Effects of Soil Amendments on Soil Properties, Soil-Borne Pathogens, and Strawberry Growth after Dazomet Fumigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Collection
2.2. Experimental Design
2.2.1. Soil Fumigation
2.2.2. Soil Amendments Application
2.3. Soil and Strawberry Plant Sampling
2.4. Soil Properties Analysis
2.5. Soil-Borne Pathogens
2.6. Strawberry Growth Indices
2.7. Data Analysis
3. Results
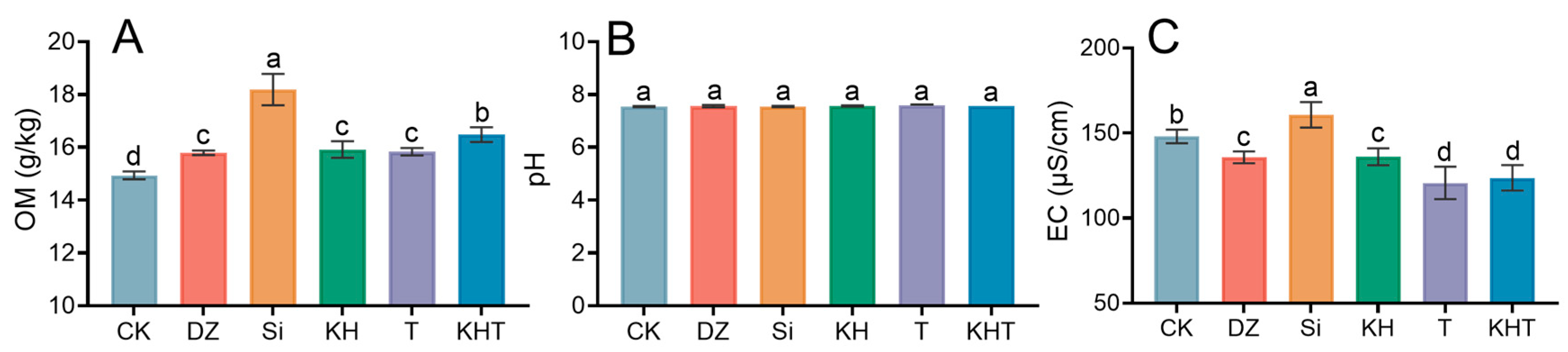
3.1. Changes in Soil N, P, and K Contents
3.2. Changes in Soil Organic Matter, pH, and Electrical Conductivity
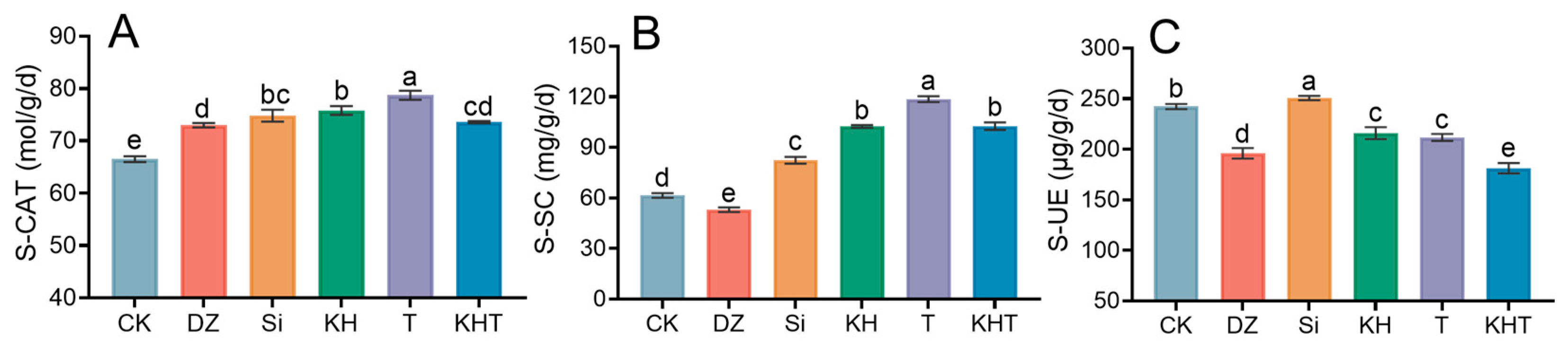
3.3. Soil Enzyme Activities
3.4. Analysis of Fusarium spp. and Phytophthora spp. in Soil
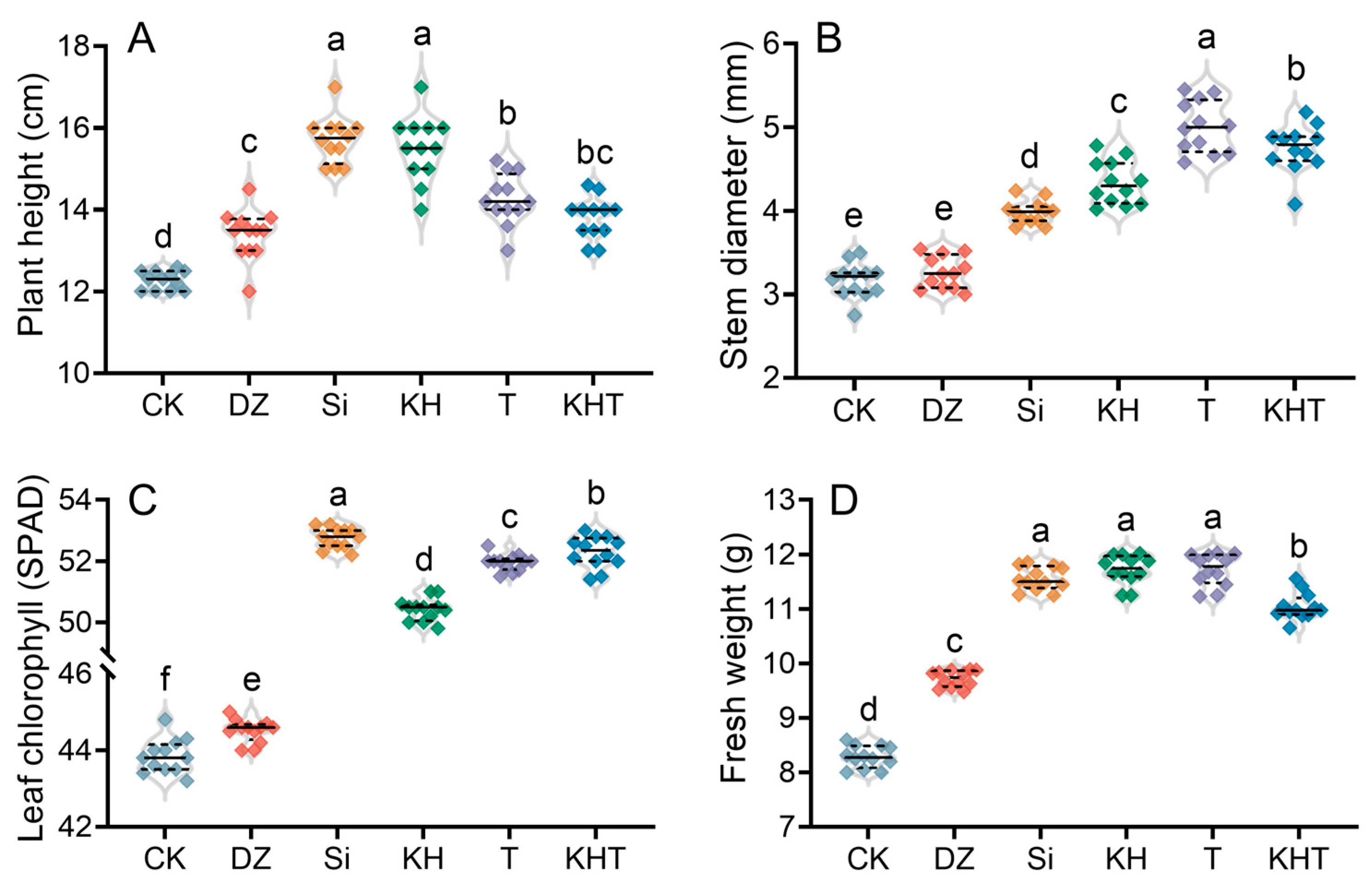
3.5. Changes in Strawberry Growth Indices
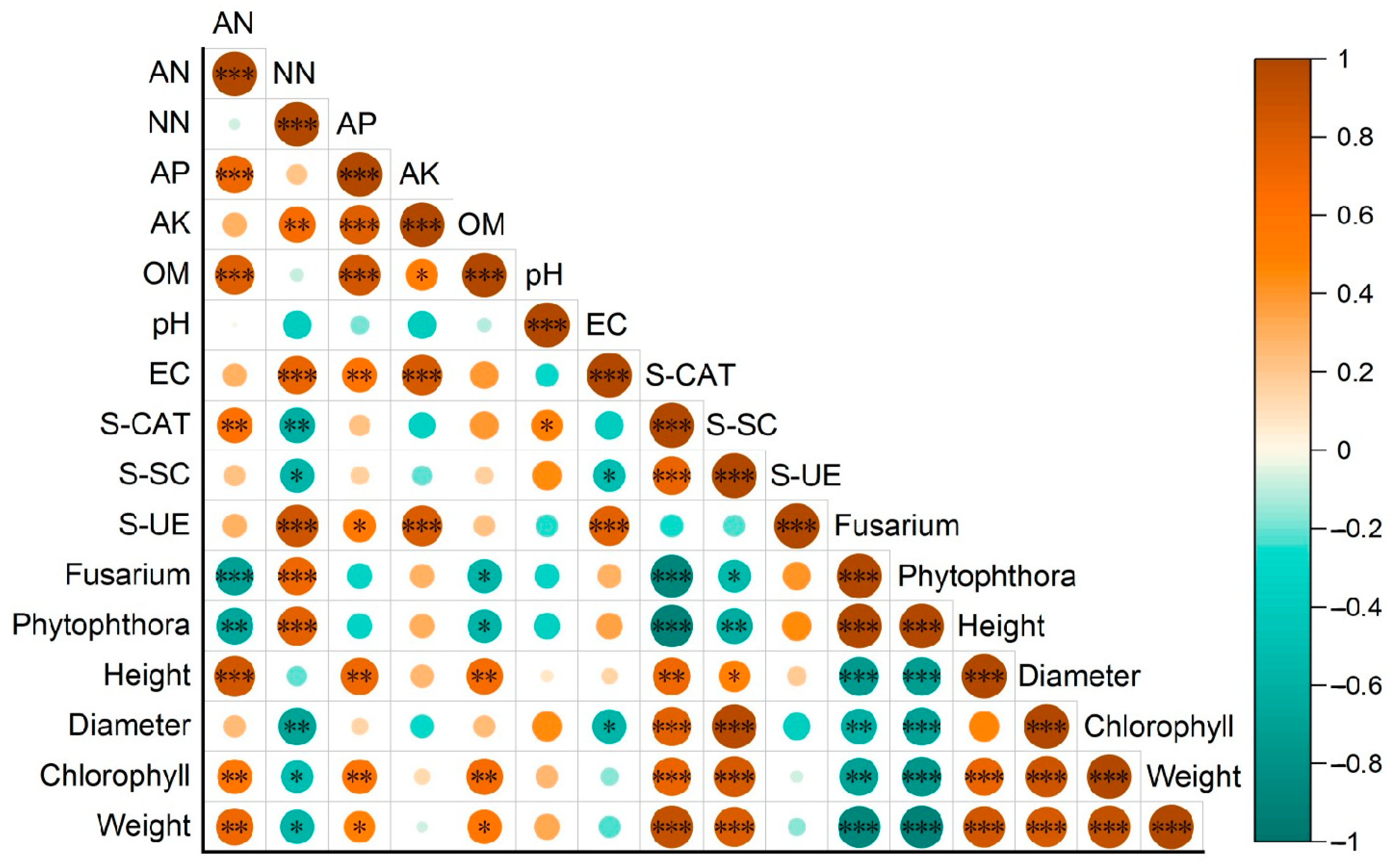
3.6. Correlation Analysis of Strawberry Growth Indexes with Soil Environmental Factors and Soil-Borne Pathogens
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Z.C.; Liang, T.; Kang, C.Y. Molecular bases of strawberry fruit quality traits: Advances, challenges, and opportunities. Plant Physiol. 2023, 193, 900–914. [Google Scholar] [CrossRef]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry as a functional food: An evidence-based review. Crit. Rev. Food Sci. Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef]
- Newerli-Guz, J.; Śmiechowska, M.; Drzewiecka, A.; Tylingo, R. Bioactive ingredients with health-promoting properties of strawberry fruit (Fragaria × ananassa Duchesne). Molecules 2023, 28, 2711. [Google Scholar] [CrossRef]
- Kang, J.X.; Wang, Z.; Zhong, Y.Y. Arthropod population dynamics and community structure characteristics in different strawberry variety orchards. Shaanxi J. Agric. Sci. 2023, 69, 68–72. [Google Scholar]
- Chen, P.; Li, H.Q.; Li, X.Y.; Zhou, X.H.; Zhang, X.X.; Zhang, A.S.; Liu, Q.Z. Transcriptomic analysis provides insight into defensive strategies in response to continuous cropping in strawberry (Fragaria × ananassa Duch.) plants. BMC Plant Biol. 2022, 22, 476. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Jing, J.Q.; Yan, M.L.; Hazard, C.; Chen, Y.H.; Guo, C.B.; Xiao, X.; Lin, J.J. Contribution of pathogenic fungi to N2O emissions increases temporally in intensively managed strawberry cropping soil. Appl. Microbiol. Biotechnol. 2021, 105, 2043–2056. [Google Scholar] [CrossRef] [PubMed]
- Pastrana, A.M.; Borrero, C.; Pérez, A.G.; Avilés, M. Soilborne pathogens affect strawberry fruit flavor and quality. Plant Sci. 2023, 326, 111533. [Google Scholar] [CrossRef] [PubMed]
- Pu, R.F.; Wang, P.P.; Guo, L.P.; Li, M.H.; Cui, X.M.; Wang, C.X.; Liu, Y.; Yang, Y. The remediation effects of microbial organic fertilizer on soil microorganisms after chloropicrin fumigation. Ecotoxicol. Environ. Saf. 2022, 231, 113188. [Google Scholar] [CrossRef]
- Zhang, D.L.; Ji, X.X.; Meng, Z.; Qi, W.Z.; Qiao, K. Effects of fumigation with 1,3-dichloropropene on soil enzyme activities and microbial communities in continuous-cropping soil. Ecotoxicol. Environ. Saf. 2019, 169, 730–736. [Google Scholar] [CrossRef]
- Fu, C.H.; Hu, B.Y.; Chang, T.T.; Hsueh, K.L.; Hsu, W.T. Evaluation of dazomet as fumigant for the control of brown root rot disease. Pest. Manag. Sci. 2012, 68, 959–962. [Google Scholar] [CrossRef]
- Li, Q.J.; Zhang, D.Q.; Cheng, H.Y.; Song, Z.X.; Ren, L.R.; Hao, B.Q.; Zhu, J.H.; Fang, W.S.; Yan, D.D.; Li, Y.; et al. Chloropicrin alternated with dazomet improved the soil’s physicochemical properties, changed microbial communities and increased strawberry yield. Ecotoxicol. Environ. Saf. 2021, 220, 112362. [Google Scholar] [CrossRef]
- Chen, H.J.; Zhao, S.; Zhao, J.M.; Zhang, K.K.; Jiang, J.; Guan, Z.Y.; Chen, S.M.; Chen, F.D.; Fang, W.M. Deep tillage combined with biofertilizer following soil fumigation improved chrysanthemum growth by regulating the soil microbiome. MicrobiologyOpen 2020, 9, e1045. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.C.; Zhang, C.L.; Li, G.T.; Lin, Q.M.; Zhao, X.R. Does soil amendment alter reactive soil N dynamics following chloropicrin fumigation? Chemosphere 2018, 212, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Ge, A.H.; Liang, Z.H.; Xiang, J.F.; Xiao, J.L.; Zhang, Y.; Liu, Y.R.; Zhang, L.M.; Shen, J.P. Fumigation practice combined with organic fertilizer increase antibiotic resistance in watermelon rhizosphere soil. Sci. Total Environ. 2022, 805, 150426. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.Y.; Zhang, D.Q.; Ren, L.R.; Song, Z.X.; Li, Q.J.; Wu, J.J.; Fang, W.S.; Huang, B.; Yan, D.D.; Li, Y.; et al. Bio-activation of soil with beneficial microbes after soil fumigation reduces soil-borne pathogens and increases tomato yield. Environ. Pollut. 2021, 283, 117160. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.D.; Doll, D.A.; Stanghellini, M.S.; Westerdahl, B.B.; Wang, D.; Hanson, B.D. Deep injection and the potential of biochar to reduce fumigant emissions and effects on nematode control. J. Environ. Manag. 2018, 223, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.J.; Zhu, J.H.; Zhang, D.Q.; Cheng, H.Y.; Hao, B.Q.; Cao, A.C.; Yan, D.D.; Wang, Q.X.; Li, Y. Beneficial effect on the soil microenvironment of Trichoderma applied after fumigation for cucumber production. PLoS ONE 2022, 17, e0266347. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Q.; Tang, T.T.; Chen, X.; Luo, X. Silicon fertilizer application promotes phytolith accumulation in rice plants. Front. Plant Sci. 2019, 10, 425. [Google Scholar] [CrossRef]
- Zhao, K.Q.; Yang, Y.; Peng, H.; Zhang, L.H.; Zhou, Y.Y.; Zhang, J.C.; Du, C.Y.; Liu, J.W.; Lin, X.; Wang, N.Y.; et al. Silicon fertilizers, humic acid and their impact on physicochemical properties, availability and distribution of heavy metals in soil and soil aggregates. Sci. Total Environ. 2022, 822, 153483. [Google Scholar] [CrossRef]
- Yuan, Z.N.; Pang, Z.Q.; Fallah, N.; Zhou, Y.M.; Dong, F.; Lin, W.X.; Hu, C.H. Silicon fertilizer mediated structural variation and niche differentiation in the rhizosphere and endosphere bacterial microbiome and metabolites of sugarcane. Front. Microbiol. 2022, 13, 1009505. [Google Scholar] [CrossRef]
- Hegab, R.H.; Eissa, D.; Abou-Shady, A. Effects of foliar application of selenium and potassium-humate on oat growth in Baloza, North Sinai, Egypt. Sci. Rep. 2022, 12, 15119. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Zhang, Y.Y.; Wang, Q.X.; Li, M.J.; Sun, H.; Liu, N.; Zhang, L.L.; Zhang, Y.; Liu, B.Z. Effects of potassium fulvic acid and potassium humate on microbial biodiversity in bulk soil and rhizosphere soil of Panax ginseng. Microbiol. Res. 2022, 254, 126914. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Q.; Lv, X.; Song, J.H.; Li, W.D.; Wang, H.J. Application of cotton straw biochar and compound Bacillus biofertilizer decrease the bioavailability of soil cd through impacting soil bacteria. BMC Microbiol. 2022, 22, 35. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.M.; Fu, M.Y.; Tang, C.J.; Mo, C.J.; Li, S.Y.; Luo, S.Y.; Qin, P.Q.; Zhao, Y.J.; Li, Y. Potential impact of polyethylene microplastics on the growth of water spinach (Ipomoea aquatica F.): Endophyte and rhizosphere effects. Chemosphere 2023, 330, 138737. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.D. Soil Agrochemical Analysis, 3rd ed.; China Agricultural Press: Beijing, China, 2010. [Google Scholar]
- Liu, Y.W.; Feng, Y.; Cheng, D.M.; Xue, J.M.; Wakelin, S.A.; Hu, H.Y.; Li, Z.J. Gentamicin degradation and changes in fungal diversity and physicochemical properties during composting of gentamicin production residue. Bioresour. Technol. 2017, 244, 905–912. [Google Scholar] [CrossRef]
- Yun, C.X.; Yan, C.R.; Xu, M.Z.; Liu, E.K.; Mormil, P.; Dong, W.Y.; Liu, Q. Effects of different soil disinfection methods on soil enzyme activities and soil-borne diseases. J. Chin. Agric. Univ. 2020, 25, 86–96. [Google Scholar]
- Subbarao, G.V.; Yoshihashi, T.; Worthington, M.; Nakahara, K.; Ando, Y.; Sahrawat, K.L.; Rao, I.M.; Lata, J.C.; Kishii, M.; Braun, H.J. Suppression of soil nitrification by plants. Plant Sci. 2015, 233, 155–164. [Google Scholar] [CrossRef]
- De Neve, S.; Csitári, G.; Salomez, J.; Hofman, G. Quantification of the effect of fumigation on short- and long-term nitrogen mineralization and nitrification in different soils. J. Environ. Qual. 2004, 33, 1647–1652. [Google Scholar] [CrossRef]
- Liang, Y.Q.; Liao, M.; Fang, Z.P.; Guo, J.W.; Xie, X.M.; Xu, C.X. How silicon fertilizer improves nitrogen and phosphorus nutrient availability in paddy soil? J. Zhejiang Univ. Sci. B 2021, 22, 521–532. [Google Scholar] [CrossRef]
- Kuang, G.; Wang, H.H.; Liu, D.Y.; Li, S.F.; Wang, B.; Yuan, Y.; Liu, G.S.; Ren, T.B. Effects of potassium humate on flue-cured tobacco quality and soil environment in western henan. Chin. Agric. Sci. Bull. 2017, 33, 60–66. [Google Scholar]
- Li, Y.; Fang, F.; Wei, J.L.; Wu, X.B.; Cui, R.Z.; Li, G.S.; Zheng, F.L.; Tan, D.S. Humic Acid Fertilizer Improved Soil Properties and Soil Microbial Diversity of Continuous Cropping Peanut: A Three-Year Experiment. Sci. Rep. 2019, 9, 12014. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhao, C.Z.; Wang, E.T.; Raza, A.; Yin, C.Y. Bacillus amyloliquefaciens as an excellent agent for biofertilizer and biocontrol in agriculture: An overview for its mechanisms. Microbiol. Res. 2022, 259, 127016. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, S.; Yadav, R.; Ramakrishna, W. Bacillus subtilis impact on plant growth, soil health and environment: Dr. Jekyll and Mr. Hyde. J. Appl. Microbiol. 2022, 132, 3543–3562. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.A.; Lou, Y.; Hafeez, R.; Li, X.; Hossain, A.; Xie, T.; Lin, L.; Li, B.; Yin, Y.; Yan, J.; et al. Functional Analysis and Genome Mining Reveal High Potential of Biocontrol and Plant Growth Promotion in Nodule-Inhabiting Bacteria Within Paenibacillus polymyxa Complex. Front. Microbiol. 2021, 11, 618601. [Google Scholar] [CrossRef] [PubMed]
- Du, J.J.; Hou, F.; Zhou, Q.X. Response of soil enzyme activity and soil bacterial community to PCB dissipation across different soils. Chemosphere 2021, 283, 131229. [Google Scholar] [CrossRef]
- Wu, L.X.; Wang, Y.; Lyu, H.; Chen, X.D. Effects of a compound Trichoderma agent on Coptis chinensis growth, nutrients, enzyme activity, and microbial community of rhizosphere soil. PeerJ 2023, 11, e15652. [Google Scholar] [CrossRef]
- Yang, R.; Wang, J.H.; Zhu, L.S.; Wang, J.; Yang, L.L.; Mao, S.S.; Conkle, J.L.; Chen, Y.Y.; Kim, Y.M. Effects of interaction between enrofloxacin and copper on soil enzyme activity and evaluation of comprehensive toxicity. Chemosphere 2021, 268, 129208. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.X.; Pei, Z.Y.; Jiang, L.Q.; Zhang, Y.; Wang, J.; Wang, J. Application of microbial organic fertilizers promotes the utilization of nutrients and restoration of microbial community structure and function in rhizosphere soils after dazomet fumigation. Front. Microbiol. 2023, 13, 1122611. [Google Scholar] [CrossRef]
- Guo, H.; Yao, J.; Cai, M.M.; Qian, Y.G.; Guo, Y.; Richnow, H.H.; Blake, R.E.; Doni, S.; Ceccanti, B. Effects of petroleum contamination on soil microbial numbers, metabolic activity and urease activity. Chemosphere 2012, 87, 1273–1280. [Google Scholar] [CrossRef]
- Huang, C.P.; Wang, L.; Gong, X.Q.; Huang, Z.T.; Zhou, M.R.; Li, J.; Wu, J.S.; Chang, S.X.; Jiang, P.K. Silicon fertilizer and biochar effects on plant and soil PhytOC concentration and soil PhytOC stability and fractionation in subtropical bamboo plantations. Sci. Total Environ. 2020, 715, 136846. [Google Scholar] [CrossRef]
- Fallah, N.; Pang, Z.Q.; Dong, F.; Zhou, Y.M.; Lin, W.X.; Fabrice, K.M.A.; Hu, C.H.; Yuan, Z.N. Niche differentiation modulates metabolites abundance and composition in silicon fertilizer amended soil during sugarcane growth. BMC Plant Biol. 2022, 22, 497. [Google Scholar] [CrossRef] [PubMed]
- Li, X.P.; Skillman, V.; Dung, J.; Frost, K. Legacy effects of fumigation on soil bacterial and fungal communities and their response to metam sodium application. Environ. Microbiome 2022, 17, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Q.; Li, W.J.; Zhang, D.Q.; Fang, W.S.; Li, Y.; Wang, Q.X.; Cao, A.C.; Yan, D.D. Long-term effects of chloropicrin fumigation on soil microbe recovery and growth promotion of Panax notoginseng. Front. Microbiol. 2023, 14, 1225944. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gao, L.M.; Dong, S.Y.; Sun, Y.M.; Shen, Q.R.; Guo, S.W. Role of silicon on plant-pathogen interactions. Front. Plant Sci. 2017, 8, 701. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.D.; Zhang, L.P.; Xu, L.J.; Wang, Z.M. Experiment on yield-increasing effect of humic acid patassium and humic acid magnesium on rice. North. Rice. 2008, 2, 40–41. [Google Scholar]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile weapons for plant disease biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Lembright, H.W. Soil fumigation: Principles and application technology. J. Nematol. 1990, 22, 632–644. [Google Scholar] [PubMed]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil amendments for immobilization of potentially toxic elements in contaminated soils: A critical review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef]
- Pu, Y.Y.; Lv, X.M.; Wu, M.C.; Wang, D.S.; Li, W.M.; Wang, S.Q.; Li, H.X.; Hu, F.; Jiao, J.G. Effects of partial substitution for chemical fertilizer by organic manure on the growth and nutrient use of watermelon under fumigation condition. J. Soil. Water Conserv. 2017, 31, 306–311. [Google Scholar]
- Liu, Y.X.; Shi, J.X.; Feng, Y.G.; Yang, X.M.; Li, X.; Shen, Q.R. Tobacco bacterial wilt can be biologically controlled by the application of antagonistic strains in combination with organic fertilizer. Biol. Fertil. Soils. 2013, 49, 447–464. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Mijangos, I.; Garbisu, C. Commercial and farm fermented liquid organic amendments to improve soil quality and lettuce yield. J. Environ. Manag. 2020, 264, 110422. [Google Scholar] [CrossRef] [PubMed]

| Ammonium Nitrogen (AN, mg/kg) | Nitrate Nitrogen (NN, mg/kg) | Available Phosphorus (AP, mg/kg) | Available Potassium (AK, mg/kg) | Organic Matter (OM, g/kg) | pH (1:2.5) | Electrical Conductivity (EC, μS/cm) |
|---|---|---|---|---|---|---|
| 0.52 | 44.02 | 56.14 | 864.17 | 16.85 | 7.37 | 303.50 |
| Soil Amendments | Composition | Source |
|---|---|---|
| Silicon fertilizer | SiO2 (17%), MgO (10%), Ca (8%), humic acid (20%), and compound amino acid (5%) | Shanxi Jifei Industry Co., Ltd., Taiyuan, Shanxi, China |
| Potassium humate | Humic acid ≥ 60% and potassium ≥ 11% | Inner Mongolia Shengtian Agricultural Technology Co., Ltd., Baotou, Inner Mongolia, China |
| Bacillus biofertilizer | Bacillus amyloliquefaciens (0.9 × 108 CFU/g), Bacillus subtilis (0.9 × 108 CFU/g), and Paenibacillus kribbensis (0.2 × 108 CFU/g) | Inner Mongolia Shengtian Agricultural Technology Co., Ltd., Baotou, Inner Mongolia, China |
| Treatments | Fusarium spp. | Phytophthora spp. | ||
|---|---|---|---|---|
| CFU/g Soil | Control Effect (%) | CFU/g Soil | Control Effect (%) | |
| CK | 1420.00 ± 11.55 a | / | 3734.00 ± 76.74 a | / |
| DZ | 180.00 ± 11.55 b | 87.32 | 860.00 ± 23.09 b | 76.97 |
| Si | 49.33 ± 5.21 c | 96.53 | 333.34 ± 24.04 c | 91.07 |
| KH | 46.67 ± 6.67 c | 96.71 | 300.00 ± 11.55 cd | 91.97 |
| T | 60.67 ± 6.36 c | 95.73 | 116.56 ± 8.82 e | 96.88 |
| KHT | 156.66 ± 12.02 b | 88.97 | 220.00 ± 11.55 de | 94.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Andom, O.; Fang, W.; Yan, D.; Li, Y.; Wang, Q.; Jin, X.; Cao, A. Effects of Soil Amendments on Soil Properties, Soil-Borne Pathogens, and Strawberry Growth after Dazomet Fumigation. Agriculture 2024, 14, 9. https://doi.org/10.3390/agriculture14010009
Li Q, Andom O, Fang W, Yan D, Li Y, Wang Q, Jin X, Cao A. Effects of Soil Amendments on Soil Properties, Soil-Borne Pathogens, and Strawberry Growth after Dazomet Fumigation. Agriculture. 2024; 14(1):9. https://doi.org/10.3390/agriculture14010009
Chicago/Turabian StyleLi, Qingjie, Okbagaber Andom, Wensheng Fang, Dongdong Yan, Yuan Li, Qiuxia Wang, Xi Jin, and Aocheng Cao. 2024. "Effects of Soil Amendments on Soil Properties, Soil-Borne Pathogens, and Strawberry Growth after Dazomet Fumigation" Agriculture 14, no. 1: 9. https://doi.org/10.3390/agriculture14010009





