Mycotoxins and Other Secondary Metabolites Are Produced by Gnomoniopsis smithogilvyi When Confronted with Biological and Chemical Control Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Gnomoniopsis smithogilvyi Isolates
2.2. Isolation and Genetic Characterisation of the BCA
2.3. Preparation of Gnomoniopsis smithogilvyi Inoculum and Control Agents
2.4. Evaluation of Biocontrol Agents on Fungal Growth
2.5. Analysis of Secondary Metabolites by Multi-Analyte LC-MS/MS
2.6. Statistical Analysis
3. Results and Discussion
3.1. Identification of Gnomoniopsis smithogilvyi Isolates
3.2. Isolation and Identification of CIMO-BCA1
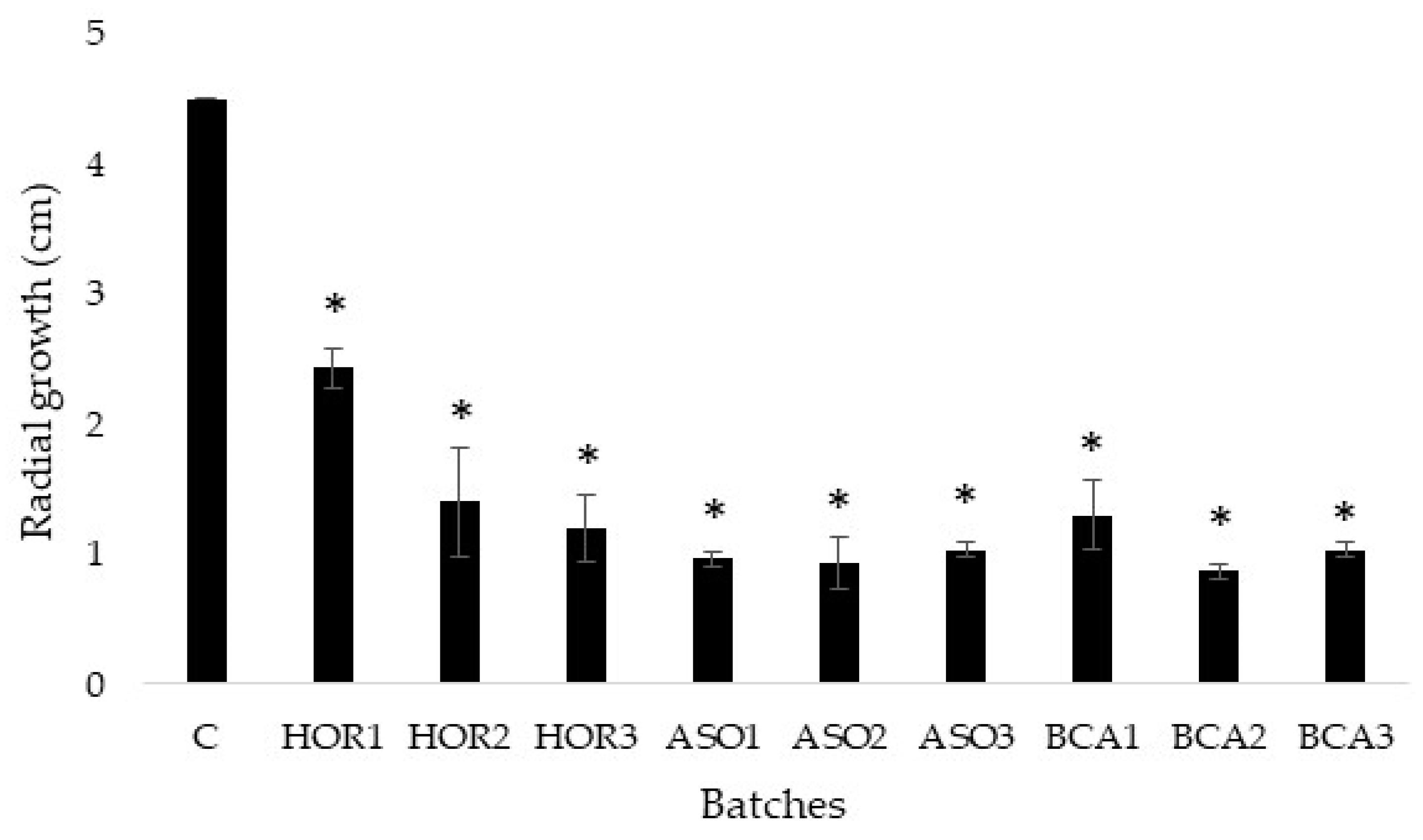
3.3. Radial Growth
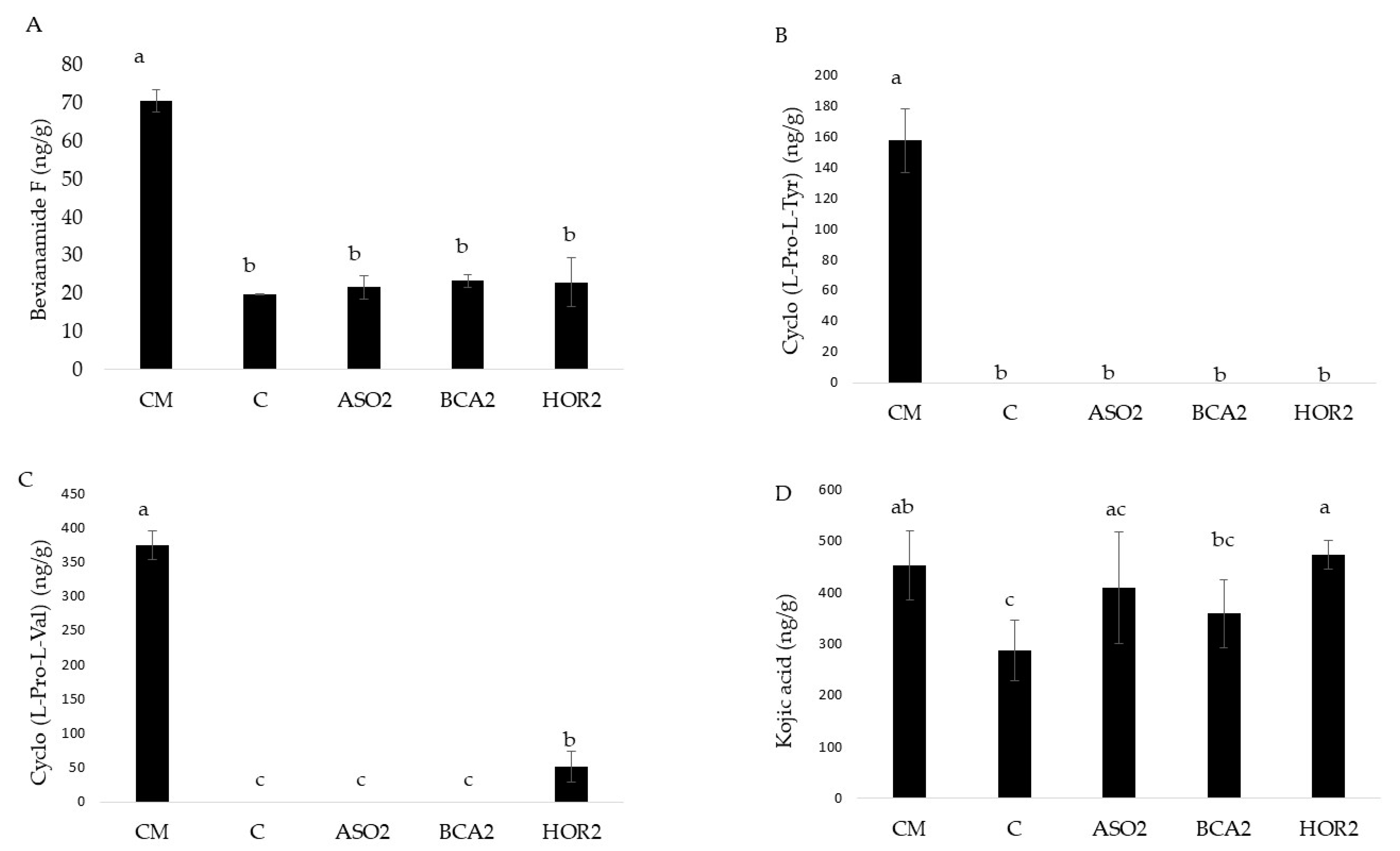
3.4. Fungal Secondary Metabolites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 1 February 2023).
- INE. Estatísticas Agrícolas 2020; Instituto Nacional de Estatística: Lisboa, Portugal, 2021; ISSN 0079-4139. [Google Scholar]
- Rodrigues, P.; Venâncio, A.; Lima, N. Incidence and diversity of the fungal genera Aspergillus and Penicillium in Portuguese almonds and chestnuts. Eur. J. Plant Pathol. 2013, 137, 197–209. [Google Scholar] [CrossRef]
- Rodrigues, P.; Venâncio, A.; Lima, N. Mycobiota and mycotoxins of almonds and chestnuts with special reference to aflatoxins. Food Res. Int. 2012, 48, 76–90. [Google Scholar] [CrossRef]
- Jiang, N.; Tian, C.M. An emerging pathogen from rotted chestnut in China: Gnomoniopsis daii sp. nov. Forests 2019, 10, 1016. [Google Scholar] [CrossRef]
- Jiang, N.; Liang, L.Y.; Tian, C.M. Gnomoniopsis chinensis (Gnomoniaceae, Diaporthales), a new fungus causing canker of Chinese chestnut in Hebei Province, China. MycoKeys 2020, 67, 19–32. [Google Scholar] [CrossRef]
- Dobry, E.; Campbell, M. Gnomoniopsis castaneae: An emerging plant pathogen and global threat to chestnut systems. Plant Pathol. 2023, 72, 218–231. [Google Scholar] [CrossRef]
- Possamai, G.; Dallemole-Giaretta, R.; Gomes-Laranjo, J.; Sampaio, A.; Rodrigues, P. Chestnut Brown Rot and Gnomoniopsis smithogilvyi: Characterization of the Causal Agent in Portugal. J. Fungi 2023, 9, 401. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, P.; Driss, J.O.; Gomes-Laranjo, J.; Sampaio, A. Impact of Cultivar, Processing and Storage on the Mycobiota of European Chestnut Fruits. Agriculture 2022, 12, 1930. [Google Scholar] [CrossRef]
- Ruocco, M.; Lombardi, N.; Lanzuise, S.; Varlese, R.; Aliberti, A.; Carpenito, S.; Woo, S.; Scala, F.; Lorito, M. New tools to improve the shelf life of chestnut fruit during storage. Acta Hortic. 2016, 1144, 309–315. [Google Scholar] [CrossRef]
- Hou, L.; Kou, X.; Li, R.; Wang, S. Thermal inactivation of fungi in chestnuts by hot air assisted radio frequency treatments. Food Control 2018, 93, 297–304. [Google Scholar] [CrossRef]
- Carocho, M.; Barreira, C.M.; Antonio, A.L.; Bento, A.; Kaluska, I.; Ferreira, I.C.F.R. Effeects of Electron-Beam Radiation on Nutritional Parameters of Portuguese Chestnuts (Castanea sativa Mill.). J. Agric. Food Chem. 2012, 60, 7754–7760. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Bianchini, L.; Caradonna, V.; Forniti, R.; Zambelli, M.; Testa, A.; Vinciguerra, V.; Botondi, R.; Lellis, V.S.C. De Ozone gas as a storage treatment to control Gnomoniopsis castanea preserving chestnut quality. J. Sci. Food Agric. 2019, 99, 6060–6065. [Google Scholar] [CrossRef] [PubMed]
- Silva-Campos, M.; Islam, M.; Cahill, D.M. Fungicide control of Gnomoniopsis smithogilvyi, casual agent of chestnut rot in Australia. Australas. Plant Pathol. 2022, 51, 483–494. [Google Scholar] [CrossRef]
- Bastianelli, G.; Morales-Rodríguez, C.; Caccia, R.; Turco, S.; Rossini, L.; Mazzaglia, A.; Thomidis, T.; Vannini, A. Use of phosphonate salts to control chestnuts ‘Brown Rot’ by Gnomoniopsis castanea in fruit orchards of Castanea sativa. Agronomy 2022, 12, 2434. [Google Scholar] [CrossRef]
- Anckaert, A.; Arias, A.A.; Hoff, G.; Calonne-Salmon, M.; Declerck, S.; Ongena, M. The use of Bacillus spp. as bacterial biocontrol agents to control plant diseases. In Microbial Bioprotectants for Plant Disease Management; Köhl, J., Ravensberg, W., Eds.; Burleigh Dodds Science Publishing: Cambridge, UK, 2022; ISBN 978 1 78676 813 1. [Google Scholar] [CrossRef]
- Fan, B.; Blom, J.; Klenk, H.P.; Borris, R. Bacillus amyloliquefaciens, Bacillus velezensis, and Bacillus siamensis form and “operational group B. amyloliquefaciens” within the B. subtilis species complex. Front. Microbiol. 2017, 8, 22. [Google Scholar] [CrossRef]
- Fousia, S.; Paplomatas, E.J.; Tjamos, S.E. Bacillus subtilis QST 713 confers protection to tomato plants against Pseudomonas syringae pv tomato and induces plant defence-related genes. J. Phytopathol. 2016, 164, 264–270. [Google Scholar] [CrossRef]
- Matzen, N.; Heick, T.M.; Jørgensen, L.N. Control of powdery mildew (Blumeria graminis spp.) in cereals by Serenade®ASO (Bacillus amyloliquefaciens (former subtilis) strain QST 713). Biol. Control 2019, 139, 104067. [Google Scholar] [CrossRef]
- Cucu, M.A.; Gilardi, G.; Pugliese, M.; Gullino, M.L.; Garibaldi, A. An assessment of the modulation of the population dynamics of pathogenic Fusarium oxysporum f. sp. lycopersici in the tomato rhizosphere by means of the application of Bacillus subtilis QST 713, Trichoderma sp. TW2 and two composts. Biol. Control 2020, 142, 104158. [Google Scholar] [CrossRef]
- Punja, Z.K.; Rodriguez, G.; Tirajoh, A. Effects of Bacillus subtilis strain QST 713 and storage temperatures on post-harvest disease development on greenhouse tomatoes. Crop Prot. 2016, 84, 98–104. [Google Scholar] [CrossRef]
- Gava, C.A.T.; Alves, Í.L.S.; Duarte, N.C. Timing the application of Bacillus subtilis QST 713 in the integrated management of the postharvest decay of mango fruits. Crop Prot. 2019, 121, 51–56. [Google Scholar] [CrossRef]
- EFSA; Anastassiadou, M.; Arena, M.; Auteri, D.; Brancato, A.; Bura, L.; Carrasco Cabrera, L.; Chaideftou, E.; Chiusolo, A.; Crivellente, F.; et al. Peer review of the pesticide risk assessment of the active substance Bacillus amyloliquefaciens strain QST 713 (formerly Bacillus subtilis strain QST 713). EFSA J. 2021, 19, e06381. [Google Scholar] [CrossRef]
- Vinale, F.; Ruocco, M.; Manganiello, G.; Guerrieri, E.; Bernardo, U.; Mazzei, P.; Piccolo, A.; Sannino, F.; Caira, S.; Woo, S.L.; et al. Metabolites produced by Gnomoniopsis castanea associated with necrosis of chestnut galls. Chem. Biol. Technol. Agric. 2014, 1, 8. [Google Scholar] [CrossRef]
- Silva-Campos, M.; Callahan, D.L.; Cahill, D.M. Metabolites derived from fungi and bacteria suppress in vitro growth of Gnomoniopsis smithogilvyi, a major threat to the global chestnut industry. Metabolomics 2022, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Pasche, S.; Crovadore, J.; Pelleteret, P.; Jermini, M.; Mauch-Mani, B.; Oszako, T.; Lefort, F. Biological control of the latent pathogen Gnomoniopsis smithogylvyi in European chestnut grafting scions using Bacillus amyloliquefaciens and Trichoderma atroviride. Dendrobiology 2016, 75, 113–122. [Google Scholar] [CrossRef]
- Rodrigues, P.; Venancio, A.; Lima, N. Toxic reagents and expensive equipment: Are they really necessary for the extraction of good quality fungal DNA? Lett. Appl. Microbiol. 2018, 66, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Ye, L.; Tong, A.H.Y.; Lok, S.; Zhang, T. Biased Diversity Metrics Revealed by Bacterial 16S Pyrotags Derived from Different Primer Sets. PLoS ONE 2013, 8, e53649. [Google Scholar] [CrossRef] [PubMed]
- De Clerck, E.; De Vos, P. Genotypic diversity among Bacillus licheniformis strains from various sources. FEMS Microbiol. Lett. 2004, 231, 91–98. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Pratush, A. Molecular diversity and functional variability of environmental isolates of Bacillus species. Springerplus 2014, 3, 312. [Google Scholar] [CrossRef]
- Sulyok, M.; Stadler, D.; Steiner, D.; Krska, R. Validation of an LC-MS/MS-based dilute-and-shoot approach for the quantification of >500 mycotoxins and other secondary metabolites in food crops: Challenges and solutions. Anal. Bioanal. Chem. 2020, 412, 2607–2620. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Cheng, S.; Cheng, H. Characterizing CMN1308, a Novel Strain of Bacillus amyloliquefaciens, for Potential Biological Control Application. Not. Bot. Horti. Agrobot. Cluj. Napoca 2016, 44, 60–66. [Google Scholar] [CrossRef]
- Ruíz-García, C.; Béjar, V.; Martínez-Checa, F.; Llamas, I.; Quesada, E. Bacillus velezensis sp. nov., a surfactant-producing bacterium isolated from the river Vélez in Málaga, southern Spain. Int. J. Syst. Evol. Microbiol. 2005, 55, 191–195. [Google Scholar] [CrossRef]
- Borriss, R.; Chen, X.H.; Rueckert, C.; Blom, J.; Becker, A.; Baumgarth, B.; Fan, B.; Pukall, R.; Schumann, P.; Spröer, C.; et al. Relationship of Bacillus amyloliquefaciens clades associated with strains DSM 7T and FZB42T: A proposal for Bacillus amyloliquefaciens subsp. amyloliquefaciens subsp. nov. and Bacillus amyloliquefaciens subsp. plantarum subsp. nov. based on complete genome sequence comparisons. Int. J. Syst. Evol. Microbiol. 2011, 61, 1786–1801. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.H.; Paul, N.C.; Deng, J.X.; Kim, Y.S.; Yun, B.S.; Yu, S.H. Biocontrol activity of Bacillus amyloliquefaciens CNU114001 against fungal plant diseases. Mycobiology 2013, 41, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Soliman, S.A.; Khaleil, M.M.; Metwally, R.A. Evaluation of the Antifungal Activity of Bacillus amyloliquefaciens and B. velezensis and Characterization of the Bioactive Secondary Metabolites Produced against Plant Pathogenic Fungi. Biology 2022, 11, 1390. [Google Scholar] [CrossRef] [PubMed]
- Ku, T.; Zhou, M.; Hou, Y.; Xie, Y.; Li, G.; Sang, N. Tebuconazole induces liver injury coupled with ROS-mediated hepatic metabolism disorder. Ecotoxicol. Environ. Saf. 2021, 220, 112309. [Google Scholar] [CrossRef] [PubMed]
- Danial, A.M.; Medina, A.; Sulyok, M.; Magan, N. Efficacy of metabolites of a Streptomyces strain (AS1) to control growth and mycotoxin production by Penicillium verrucosum, Fusarium verticillioides and Aspergillus fumigatus in culture. Mycotoxin Res. 2020, 36, 225–234. [Google Scholar] [CrossRef]
- Szulc, J.; Ruman, T. Laser Ablation Remote-Electrospray Ionisation Mass Spectrometry (LARESI MSI) Imaging- New Method for Detection and Spatial Localization of Metabolites and Mycotoxins Produced by Moulds. Toxins 2020, 12, 720. [Google Scholar] [CrossRef]
- Wattana-Amorn, P.; Charoenwongsa, W.; Williams, C.; Crump, M.P.; Apichaisataienchote, B. Antibacterial activity of cyclo(L-Pro-L-Tyr) and cyclo(D-Pro-L-Tyr) from Streptomyces sp. strain 22-4 against phytopathogenic bacteria. Nat. Prod. Res. 2016, 30, 1980–1983. [Google Scholar] [CrossRef]
- Kumar, S.N.; Nambisan, B. Antifungal activity of diketopiperazines and stilbenes against plant pathogenic fungi in vitro. Appl. Biochem. Biotechnol. 2014, 172, 741–754. [Google Scholar] [CrossRef]
- Ritthibut, N.; Lim, S.T.; Oh, S.J. In vitro cosmeceutical activity of alcoholic extract from chestnut inner shell fermented with Aspergillus sojae. Food Sci. Biotechnol. 2022, 31, 443–450. [Google Scholar] [CrossRef]
- Zhu, G.Y.; Shi, X.C.; Wang, S.Y.; Wang, B.; Laborda, P. Antifungal Mechanism and Efficacy of Kojic Acid for the Control of Sclerotinia sclerotiorum in Soybean. Front. Plant Sci. 2022, 13, 845698. [Google Scholar] [CrossRef]
- Zilles, J.C.; dos Santos, F.L.; Kulkamp-Guerreiro, I.C.; Contri, R.V. Biological activities and safety data of kojic acid and its derivatives: A review. Exp. Dermatol. 2022, 31, 1500–1521. [Google Scholar] [CrossRef] [PubMed]
- Ichinomiya, M.; Kawamoto, A.; Yamaguchi, T.; Iwashita, K.; Nagashima, H.; Hatabayashi, H.; Nakajima, H.; Yabe, K. Detoxication of Citrinin with Kojic Acid by the Formation of the Citrinin-Kojic Acid Adduct, and the Enhancement of Kojic Acid Production by Citrinin via Oxidative Stress in Aspergillus parasiticus. J. Fungi 2022, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Deng, Q.; Sun, M.; Xu, J. Cytospyrone and Cytospomarin: Two new polyketides isolated from mangrove endophytic fungus, Cytospora sp. Molecules 2020, 25, 4224. [Google Scholar] [CrossRef]
- Flores, A.C.; Pamphile, J.A.; Sarragiotto, M.H.; Clemente, E. Production of 3-nitropropionic acid by endophytic fungus Phomopsis longicolla isolated from Trichilia elegans A. JUSS ssp. elegans and evaluation of biological activity. World J. Microbiol. Biotechnol. 2013, 29, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Furian, A.F.; Fighera, M.R.; Royes, L.F.F.; Oliveira, M.S. Recent advances in assessing the effects of mycotoxins using animal models. Curr. Opin. Food Sci. 2022, 47, 100874. [Google Scholar] [CrossRef]
- Skogvold, H.; Yazdani, M.; Sandås, E.M.; Østeby Vassli, A.; Kristensen, E.; Haarr, D.; Rootwelt, H.; Elgstøen, K.B.P. A pioneer study on human 3-nitropropionic acid intoxication: Contributions from metabolomics. J. Appl. Toxicol. 2022, 42, 818–829. [Google Scholar] [CrossRef]
- Salman, M.; Sharma, P.; Alam, M.I.; Tabassum, H.; Parvez, S. Naringenin mitigates behavioral alterations and provides neuroprotection against 3-nitropropinoic acid-induced Huntington’s disease like symptoms in rats. Nutr. Neurosci. 2022, 25, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Wicklow, D.T.; Rogers, K.D.; Dowd, P.F.; Gloer, J.B. Bioactive metabolites from Stenocarpella maydis, a stalk and ear rot pathogen of maize. Fungal Biol. 2011, 115, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Botha, C.J.; Ackerman, L.G.J.; Masango, M.G.; Arnot, L.F. Failure of diplodiatoxin to induce diplodiosis in juvenile goats. Onderstepoort J. Vet. Res. 2020, 87, a1712. [Google Scholar] [CrossRef]
- Masango, M.G.; Ellis, C.E.; Botha, C.J. Characterization of cell death caused by diplodiatoxin and dipmatol, toxic metabolites of Stenocarpella maydis. Toxicon 2015, 102, 14–24. [Google Scholar] [CrossRef]
- Lim, F.Y.; Hou, Y.; Chen, Y.; Oh, J.H.; Lee, I.; Bugni, T.S.; Keller, N.P. Genome-based cluster delection reveals an endocrocin biosynthetic pathway in Aspergillus fumigatus. Appl. Environ. Microbiol. 2012, 78, 4117–4125. [Google Scholar] [CrossRef]
- Berthier, E.; Lim, F.Y.; Deng, Q.; Guo, C.J.; Kontoyiannis, D.P.; Wang, C.C.C.; Rindy, J.; Beebe, D.J.; Huttenlocher, A.; Keller, N.P. Low-volume toolbox for the discoery of immunosuppressive fungal secondary metabolites. PLoS Pathog. 2013, 9, e1003289. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, M.; Rodríguez, A.; Núñez, F.; Silva, A.; Andrade, M.J. In vitro antifungal effects of spices on ochratoxin A production and related gene expression in Penicillium nordicum on a dry-cured fermented sausage medium. Food Control 2020, 114, 107222. [Google Scholar] [CrossRef]
- da Cruz Cabral, L.; Rodríguez, A.; Andrade, M.J.; Patriarca, A.; Delgado, J. Effect of Debaryomyces hansenii and the antifungal PgAFP protein on Alternaria spp. growth, toxin production, and RHO1 gene expression in a tomato-based medium. Food Microbiol. 2021, 97, 103741. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Heydt, M.; Baxter, E.; Geisen, R.; Magan, N. Physiological relationship between food preservatives, environmental factors, ochratoxin and otapksPV gene expression by Penicillium verrucosum. Int. J. Food Microbiol. 2007, 119, 277–283. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar]
- Kimura, M. A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA 11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 33, 1870–1874. [Google Scholar] [CrossRef]

| Antifungal Agent | Batch Denomination | Concentration of Antifungal Agent (µL/mL or cfu/mL) | Concentration of Active Substance (g/L) |
|---|---|---|---|
| Serenade® ASO | ASO1 | 5.5 | 0.074 |
| ASO2 | 10.0 | 0.134 | |
| ASO3 | 16.0 | 0.214 | |
| Horizon® | HOR1 | 0.2 | 0.050 |
| HOR2 | 0.4 | 0.100 | |
| HOR3 | 0.6 | 0.150 | |
| CIMO-BCA1 | BCA1 | 105 | - |
| BCA2 | 107 | - | |
| BCA3 | 109 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, M.; Agostini, I.; Silva, S.; Dallemole-Giaretta, R.; Sulyok, M.; Sampaio, A.; Rodrigues, P. Mycotoxins and Other Secondary Metabolites Are Produced by Gnomoniopsis smithogilvyi When Confronted with Biological and Chemical Control Agents. Agriculture 2023, 13, 1166. https://doi.org/10.3390/agriculture13061166
Álvarez M, Agostini I, Silva S, Dallemole-Giaretta R, Sulyok M, Sampaio A, Rodrigues P. Mycotoxins and Other Secondary Metabolites Are Produced by Gnomoniopsis smithogilvyi When Confronted with Biological and Chemical Control Agents. Agriculture. 2023; 13(6):1166. https://doi.org/10.3390/agriculture13061166
Chicago/Turabian StyleÁlvarez, Micaela, Isadora Agostini, Sofia Silva, Rosangela Dallemole-Giaretta, Michael Sulyok, Ana Sampaio, and Paula Rodrigues. 2023. "Mycotoxins and Other Secondary Metabolites Are Produced by Gnomoniopsis smithogilvyi When Confronted with Biological and Chemical Control Agents" Agriculture 13, no. 6: 1166. https://doi.org/10.3390/agriculture13061166






