Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research
Abstract
:1. Introduction
2. Fusarium: Overview and Taxonomy
3. Identification of Phytopathogenic Fusarium Fungal Species
3.1. Morphological Characters for Identifying Phytopathogenic Fusarium Species
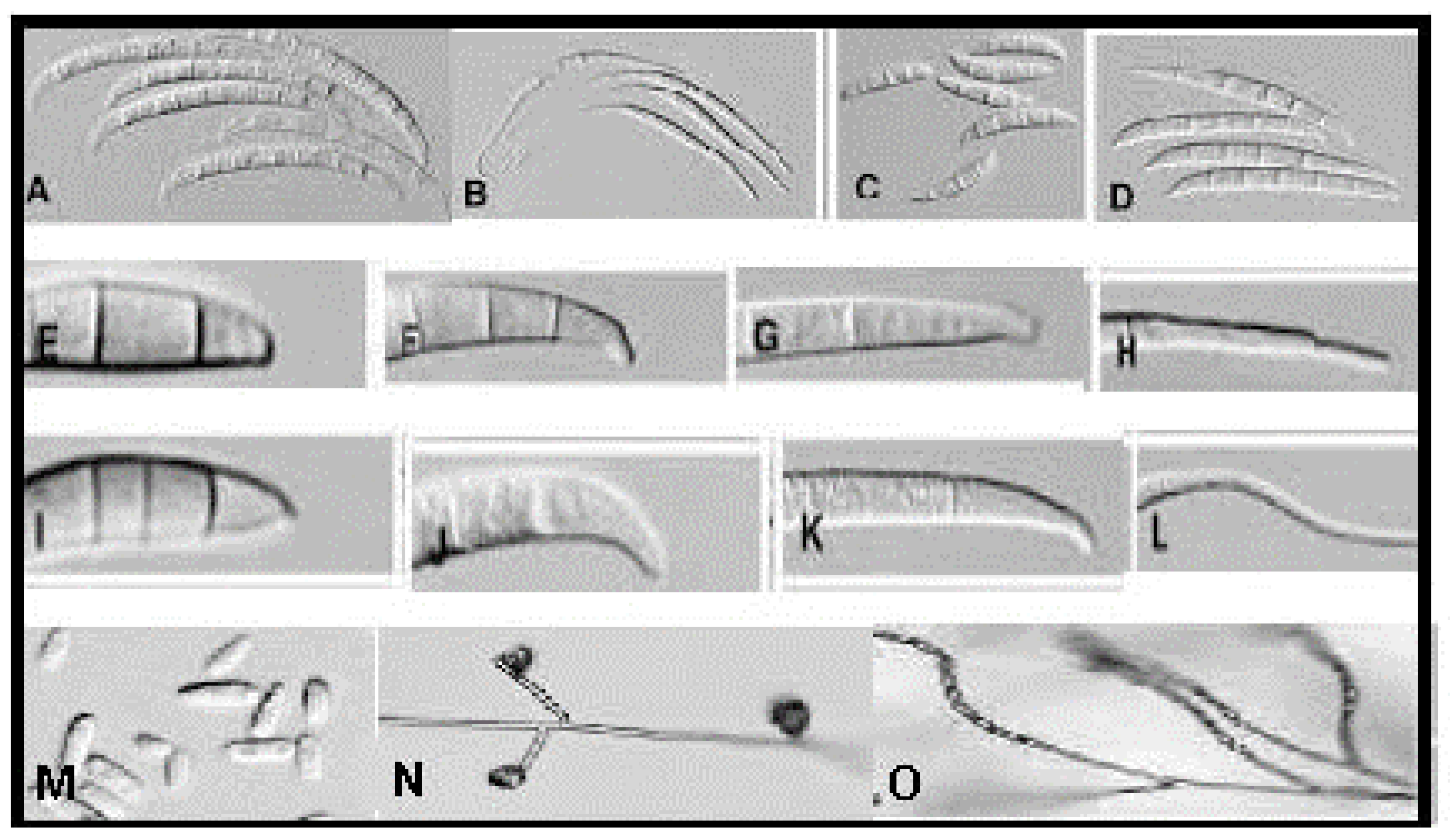
3.1.1. Universally Found Characters
- (i)
- The macroconidium is the single most important cultural characteristic for the identification of a culture of Fusarium species. The most distinctive characteristic of the macroconidia is its shape, followed by the size and number of septa, and finally, the nature of apical, basal, or foot cells [12]. Regarding shape, most Fusarium produce sickle-shaped macroconidia that can be characterized into three types: (1) straight macroconidia, which can appear virtually needle-like if they are thin, e.g., F. avenacum; (2) microconidia having dorsivental curvature along all or a portion of the spore (these spores are almost of the same width along their entire length, e.g., F. equiseti); and (3) microconidia in which the dorsal side is more curved than the ventral side, e.g., F. crookwellence (Figure 1). Macroconidia can be long (F. armeniacum) or short (F. culmorum), but usually spore size is a relatively constant character and major variations indicate improper culture conditions. Typically, Fusarium macroconidia are 3–5-septate. The number of septa ought to be determined depending on the range and the average number of septa per spore [12].
- (ii)
- Chlamydospores are verrucose (rough) or smooth-walled structures produced individually, e.g., F. solani; as doubles or pairs, e.g., F. compactum; as clumps, e.g., F. scirpi; or as chains, e.g., F. compactum. Chlamydospores are produced rarely and take a longer time (more than 6 weeks) when compared to macro- or microconidia. The presence of chlamydospores in the aerial mycelia or embedded on the agar surface is another important criterion used in the identification of Fusarium species [12].
3.1.2. Other Important Characteristics
3.2. Molecular Tools for Identifying Phytopathogenic Fusarium Species Based on Genetic Diversity
- (i)
- Random Amplified Polymorphic DNA (RAPD)
- (ii)
- Restriction fragment length polymorphism (RFLP)
- (iii)
- Amplified fragment length polymorphism (AFLP)
- (iv)
- Inter-genic Spacers (IGS)
- (v)
- β-tubulin
- (vi)
- Translation elongation factors
- (vii)
- Internal Transcribed Spacers (ITS)
- (viii)
- The matrix-assisted laser desorption/ionization time-of-flight mass spectrometry
4. The Pathogen—Fusarium
4.1. Fusarium as a Plant Pathogen
- (i)
- Adhesion
- (ii)
- Entry
- (iii)
- Colonization, Adaptation, and Propagation
- (iv)
- Disease development
| Pathogen | Host Plant | Infection | Reference |
|---|---|---|---|
| F. avenaceum | Wheat | Fusarium head blight (FHB) | [95] |
| F. oxysporium | Oriental lilium plant | Root and bulb disease | [96] |
| F. oxysporum | Potato | Stem-end rot | [97] |
| F. oxysporum | Banana | Fusarium wilt | [98] |
| F. oxysporium | Pineapple | Dieback | [99] |
| F. oxysporium | Avocado | Stem-end rot | [100] |
| F. fujikuroi | Rice | Bakane | [101] |
| F. graminearum | Oil palm | Fusarium wilt | [102] |
| F. graminearum | Banana | Crown rot | [103] |
| F. graminearum | Wheat and barley | Fusarium head blight (FHB) | [104] |
| F. proliferatum | Oriental lilium plant | Root and bulb disease | [96] |
| F. proliferatum | Mango | Leaf spot | [105] |
| F. proliferatum | Pineapple | Fruitlet core rot | [106] |
| F. proliferatum | Chilli pepper (Capsicum annuum L.) | Fruit rot | [107] |
| F. solani | Paprika | Fusarium rot | [108] |
| F. solani | Avocado | Stem-end rot | [109] |
| F. solani | Papaya | Root rot | [110] |
| F. verticilloides | Maize | Fusarium ear rot | [111] |
| F. verticilloides | Mango | Leaf spot | [112] |
| F. verticilloides | Banana | Crown rot | [113] |
| F. commune | Chinese water plant (Eleocharis dulcis) | Fusarium wilt | [114] |
| F. equiseti | Papaya | Fruit rot | [115] |
| F. equiseti | Avocado | Stem-end rot | [116] |
| F. sporotrichioides | Avocado | Stem-end rot | [117] |
4.2. Fusarium as Human and Animal Pathogen
- (i)
- Adhesion
- (ii)
- Entry
- (iii)
- Colonization, Adaptation, and Propagation
- (iv)
- Disease development, Dissemination
5. Plant Pathologies Caused by Fusarium Species
6. Host–Pathogen Interaction in Fusarium Infections
- (i)
- Host-specific toxins in Fusarium infections
- (ii)
- Effector Proteins
- (iii)
- Cell-Wall-Degrading Enzymes

7. Control and Management of Phytopathogenic Fusarium spp.
8. Phytopathogenic Fusarium spp. and Global Food Security
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, B.; Xie, J.; Fu, Y.; Cheng, J.; Li, B.; Chen, T.; Zhao, Y.; Gao, Z.; Yang, P.; Barbetti, M.J.; et al. A cosmopolitan fungal pathogen of dicots adopts an endophytic lifestyle on cereal crops and protects them from major fungal diseases. ISME J. 2020, 14, 3120–3135. [Google Scholar] [CrossRef] [PubMed]
- Wu, F. Perspective: Time to face the fungal threat. Nature 2014, 516, S7. [Google Scholar] [CrossRef] [PubMed]
- Ekwomadu, T.I.; Gopane, R.E.; Mwanza, M. Occurrence of filamentous fungi in maize destined for human consumption in South Africa. Food Sci. Nutr. 2018, 6, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.J.; Geiser, D.M.; Proctor, R.H.; Rooney, A.P.; O’Donnell, K.; Trail, F.; Gardiner, D.M.; Manners, J.M.; Kazan, K. Fusarium pathogenomics. Annu. Rev. Microbiol. 2013, 67, 399–416. [Google Scholar] [CrossRef]
- Ekwomadu, T.I. Occurrence and Variation of Fusarium Free and Masked Mycotoxins in Maize from Agriculture Regions of South Africa. Doctoral Dissertation, North-West University, Potchefstroom, South Africa, 2019. [Google Scholar]
- Dean, R.; van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The top 10 fungal pathogens in molecular plant pathology. Mol. Plant. Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Burgess, L.W.; Bryden, W.L. Fusarium: A ubiquitous fungus of global significance. Microbiol. Aust. 2012, 33, 22–25. [Google Scholar] [CrossRef]
- Rampersad, S.N. Pathogenomics and Management of Fusarium Diseases in Plants. Pathogens 2020, 9, 340. [Google Scholar] [CrossRef]
- Nelson, P.E.; Toussoun, T.A.; Cook, R.J. Fusarium: Diseases, Biology, and Taxonomy; The Pennsylvania State University: State College, PA, USA, 1981. [Google Scholar]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: State College, PA, USA, 1983. [Google Scholar]
- Logrieco, A.; Bottalico, A.; Mule, G.; Moretti, A.; Perrone, G. Epidemiology of toxigenic fungi and their associated mycotoxins for some Mediterranean crops. Eur. J. Plant Pathol. 2003, 109, 645–667. [Google Scholar] [CrossRef]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Publishing: Hoboken, NJ, USA, 2006. [Google Scholar]
- Summerell, B.A.; Laurence, M.H.; Liew, E.C.Y.; Leslie, J.F. Biogeography and phylogeography of Fusarium: A review. Fungal Divers. 2010, 44, 3–13. [Google Scholar] [CrossRef]
- Wollenweber, H.W.; Reinking, O.A. Die Usarien, HhreBeschreibung, Schadwirkung, und Bekämpfung; Verlag Paul Parey: Berlin, Germany, 1983. [Google Scholar]
- Moss, M.O.; Thrane, U. Fusarium taxonomy with relation to trichothecene formation. Toxicol. Lett. 2004, 153, 23–28. [Google Scholar] [CrossRef]
- Booth, C. The Genus Fusarium; Commonwealth Mycological Institute, The Eastern Press Limited: London, UK, 1971. [Google Scholar]
- Munkvold, G. Fusarium Species and their Associated Mycotoxins. In Mycotoxigenic Fungi; Moretti, A., Susca, A., Eds.; Springer: New York, NY, USA, 2017; pp. 51–106. ISBN 978-1-4939-6705-6. [Google Scholar]
- Jay, J.M. Modern Food Microbiology, 3rd ed.; CBS Publishers and Distributors: New Delhi, India, 1987; pp. 541–551. [Google Scholar]
- Joffe, A.Z. A modern system of Fusarium taxonomy. Mycopathologia 1974, 53, 201–228. [Google Scholar] [CrossRef] [PubMed]
- Arici, S.E.; Koc, N.K. RAPD–PCR analysis of genetic variation among isolates of Fusarium graminearum and Fusarium culmorum from wheat in Adana Turkey. Pak. J. Biol. Sci. 2010, 13, 138–142. [Google Scholar] [PubMed]
- Martínez-García, L.B.; Armas, C.; Miranda, J.D.; Padilla, F.M.; Pugnaire, F.I. Shrubs influence arbuscular mycor-rhizal fungi communities in a semi-arid environment. Soil Biol. Biochem. 2011, 43, 682–689. [Google Scholar] [CrossRef]
- Leissner, C.E.W.; Niessen, M.L.; Vogel, R.F. Use of the AFLP technique for the identification and discrimination of Fusarium graminearum. Cereal Res. Commun. 1997, 25, 555–556. [Google Scholar] [CrossRef]
- Schmidt, H.; Adler, A.; Holst-Jensen, A.; Klemsdal, S.; Logrieco, A.; Mach, R.; Nirenberg, H.; Thrane, U.; Torp, M.; Vogel, R.; et al. An integrated taxonomic study of Fusarium langsethiae, Fusarium poae and Fusarium sporotrichioides based on the use of composite datasets. Int. J. Food Microbiol. 2004, 95, 341–349. [Google Scholar] [CrossRef]
- Konstantinova, P.; Yli-Mattila, T. IGS-RFLP analysis and development of molecular markers for identification of Fusarium poae, F. langsethiae, F. sporotrichioides and F. kyushuense. Int. J. Food Microbiol. 2004, 95, 321–331. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Mach, R.; Alekhina, I.; Bulat, S.; Koskinen, S.; Kullnig-Gradinger, C.; Kubicek, C.; Klemsdal, S. Phylogenetic relationship of Fusarium langsethiae to Fusarium poae and Fusarium sporotrichioides as inferred by IGS, ITS, β-tubulin sequences and UP-PCR hybridization analysis. Int. J. Food Microbiol. 2004, 95, 267–285. [Google Scholar] [CrossRef]
- Knutsen, A.; Torp, M.; Holst-Jensen, A. Phylogenetic analyses of the Fusarium poae, Fusarium sporotrichioides and Fusarium langsethiae species complex based on partial sequences of the translation elongation factor-1 alpha gene. Int. J. Food Microbiol. 2004, 95, 287–295. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Hatsch, D.; Phalip, V.; Jeltsch, J.M. Use of genes encoding cellobiohydrolase-C and topoisomerase II as targets for phylogenetic analyses and identification of Fusarium. Res. Microbiol. 2004, 155, 290–296. [Google Scholar] [CrossRef]
- Mulè, G.; Susca, A.; Stea, G.; Moretti, A. Specific detection of the toxigenic species Fusarium proliferatum and F. oxysporum from asparagus plants using primers based on calmodulin gene sequences. FEMS Microbiol. Lett. 2004, 230, 235–240. [Google Scholar] [CrossRef]
- Wigmann, F.; Behr, J.; Vogel, R.F.; Niessen, L. MALDI-TOF MS fingerprinting for identification and differentiation of species within the Fusarium fujikuroi species complex. Appl. Microbiol. Biotechnol. 2019, 103, 5323–5337. [Google Scholar] [CrossRef] [PubMed]
- Dassanayake, R.S.; Samaranayake, L.P. Amplification-Based Nucleic Acid Scanning Techniques to Assess Genetic Polymorphism in Candida. Crit. Rev. Microbiol. 2003, 29, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.S.; Gurusubramanian, G. Random amplified polymorphic DNA (RAPD) markers and its applications. Sci. Vis. 2011, 3, 116–124. [Google Scholar]
- Nicholson, P.; Simpson, D.R.; Weston, G.; Rezanoor, H.N.; Lees, A.K.; Parry, D.W.; Joyce, D. Detection and quantification of Fusarium culmorum and Fusarium graminearum in cereals using PCR assays. Physiol. Mol. Plant Pathol. 1998, 53, 17–37. [Google Scholar] [CrossRef]
- Fungaro, M.H.P.; Magnani, M.; Vilas-Boas, L.A.; Vissotto, P.C.; Furlaneto, M.C.; Vieira, M.L.C.; Taniwaki, M.H. Genetic relationships among Brazilian strains of Aspergillus ochraceus based on RAPD and ITS sequences. Can. J. Microbiol. 2004, 50, 985–988. [Google Scholar] [CrossRef]
- Yli-Mattila, T.; Paavanen, S.; Hannukkala, A.; Parikka, P.; Tahvonen, R.; Karjalainen, R. Isozyme and RAPD-PCR analyses of Fusarium avenaceum strains from Finland. Plant Pathol. 1996, 45, 126–134. [Google Scholar] [CrossRef]
- Kernnyi, Z.; Tabarhegyi, E.; Pomazi, A.; Hornok, L. Variability among strains of Fusarium Poae assessed by vege-tative compatibility and RAPD polymorphism. Plant Pathol. 1997, 46, 882–889. [Google Scholar] [CrossRef]
- Hue, F.-X.; Huerre, M.; Rouffault, M.A.; de Bievre, C. Specific Detection of Fusarium Species in Blood and Tissues by a PCR Technique. J. Clin. Microbiol. 1999, 37, 2434–2438. [Google Scholar] [CrossRef]
- Paavanen-Huhtala, S.; Hyvönen, J.; Bulat, S.; Yli-Mattila, T. RAPD-PCR, isozyme, rDNA RFLP and rDNA sequence analyses in identification of Finnish Fusarium oxysporum isolates. Mycol. Res. 1999, 103, 625–634. [Google Scholar] [CrossRef]
- Ellsworth, D.L.; Rittenhouse, K.D.; Honeycutt, R.L. Artifactual variation in randomly amplified polymorphic DNA banding patterns. BioTechniques 1993, 14, 214–217. [Google Scholar]
- Khandka, D.K.; Tuna, M.; Tal, M.; Nejidat, A.; Golan-Goldhirsh, A. Variability in the pattern of random amplified polymorphic DNA. Electrophoresis 1997, 18, 2852–2856. [Google Scholar] [CrossRef]
- Capote, N.; Pastrana, A.M.; Aguado, A.; Sánchez-Torres, P. Molecular Tools for Detection of Plant Pathogenic Fungi and Fungicide Resistance. In Plant Pathology; Cumagun, C.J., Ed.; InTechOpen: London, UK, 2012; ISBN 978-953-51-0489-6. [Google Scholar]
- Thies, J.E. Soil Microbial Community Analysis using Terminal Restriction Fragment Length Polymorphisms. Soil Sci. Soc. Am. J. 2007, 71, 579–591. [Google Scholar] [CrossRef]
- Kim, Y.T.; Cho, M.; Jeong, J.Y.; Lee, H.B.; Kim, S.B. Application of Terminal Restriction Fragment Length Polymorphism (T-RFLP) analysis to monitor effect of biocontrol agents on rhizosphere microbial community of hot pepper (Capsicum annuum L.). J. Microbiol. 2010, 48, 566–572. [Google Scholar] [CrossRef]
- Hyakumachi, M.; Priyatmojo, A.; Kubota, M.; Fukui, H. New anastomosis groups, AG-T and AG-U, of binucleate Rhizoctonia spp. causing root and stem rot of cutflower and miniature roses. Phytopathology 2005, 95, 784–792. [Google Scholar] [CrossRef]
- Vos, P.; Hogers, R.; Bleeker, M.; Reijans, M.; Van De Lee, T.; Hornes, M.; Friters, A.; Pot, J.; Paleman, J.; Kuiper, M.; et al. AFLP: A new technique for DNA fingerprinting. Nucleic Acids Res. 1995, 21, 4407–4414. [Google Scholar] [CrossRef]
- McDonald, B.A.; Kimunye, J.N.; Muzhinji, N.; Mostert, D.; Viljoen, A.; der Merwe, A.E.B.-V.; Everhart, S.; Gambhir, N.; Stam, R.; Wyman, C.R.; et al. The Population Genetics of Fungi: Tools and Techniques. Phytopathology 1997, 87, 448–453. [Google Scholar] [CrossRef]
- Gril, T.; Celar, F.; Munda, A.; Javornik, B.; Jakse, J. AFLP Analysis of Intraspecific Variation Between Monilinia laxa Isolates from Different Hosts. Plant Dis. 2008, 92, 1616–1624. [Google Scholar] [CrossRef]
- Stewart, J.E.; Kim, M.-S.; James, R.L.; Dumroese, R.K.; Klopfenstein, N.B.; Hassan, O.; Lim, T.-H.; Chang, T.; Zhu, Z.; Zheng, L.; et al. Molecular Characterization of Fusarium oxysporum and Fusarium commune Isolates from a Conifer Nursery. Phytopathology 2006, 96, 1124–1133. [Google Scholar] [CrossRef]
- Baayen, R.P.; O’Donnell, K.; Bonants, P.J.M.; Cigelnik, E.; Kroon, L.P.N.M.; Roebroeck, E.J.A.; Waalwijk, C. Gene genealogies and AFLP analyses in the Fusarium oxysporum complex identify monophyletic and nonmonophyletic formae speciales causing wilt and rot disease. Phytopathology 2000, 90, 891–900. [Google Scholar] [CrossRef]
- Groenewald, S.; Van Den Berg, N.; Marasas, W.F.; Viljoen, A. The application of high-throughput AFLP’s in assessing genetic diversity in Fusarium oxysporum f. sp. cubense. Mycol. Res. 2006, 110, 297–305. [Google Scholar] [CrossRef]
- Fourie, G.; Steenkamp, E.T.; Ploetz, R.C.; Gordon, T.R.; Viljoen, A. Current status of the taxonomic position of Fusarium oxysporum formae specialis cubense within the Fusarium oxysporum complex. Infect. Genet. Evol. 2011, 11, 533–542. [Google Scholar] [CrossRef]
- Taylor, J.W.; Jacobson, D.J.; Kroken, S.; Kasuga, T.; Geiser, D.M.; Hibbett, D.S.; Fisher, M.C. Phylogenetic Species Recognition and Species Concepts in Fungi. Fungal Genet. Biol. 2000, 31, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Hillis, D.M.; Dixon, M.T. Ribosomal DNA: Molecular Evolution and Phylogenetic Inference. Q. Rev. Biol. 1991, 66, 411–453. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Tewari, J.P.; Clear, R.M.; Turkington, T.K. Genetic diversity and recombination within populations of Fusarium pseudograminearum from western Canada. Int. Microbiol. 2006, 9, 65–68. [Google Scholar] [PubMed]
- Mbofung, G.C.Y.; Hong, S.G.; Pryor, B.M.; Herrero, M.L.; Nagy, N.E.; Solheim, H.; Hafez, M.; Abdelmagid, A.; Aboukhaddour, R.; Adam, L.R.; et al. Phylogeny of Fusarium oxysporum f. sp. lactucae Inferred from Mitochondrial Small Subunit, Elongation Factor 1-α, and Nuclear Ribosomal Intergenic Spacer Sequence Data. Phytopathology 2007, 97, 87–98. [Google Scholar] [CrossRef]
- Samson, R.A.; Seifert, K.A.; Kuijpers, A.F.A.; Houbraken, J.A.M.P.; Frisvad, J.C. Phylogenetic analysis of Penicillium subgenus Penicillium using partial B-tubulin sequences. Stud. Mycol. 2004, 49, 175–200. [Google Scholar]
- Amrani, L.; Corio-Costet, M.-F. A single nucleotide in the β-tubulin gene distinguishing two genotypes of Erysiphe necator expressing different symptoms on grapevine. Plant Pathol. 2006, 55, 505–512. [Google Scholar] [CrossRef]
- Geiser, D.M.; Jiménez-Gasco, M.d.M.; Kang, S.; Makalowska, I.; Veeraraghavan, N.; Ward, T.J.; Zhang, N.; Kuldau, G.A.; O’Donnell, K. FUSARIUM-ID v. 1.0: A DNA Sequence Database for Identifying Fusarium. Eur. J. Plant Pathol. 2004, 110, 473–479. [Google Scholar] [CrossRef]
- O’Donnell, K.; Kistler, H.C.; Cigelnik, E.; Ploetz, R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA 1998, 9, 2044–2049. [Google Scholar] [CrossRef]
- Bruns, T.D.; Shefferson, R.P. Evolutionary studies of ectomycorrhizal fungi: Recent advances and future directions. Can. J. Bot. 2004, 82, 1122–1132. [Google Scholar] [CrossRef]
- Young-Mi, L.; Choi, Y.-K.; Min, B.-R. PCR-RFLP and sequence analysis of the rDNA ITS region in the Fusarium spp. J. Microbiol. 2000, 38, 66–73. [Google Scholar]
- Durán, A.; Gryzenhout, M.; Drenth, A.; Slippers, B.; Ahumada, R.; Wingfield, B.D.; Wingfield, M.J. AFLP analysis reveals a clonal population of Phytophthora pinifolia in Chile. Fungal Biol. 2010, 114, 746–752. [Google Scholar] [CrossRef]
- Sanitá-Lima, M.; de Lucas, R.C.; Lima, N.; Polizeli, M.L.T.; Santos, C. Fungal community ecology using MALDI-TOF MS demands curated mass spectral databases. Front. Microbiol. 2019, 10, 315. [Google Scholar] [CrossRef]
- Quéro, L.; Girard, V.; Pawtowski, A.; Tréguer, S.; Weill, A.; Arend, S.; Cellière, B.; Polsinelli, S.; Monnin, V.; van Belkum, A.; et al. Development and application of MALDI-TOF MS for identification of food spoilage fungi. Food Microbiol. 2019, 81, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Huschek, D.; Witzel, K. Rapid dereplication of microbial isolates using matrix-assisted laser desorption ionization time-of-flight mass spectrometry: A mini-review. J. Adv. Res. 2019, 19, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Tupaki-Sreepurna, A.; Kindo, A.J. Fusarium The versatile pathogen. Indian J. Med. Microbiol. 2018, 36, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Elvers, K.T.; Leeming, K.; Moore, C.P.; Lappin-Scott, H.M. Bacterial-fungal biofilms in flowing water pho-to-processing tanks. J. Appl. Microbiol. 1998, 84, 607–618. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.M.; Armbruster, C.R.; Arduino, M.J. Plumbing of hospital premises is a reservoir for opportunistically pathogenic microorganisms: A review. Biofouling 2013, 29, 147–162. [Google Scholar] [CrossRef]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Gale, L.R.; Chen, L.F.; Hernick, C.A.; Takamura, K.; Kistler, H.C. Population Analysis of Fusarium graminearum from Wheat Fields in Eastern China. Phytopathology 2002, 92, 1315–1322. [Google Scholar] [CrossRef]
- Nucci, M.; Anaissie, E. Fusarium Infections in Immunocompromised Patients. Clin. Microbiol. Rev. 2007, 20, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Nelson, P.E.; Dignani, M.C.; Anaissie, E.J. Taxonomy, biology, and clinical aspects of Fusarium species. Clin. Microbiol. Rev. 1994, 7, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Guarro, J. Fusariosis, a complex infection caused by a high diversity of fungal species refractory to treatment. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M.; Lass-Flörl, C. Changing epidemiology of systemic fungal infections. Clin. Microbiol. Infect. 2008, 14, 5–24. [Google Scholar] [CrossRef]
- Lysøe, E.; Klemsdal, S.S.; Bone, K.R.; Frandsen, R.J.N.; Johansen, T.; Thrane, U.; Giese, H. The PKS4 Gene of Fusarium graminearum Is Essential for Zearalenone Production. Appl. Environ. Microbiol. 2006, 72, 3924–3932. [Google Scholar] [CrossRef]
- Schollenberger, M.; Muller, H.M.; Rufle, M.; Suchy, S.; Drochner, W. Redistribution of 16 Fusarium toxins during comer cial dry milling of maize. Cereal Chem. 2008, 85, 557–560. [Google Scholar] [CrossRef]
- Marasas, W.F.O.; Wehner, F.C.; van Rensburg, S.J.; van Schalkwyk, D.J. Mycoflora of corn produced in human esoph ageal cancer areas in Transkei, Southern Africa. Phytopathology 1981, 71, 792–796. [Google Scholar] [CrossRef]
- Rheeder, J.P.; Marasas, W.F.O.; Thiel, P.G.; Sydenham, E.W.; Shephard, G.S.; van Schalkwyk, D.J. Fusarium moniliform and fumonisins in corn in relation to human oesophageal cancer in Transkei. Phytopathology 1992, 82, 353–357. [Google Scholar] [CrossRef]
- Cotten, T.K.; Munkvold, G.P. Survival of Fusarium moniliforme, F. proliferatum and F. subglutinans in maize stalk residue. Ecol. Popul. Biol. 1998, 88, 550–555. [Google Scholar] [CrossRef]
- Lucas, J. Plant Diseases. In Plant Pathology and Plant Pathogens; Blackwell Publishing: Malden, MA, USA, 1998. [Google Scholar]
- Bishop, C.; Cooper, R. An ultrastructural study of root invasion in three vascular wilt diseases. Physiol. Plant Pathol. 1983, 22, 15-IN13. [Google Scholar] [CrossRef]
- Vidhyasekaran, P. Fungal Pathogenesis in Plants and Crops. In Molecular Biology and Host Defense Mechanisms; Marcel Dekker Inc.: New York, NY, USA, 1997; p. 553. [Google Scholar]
- Recorbet, G.; Alabouvette, C. Adhesion of Fusarium oxysporum conidia to tomato roots. Lett. Appl. Microbiol. 1997, 25, 375–379. [Google Scholar] [CrossRef]
- Mendgen, K.; Hahn, M.; Deising, H. Morphogenesis and mechanisms of penetration by plant pathogenic fungi. Annu. Rev. Phytopathol. 1996, 34, 367–386. [Google Scholar] [CrossRef]
- Koretsky, L.S. The influence of Fusarium oxysporum infection and low temperatures on the activity of soybean esterase and PR proteins. Búvisindi Icel. Agric. Sci. 2001, 14, 67–73. [Google Scholar]
- Knogge, W. Fungal Infection of Plants. Plant Cell 1996, 8, 1711–1722. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.D. Deconstructing the cell wall. Plant Physiol. 1994, 104, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Bishop, C.; Cooper, R. An ultrastructural study of vascular colonization in three vascular wilt diseases I. Colonization of susceptible cultivars. Physiol. Plant Pathol. 1983, 23, 323–343. [Google Scholar] [CrossRef]
- Beckman, C.H.; Mace, M.E.; Halmos, S.; McGahan, M.W. Physical barriers associated with resistance in Fusarium wilt of bananas. Phytopathology 1961, 51, 507–515. [Google Scholar]
- Beckman, C.H.; Halmos, S.; Mace, M.E. The interaction of host, pathogen and soil temperature in relation to sus-ceptibility to Fusarium wilt of bananas. Phytopathology 1962, 52, 134–140. [Google Scholar]
- Beckman, C.H. The Nature of Wilt Diseases of Plants; American Phytopathological Society: St. Paul, MN, USA, 1987; p. 175. [Google Scholar]
- MacHardy, W.E.; Beckman, C.H. Vascular wilt Fusaria: Infections and Pathogenesis. In Fusarium: Diseases, Biology and Taxonomy; Nelson, P.E., Toussoun, T.A., Cook, R.J., Eds.; The Pennysylvania State University Press: State College, PA, USA, 1981; pp. 365–390. [Google Scholar]
- Schnathorst, W.C. Life cycle and epidemiology of Verticillium. In Fungal Wilt Diseases of Plants; Mace, M.E., Ed.; Academic Press, Inc.: New York, NY, USA, 1981; pp. 81–111. [Google Scholar]
- Huisman, O.C. Interactions of root growth dynamics to epidemiology of root invading fungi. Annu. Rev. Phytopathol. 1982, 20, 303–327. [Google Scholar] [CrossRef]
- Moradi, M.; Oerke, E.-C.; Steiner, U.; Tesfaye, D.; Schellander, K.; Dehne, H.-W. Microbiological and Sybr® Green real-time PCR detection of major Fusarium head blight pathogens on wheat ears. Microbiology 2010, 79, 646–654. [Google Scholar] [CrossRef]
- Prados-Ligero, A.M.; Basallote-Ureba, M.J.; Melero-Vara, J.M. First report of Fusarium oxysporum f. sp. lilii and F. proliferatum affecting Lilium crops in Spain. Trop. Plant Pathol. 2008, 33, 235–236. [Google Scholar] [CrossRef]
- Aktaruzzaman, M.; Xu, S.J.; Kim, J.Y. First report of potato stem-end rot caused by Fusarium oxysporum in Korea. Mycobiology 2014, 2, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Viljoen, A. The status of Fusarium wilt (Panama disease) of banana in South Africa. S. Afr. J. Sci. 2002, 98, 341–344. [Google Scholar]
- Green, J.; Nelson, S. Heart and root rots of pineapples. Plant Dis. 2015, 106, 1–7. [Google Scholar]
- Nilmini, R.K.; Panapitiya, D.; Abeywickrama, K.; Kuruppu, M. Morphological and molecular identification of fungal species associated with postharvest stem-end rot disease of avocado in Sri Lanka. Sri Lanka J. Food Agric. 2020, 6, 47–56. [Google Scholar] [CrossRef]
- Wulff, E.G.; Sørensen, J.L.; Lübeck, M.; Nielsen, K.F.; Thrane, U.; Torp, J. Fusarium spp. associated with rice Bakanae: Ecology, genetic diversity, pathogenicity and toxigenicity. Environ. Microbiol. 2010, 12, 649–657. [Google Scholar] [CrossRef]
- Flood, J.A. Review of Fusarium Wilt of Oil Palm Caused by Fusarium oxysporum f. sp. Elaeidis. Phytopathology 2006, 6, 660–662. [Google Scholar] [CrossRef]
- Umaña-Rojas, G.; García, J. Frequency of organisms associated with crown rot of bananas in integrated and organic production systems. Acta Hortic. 2011, 906, 211–217. [Google Scholar] [CrossRef]
- Brandfass, C.; Karlovsky, P. Simultaneous detection of Fusarium culmorum and F. graminearum in plant material by duplex PCR with melting curve analysis. BMC Microbiol. 2006, 6, 4. [Google Scholar] [CrossRef]
- Omar, N.H.; Mohd, M.; Nor, N.M.I.M.; Zakaria, L. Characterization and pathogenicity of Fusarium species associated with leaf spot of mango (Mangifera indica L.). Microb. Pathog. 2018, 114, 362–368. [Google Scholar] [CrossRef]
- Barral, B.; Chillet, M.; Doizy, A.; Grassi, M.; Ragot, L.; Léchaudel, M.; Durand, N.; Rose, L.J.; Viljoen, A.; Schorr-Galindo, S. Diversity and Toxigenicity of Fungi that Cause Pineapple Fruitlet Core Rot. Toxins 2020, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N.; Teelucksingh, L.D. First report of Fusarium proliferatum infecting pimento chili peppers in Trinidad. Plant Dis. 2011, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Jee, H.J.; Ryu, K.Y.; Shim, C.K.; Nam, K.W. Occurrence of stem and fruit rot paprika caused by Nectria haemato-cocca. Plant Pathol. J. 2005, 21, 317–321. [Google Scholar] [CrossRef]
- Wanjiku, E.K.; Waceke, J.W.; Wanjala, B.W.; Mbaka, J.N. Identification and Pathogenicity of Fungal Pathogens Associated with Stem End Rots of Avocado Fruits in Kenya. Int. J. Microbiol. 2020, 2020, 4063697. [Google Scholar] [CrossRef]
- Singh, S.K.; Kumar, R. Etiology, symptoms and molecular characterization of papaya root rot—A new and serious threat. Indian Phytopathol. 2015, 68, 348–349. [Google Scholar]
- Boutigny, A.-L.; Beukes, I.; Small, I.; Zühlke, S.; Spiteller, M.; Van Rensburg, B.J.; Flett, B.; Viljoen, A. Quantitative detection of Fusarium pathogens and their mycotoxins in South African maize. Plant Pathol. 2012, 61, 522–531. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, Z.; Li, Q.; Tang, L.; Guo, T.; Huang, S.; Mo, J.; Hsiang, T.; Luo, S. Fusarium species associated with leaf spots of mango in China. Microb. Pathog. 2021, 150, 104736. [Google Scholar] [CrossRef]
- Marin, D.H.; Sutton, T.B.; Blankenship, S.M.; Swallow, W.H. Pathogenicity of fungi associated with crown rot of bananas in Latin America on Grande Naine and disease-resistant hybrid bananas. Plant Dis. 1996, 80, 525–528. [Google Scholar] [CrossRef]
- Zhu, Z.; Zheng, L.; Pan, L.; Hsiang, T.; Huang, J. Identification and characterization of Fusarium species associated with wilt of Eleocharis dulcis (Chinese water chestnut) in China. Plant Dis. 2014, 98, 977–987. [Google Scholar] [CrossRef]
- Sharddha, G.; Lal, A.A. Eco-friendly management of post-harvest fungal pathogen causing Fusarium rot of papaya (Carica papaya L.) in Allahabad. Natl. Acad. Sci. Lett. 2010, 33, 227–233. [Google Scholar]
- Suratos, S.C.M. Interaction of molds associated with stem-end rot in avocado (Persea Americana Mill.) fruit. CLSU Sci. J. 2005, 25, 63–64. [Google Scholar]
- Darvas, J.M.; Kotze, J.M. Post-harvest diseases of avocados. S. Afr. Avocado Grow. Assoc. Yearb. 1981, 4, 63–66. [Google Scholar]
- Flückiger, U.; Marchetti, O.; Bille, J.; Eggimann, P.; Zimmerli, S.; Imhof, A.; Garbino, J.; Ruef, C.; Pittet, D.; Täuber, M.; et al. Treatment options of invasive fungal infections in adults. Swiss Med. Wkly. 2006, 136, 447–463. [Google Scholar]
- Stempel, J.M.; Hammond, S.P.; Sutton, D.A.; Weiser, L.M.; Marty, F.M. Invasive Fusariosis in the Voriconazole Era: Single-Center 13-Year Experience. Open Forum Infect. Dis. 2015, 2, ofv099. [Google Scholar] [CrossRef] [PubMed]
- Nucci, M.; Anaissie, E. Cutaneous Infection by Fusarium Species in Healthy and Immunocompromised Hosts: Implications for Diagnosis and Management. Clin. Infect. Dis. 2002, 35, 909–920. [Google Scholar] [CrossRef]
- Pontón, J.; Rüchel, R.; Clemons, K.V.; Coleman, D.C.; Grillot, R.; Guarro, J.; Aldebert, D.; Ambroise-Thomas, P.; Cano, J.; Carrillo-Muñoz, A.J.; et al. Emerging pathogens. MedicalMycology 2000, 38, 225–236. [Google Scholar]
- Sullivan, D.J.; Moran, G.P. (Eds.) Human Pathogenic Fungi: Molecular Biology and Pathogenic Mechanisms; Caister Academic Press: Norfolk, UK, 2014. [Google Scholar]
- Gnat, S.; Łagowski, D.; Nowakiewicz, A.; Dyląg, M. A global view on fungal infections in humans and animals: Opportunistic infections and microsporidioses. J. Appl. Microbiol. 2021, 131, 2095–2113. [Google Scholar] [CrossRef]
- Kobayashi, G.S. Disease of Mechanisms of Fungi. In Medical Microbiology, 4th ed.; Baron, S., Ed.; University of Texas Medical Branch: Galveston, TX, USA, 1996; Chapter 74. [Google Scholar]
- Van Burik, J.A.; Magee, P.T. Aspects of fungal pathogenesis in humans. Annu. Rev. Microbiol. 2001, 55, 743–772. [Google Scholar] [CrossRef]
- Vennewald, I.; Wollina, U. Cutaneous infections due to opportunistic molds: Uncommon presentations. Clin. Dermatol. 2005, 23, 565–571. [Google Scholar] [CrossRef]
- Lukaszuk, C.R.; Kułak, W. Effects of fungal air pollution on human health. Prog. Health Sci. 2011, 1, 156–164. [Google Scholar]
- Gurdaswani, V.; Ghag, S.B. Chapter 2—Toxins from Fusarium Species and their Role in Animal and Plant Diseases. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J., Gehlot, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–27. ISBN 9780128210062. [Google Scholar]
- Perincherry, L.; Lalak-Kańczugowska, J.; Stępień, Ł. Fusarium-Produced Mycotoxins in Plant-Pathogen Interactions. Toxins 2019, 11, 664. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Buchenauer, H. Ultrastructural and Cytochemical Studies on Cellulose, Xylan and Pectin Degradation in Wheat Spikes Infected by Fusarium culmorum. J. Phytopathol. 2000, 148, 263–275. [Google Scholar] [CrossRef]
- Kang, Z.; Zingen-Sell, I.; Buchenauer, H. Infection of wheat spikes by Fusarium avenaceum and alterations of cell wall components in the infected tissue. Eur. J. Plant Pathol. 2005, 111, 19–28. [Google Scholar] [CrossRef]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef]
- Jajić, I.; Dudaš, T.; Krstović, S.; Krska, R.; Sulyok, M.; Bagi, F.; Savić, Z.; Guljaš, D.; Stankov, A. Emerging Fusarium Mycotoxins Fusaproliferin, Beauvericin, Enniatins, and Moniliformin in Serbian Maize. Toxins 2019, 11, 357. [Google Scholar] [CrossRef]
- Lyu, X.; Shen, C.; Fu, Y.; Xie, J.; Jiang, D.; Li, G.; Cheng, J. Comparative genomic and transcriptional analyses of the car-bohydrate-active enzymes and secretomes of phytopathogenic fungi reveal their significant roles during infection and development. Sci. Rep. 2015, 5, 15565. [Google Scholar] [CrossRef]
- De Guillen, K.; Ortiz-Vallejo, D.; Gracy, J.; Fournier, E.; Kroj, T.; Padilla, A. Structure Analysis Uncovers a Highly Diverse but Structurally Conserved Effector Family in Phytopathogenic Fungi. PLoS Pathog. 2015, 11, e1005228. [Google Scholar] [CrossRef]
- Tan, K.-C.; Oliver, R.P. Regulation of proteinaceous effector expression in phytopathogenic fungi. PLoS Pathog. 2017, 13, e1006241. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, H.; Wang, C.; Xu, J.-R. Comparative analysis of fungal genomes reveals different plant cell wall degrading capacity in fungi. BMC Genom. 2013, 14, 274. [Google Scholar] [CrossRef]
- Guerriero, G.; Hausman, J.-F.; Strauss, J.; Ertan, H.; Siddiqui, K.S. Destructuring plant biomass: Focus on fungal and extremophilic cell wall hydrolases. Plant Sci. 2015, 234, 180–193. [Google Scholar] [CrossRef]
- Lara-Márquez, A.; Zavala-Páramo, M.G.; López-Romero, E.; Calderón-Cortés, N.; López-Gómez, R.; Conejo-Saucedo, U.; Cano-Camacho, H. Cloning and characterization of a pectin lyase gene from Colletotrichum lindemuthianum and comparative phyloge-netic/structural analyses with genes from phytopathogenic and saprophytic/opportunistic microorganisms. BMC Microbiol. 2011, 11, 260. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Świderska-Burek, U.; Jarosz-Wilkołazka, A.; Paszczyński, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef]
- Beliën, T.; Van Campenhout, S.; Van Acker, M.; Robben, J.; Courtin, C.M.; Delcour, J.A.; Volckaert, G. Mutational analysis of endoxylanases XylA and XylB from the phytopathogen Fusarium graminearum reveals comprehensive insights into their inhibitor insensitivity. Appl. Environ. Microbiol. 2007, 73, 4602–4608. [Google Scholar] [CrossRef]
- Félix, C.; Libório, S.; Nunes, M.; Félix, R.; Duarte, A.S.; Alves, A.; Esteves, A.C. Lasiodiplodia theobromae as a Producer of Biotechnologically Relevant Enzymes. Int. J. Mol. Sci. 2018, 19, 29. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Zhang, B.; Gai, Y.; Sun, X.; Chung, K.R.; Li, H. Cellwall-degrading enzymes required for virulence in the host selective toxin producing necrotroph alternaria alternata of citrus. Front. Microbiol. 2019, 10, 2514. [Google Scholar] [CrossRef]
- Di Pietro, A.; García-Maceira, F.I.; Méglecz, E.; Roncero, M.I.G. A MAP kinase of the vascular wilt fungus Fusarium oxysporum is essential for root penetration and pathogenesis. Mol. Microbiol. 2001, 39, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Akiyama, K.; Mae, K.; Ohguchi, T.; Takata, R. Targeteddisruption of a G protein a subunit gene results in reduced pathogenicityin Fusarium oxysporum. Curr. Genet. 2002, 41, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Akiyama, K.; Takata, R.; Ohguchi, T. Signaling via the G-protein subunit FGA2 is necessary for pathogenesis in Fusarium oxysporum. FEMS Microbiol. Lett. 2005, 243, 165–172. [Google Scholar] [CrossRef]
- Martínez-Rocha, A.L.; Roncero, M.I.G.; López-Ramirez, A.; Mariné, M.; Guarro, J.; Martínez-Cadena, G.; Di Pietro, A. Rho1 has, distinct functions in morphogenesis, cell wall biosynthesis and virulence of Fusarium oxysporum. Cell. Microbiol. 2008, 10, 1339–1351. [Google Scholar] [CrossRef]
- López-Berges, M.S.; Hera, C.; Sulyok, M.; Schäfer, K.; Capilla, J.; Guarro, J.; Di Pietro, A. The velvet complex governs mycotoxin production and virulence of Fusarium oxysporum on plant and mammalian hosts. Mol. Microbiol. 2013, 87, 49–65. [Google Scholar] [CrossRef]
- Carreras-Villaseñor, N.; Rodríguez-Haas, J.B.; Martínez-Rodríguez, L.A.; Pérez-Lira, A.J.; Ibarra-Laclette, E.; Villafán, E.; Castillo-Díaz, A.P.; Ibarra-Juárez, L.A.; Carrillo-Hernández, E.D.; Sánchez-Rangel, D. Characterization of Two Fusarium solani Species Complex Isolates from the Ambrosia Beetle Xylosandrus morigerus. J. Fungi 2022, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Muimba-Kankolongo, A. Chapter 10—Leguminous Crops Food Crop Production by Smallholder Farmers in Southern Africa; Academic Press: Cambridge, MA, USA, 2018; pp. 173–203. [Google Scholar]
- Knights, E.J.; Hobson, K.B. Chickpea Overview. In Reference Module in Food Science, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 1. [Google Scholar] [CrossRef]
- Nikitin, D.A.; Ivanova, E.A.; Semenov, M.V.; Zhelezova, A.D.; Ksenofontova, N.A.; Tkhakakhova, A.K.; Kholodov, V.A. Diversity, Ecological Characteristics and Identification of Some Problematic Phytopathogenic Fusarium in Soil: A Review. Diversity 2023, 15, 49. [Google Scholar] [CrossRef]
- Goncharov, A.A.; Glebova, A.A.; Tiunov, A.V. Trophic interactions between Fusarium species and soil fauna: A meta-analysis of experimental studies. Appl. Soil Ecol. 2020, 145, 103–302. [Google Scholar] [CrossRef]
- Egel, D.S.; Martyn, R.D. Fusarium wilt of watermelon and other cucurbits. Plant Health Instr. 2007, 10, 1094. [Google Scholar] [CrossRef]
- Shah, L.; Ali, A.; Yahya, M.; Zhu, Y.; Wang, S.; Si, H.; Rahman, H.; Ma, C. Integrated control of fusarium head blight and deoxynivalenol mycotoxin in wheat. Plant Pathol. 2018, 67, 532–548. [Google Scholar] [CrossRef]
- Khokhar, M.; Hooda, K.; Sharma, S.; Singh, V.; Saini, A. Fusarium stalk rot: A major threat to maize production in India. Maize J. 2013, 1, 1–6. [Google Scholar]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to animal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Gordon, T.R.; Martyn, R.D. The Evolutionary Biology of Fusarium oxysporum. Annu. Rev. Phytopathol. 1997, 35, 111–128. [Google Scholar] [CrossRef]
- Fones, H.N.; Bebber, D.P.; Chaloner, T.M.; Kay, W.T.; Steinberg, G.; Gurr, S.J. Threats to global food security from emerging fungal and oomycete crop pathogens. Nat. Food 2020, 1, 332–342. [Google Scholar] [CrossRef]
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Worldwide emergence of resistance to antifungal drugs challenges human health and food security. Science 2018, 360, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Bebber, D.P.; Ramotowski, M.A.T.; Gurr, S.J. Crop pests and pathogens move polewards in a warming world. Nat. Clim. Chang. 2013, 3, 985–988. [Google Scholar] [CrossRef]
- Lević, J. Species of Genus FUSARIUM in the Fields of Agriculture, Veterinary and Human Medicine; Monograph, Maize Research Institute Zemun Polje and Serbian Genetic Society: Belgrade, Serbia, 2008. [Google Scholar]
- Spadaro, D.; Gullino, M.L. Improving the efficacy of biocontrol agents against soilborne pathogens. Crop. Prot. 2005, 24, 601–613. [Google Scholar] [CrossRef]
| Fusarium Species | Major Mycotoxins Produced |
|---|---|
| F. cerealis | NIV, FX, ZEA |
| F. culmorium | DON, 3-AcDON, 15-AcDON, NIV, FX, ZEA |
| F. equiseti | ZEA, DAS |
| F. graminearium | DON, 15-AcDON, NIV, FX, ZEA |
| F. oxysporium | Moniliformin, fusaric acid |
| F. poae | T-2 toxin, HT-2 toxin, NIV, DAS, FX |
| F. ploriferatum | Fumonisin, fusarin C, moniliformin |
| F. solani | Fusaric acid, solaniol |
| F. sporotrichioides | T-2 toxin, HT-2 toxin, NEO, DAS, FX, ZEA |
| F. verticiloides | Fumonisin, fusarin C, moniliformin |
| F. sambucinum | Sambutoxin, DON, DAS, T-2 toxin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ekwomadu, T.I.; Mwanza, M. Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture 2023, 13, 1810. https://doi.org/10.3390/agriculture13091810
Ekwomadu TI, Mwanza M. Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research. Agriculture. 2023; 13(9):1810. https://doi.org/10.3390/agriculture13091810
Chicago/Turabian StyleEkwomadu, Theodora Ijeoma, and Mulunda Mwanza. 2023. "Fusarium Fungi Pathogens, Identification, Adverse Effects, Disease Management, and Global Food Security: A Review of the Latest Research" Agriculture 13, no. 9: 1810. https://doi.org/10.3390/agriculture13091810






