± 7.78 |
| Mean | 10.3 | 34.1 | 14.2 | 184.1 | 99.4 |
| R A2B2 | 12.5 ± 0.85 | 42.7 ± 0.28 | 19.7 ± 1.77 | 32.8 ± 1.91 | 117.8 ± 6.64 |
| S A2B2 | 12.3 ± 0.85 | 95.6 ± 0.56 | 22.5 ± 3.68 | 44.7 ± 1.77 | 148.5 ± 2.90 |
| T A2B2 | 12.8 ± 0.71 | 41.5 ± 0.14 | 18.6 ± 3.82 | 32.6 ± 0.21 | 105.7 ± 4.38 |
| Mean | 12.5 | 59.9 | 20.3 | 36.7 | 124.0 |
| p = 0.005 * | p = 0.21 | p = 0.04 * | p = 0.49 | p = 0.49 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanarek, P.; Breza-Boruta, B.; Pawłowski, M.; Kobierski, M. Evaluation of Microbiological and Chemical Properties of Soils as a Result of Anthropogenic Denudation. Agriculture 2023, 13, 2247. https://doi.org/10.3390/agriculture13122247
Kanarek P, Breza-Boruta B, Pawłowski M, Kobierski M. Evaluation of Microbiological and Chemical Properties of Soils as a Result of Anthropogenic Denudation. Agriculture. 2023; 13(12):2247. https://doi.org/10.3390/agriculture13122247
Chicago/Turabian StyleKanarek, Piotr, Barbara Breza-Boruta, Mateusz Pawłowski, and Mirosław Kobierski. 2023. "Evaluation of Microbiological and Chemical Properties of Soils as a Result of Anthropogenic Denudation" Agriculture 13, no. 12: 2247. https://doi.org/10.3390/agriculture13122247