Potential of Limestonevirus Bacteriophages for Ecological Control of Dickeya solani Causing Bacterial Potato Blackleg
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteriophages and Bacterial Strains
2.2. Determination of Varietal Sensitivity to D. solani
2.3. Greenhouse Experiments of the Efficacy of Lytic Phages on Dickeya solani
2.4. Field Trials on the Efficacy of Lytic Phages on D. solani
2.5. Statistical Assessment of the Experiments
3. Results
3.1. Determination of Potato Varietal Sensitivity to D. solani
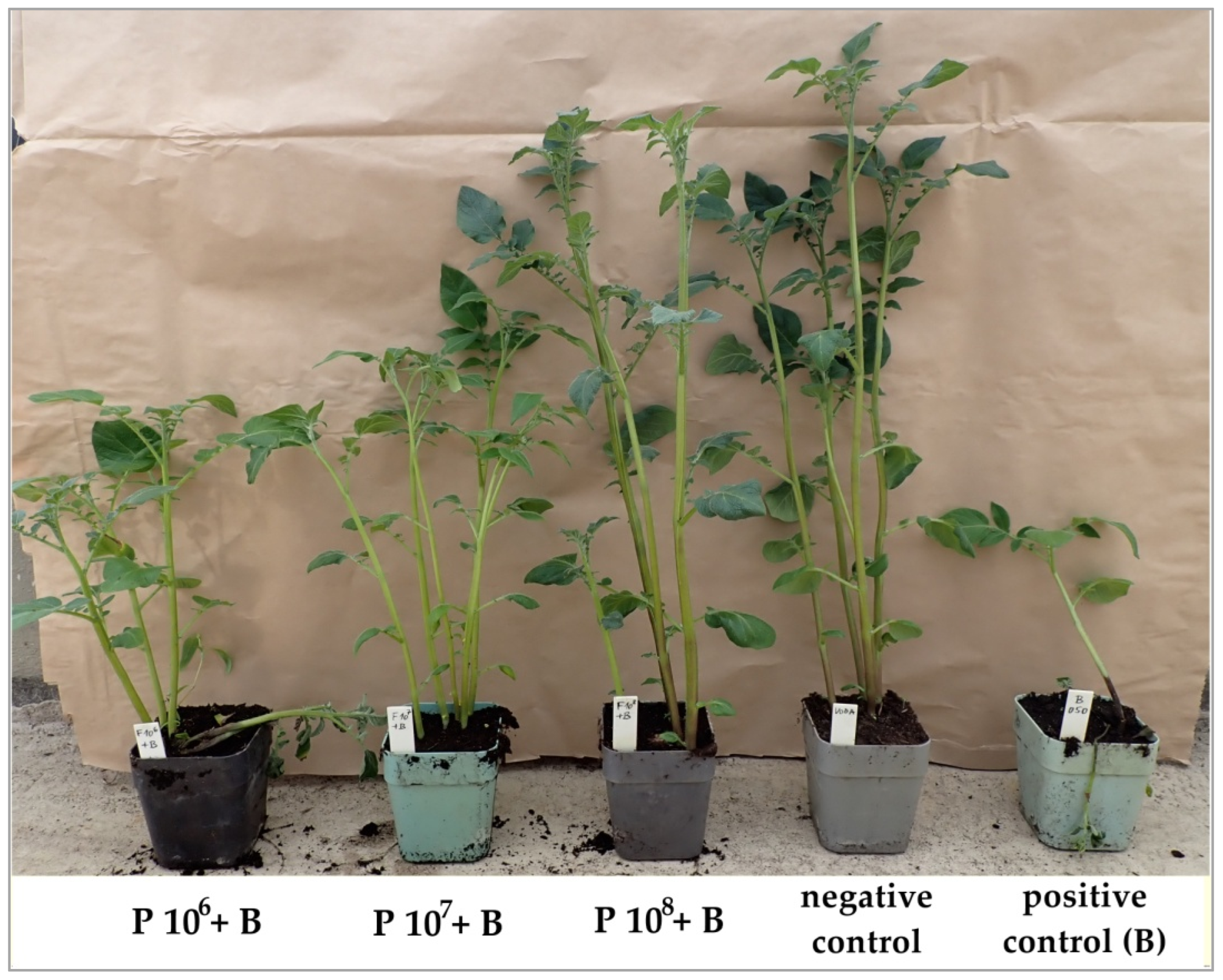
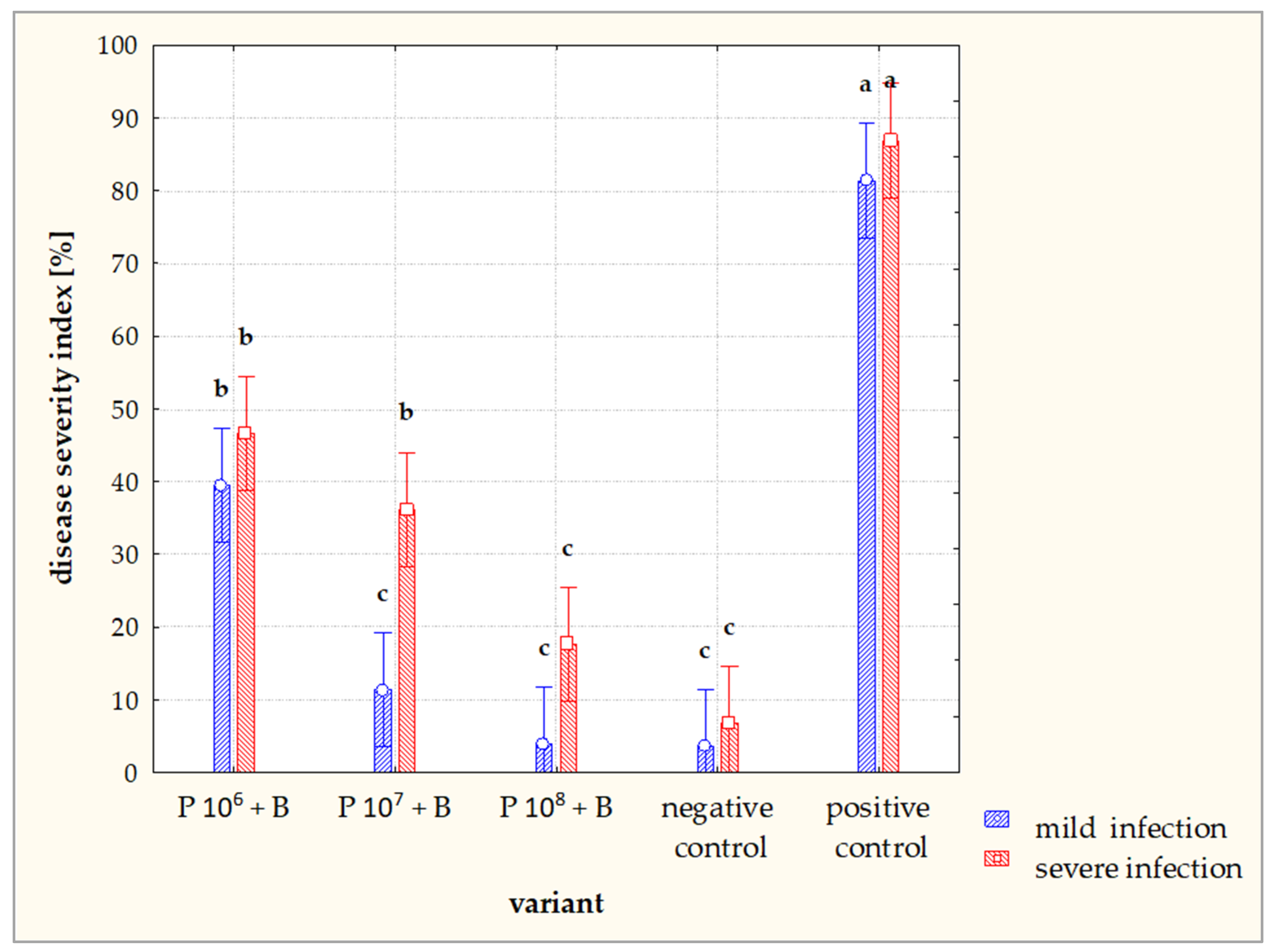
3.2. Greenhouse Tests of Phage Efficacy on D. solani
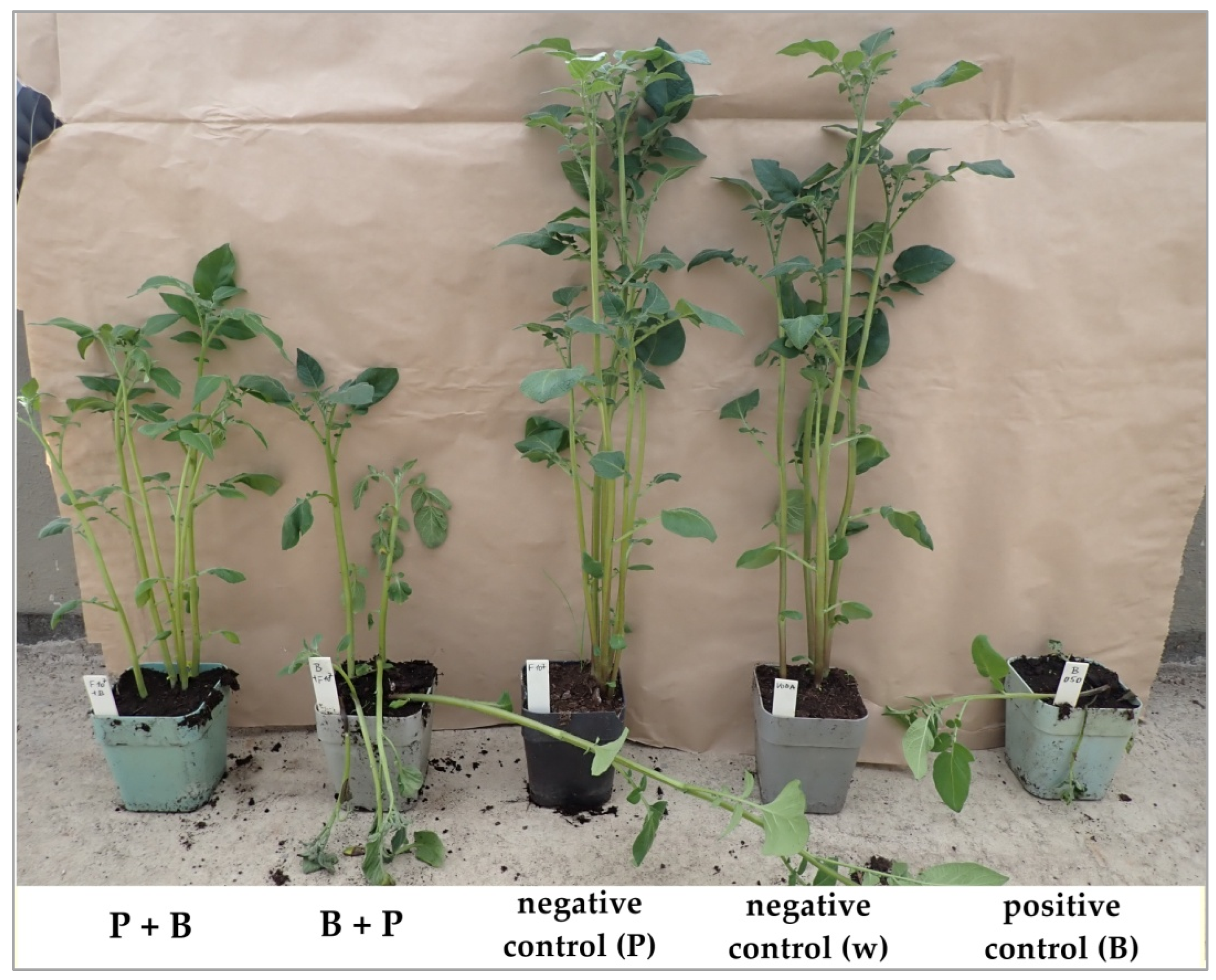
3.3. Field Trials on the Efficacy of Lytic Phages on D. solani
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Czajkowski, R.; Pérombelon, M.C.M.; van Veen, J.A.; van der Wolf, J.M. Control of Blackleg and Tuber Soft Rot of Potato Caused by Pectobacterium and Dickeya Species: A Review: Control of Dickeya and Pectobacterium Species in Potato. Plant Pathol. 2011, 60, 999–1013. [Google Scholar] [CrossRef]
- Skelsey, P.; Humphris, S.N.; Campbell, E.J.; Toth, I.K. Threat of Establishment of Non-Indigenous Potato Blackleg and Tuber Soft Rot Pathogens in Great Britain under Climate Change. PLoS ONE 2018, 13, e0205711. [Google Scholar] [CrossRef] [PubMed]
- Pérombelon, M.C.M. Potato Diseases Caused by Soft Rot Erwinias: An Overview of Pathogenesis. Plant Pathol. 2002, 51, 1–12. [Google Scholar] [CrossRef]
- Golanowska, M.; Łojkowska, E. A Review on Dickeya solani, a New Pathogenic Bacterium Causing Loss in Potato Yield in Europe. BioTechnologia J. Biotechnol. Comput. Biol. Bionanotechnol. 2016, 97, 109–127. [Google Scholar] [CrossRef]
- Adriaenssens, E.M.; Van Vaerenbergh, J.; Vandenheuvel, D.; Dunon, V.; Ceyssens, P.-J.; De Proft, M.; Kropinski, A.M.; Noben, J.-P.; Maes, M.; Lavigne, R. T4-Related Bacteriophage LIMEstone Isolates for the Control of Soft Rot on Potato Caused by ‘Dickeya Solani’. PLoS ONE 2012, 7, e33227. [Google Scholar] [CrossRef]
- Reverchon, S.; Nasser, W. Dickeya Ecology, Environment Sensing and Regulation of Virulence Programme. Environ. Microbiol. Rep. 2013, 5, 622–636. [Google Scholar] [CrossRef] [PubMed]
- Toth, I.K.; Van Der Wolf, J.M.; Saddler, G.; Lojkowska, E.; Hélias, V.; Pirhonen, M.; Tsror (Lahkim), L.; Elphinstone, J.G. Dickeya Species: An Emerging Problem for Potato Production in Europe. Plant Pathol. 2011, 60, 385–399. [Google Scholar] [CrossRef]
- Day, A.; Ahn, J.; Fang, X.; Salmond, G.P.C. Environmental Bacteriophages of the Emerging Enterobacterial Phytopathogen, Dickeya solani, Show Genomic Conservation and Capacity for Horizontal Gene Transfer between Their Bacterial Hosts. Front. Microbiol. 2017, 8, 1654. [Google Scholar] [CrossRef] [PubMed]
- Sławiak, M.; Van Beckhoven, J.R.C.M.; Speksnijder, A.G.C.L.; Czajkowski, R.; Grabe, G.; Van Der Wolf, J.M. Biochemical and Genetical Analysis Reveal a New Clade of Biovar 3 Dickeya Spp. Strains Isolated from Potato in Europe. Eur. J. Plant Pathol. 2009, 125, 245–261. [Google Scholar] [CrossRef]
- Van Der Wolf, J.M.; Nijhuis, E.H.; Kowalewska, M.J.; Saddler, G.S.; Parkinson, N.; Elphinstone, J.G.; Pritchard, L.; Toth, I.K.; Lojkowska, E.; Potrykus, M.; et al. Dickeya solani Sp. Nov., a Pectinolytic Plant-Pathogenic Bacterium Isolated from Potato (Solanum tuberosum). Int. J. Syst. Evol. Microbiol. 2014, 64, 768–774. [Google Scholar] [CrossRef]
- Parkinson, N.; Pritchard, L.; Bryant, R.; Toth, I.; Elphinstone, J. Epidemiology of Dickeya dianthicola and Dickeya solani in Ornamental Hosts and Potato Studied Using Variable Number Tandem Repeat Analysis. Eur. J. Plant Pathol. 2015, 141, 63–70. [Google Scholar] [CrossRef]
- Czajkowski, R.; De Boer, W.J.; Van Der Zouwen, P.S.; Kastelein, P.; Jafra, S.; De Haan, E.G.; Van Den Bovenkamp, G.W.; Van Der Wolf, J.M. Virulence of ‘Dickeya solani’ and Dickeya dianthicola Biovar-1 and -7 Strains on Potato (Solanum tuberosum). Plant Pathol. 2013, 62, 597–610. [Google Scholar] [CrossRef]
- Czajkowski, R.; De Boer, W.J.; Velvis, H.; Van Der Wolf, J.M. Systemic Colonization of Potato Plants by a Soilborne, Green Fluorescent Protein-Tagged Strain of Dickeya sp. Biovar 3. Phytopathology 2010, 100, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Fikowicz-Krosko, J.; Czajkowski, R. Fast and Reliable Screening System to Preselect Candidate Dickeya Solani Tn5 Mutants in Plant Tissue-Induced Genes. Eur. J. Plant Pathol. 2017, 149, 1023–1027. [Google Scholar] [CrossRef]
- Golanowska, M.; Potrykus, M.; Motyka-Pomagruk, A.; Kabza, M.; Bacci, G.; Galardini, M.; Bazzicalupo, M.; Makalowska, I.; Smalla, K.; Mengoni, A.; et al. Comparison of Highly and Weakly Virulent Dickeya Solani Strains, With a View on the Pangenome and Panregulon of This Species. Front. Microbiol. 2018, 9, 1940. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Schloop, A.; Swingle, B.; Perry, K.L. Pectobacterium and Dickeya Responsible for Potato Blackleg Disease in New York State in 2016. Plant Dis. 2018, 102, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, R.; Ozymko, Z.; Zwirowski, S.; Lojkowska, E. Complete Genome Sequence of a Broad-Host-Range Lytic Dickeya Spp. Bacteriophage ϕD5. Arch. Virol. 2014, 159, 3153–3155. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 Plant Pathogenic Bacteria in Molecular Plant Pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.B.; Jackson, L.E.; Balogh, B.; Obradovic, A.; Iriarte, F.B.; Momol, M.T. Bacteriophages for Plant Disease Control. Annu. Rev. Phytopathol. 2007, 45, 245–262. [Google Scholar] [CrossRef]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and Bacterial Plant Diseases. Front. Microbiol. 2017, 8, 212667. [Google Scholar] [CrossRef]
- Jones, J.B.; Vallad, G.E.; Iriarte, F.B.; Obradović, A.; Wernsing, M.H.; Jackson, L.E.; Balogh, B.; Hong, J.C.; Momol, M.T. Considerations for Using Bacteriophages for Plant Disease Control. Bacteriophage 2012, 2, e23857. [Google Scholar] [CrossRef]
- Carstens, A.; Djurhuus, A.; Kot, W.; Jacobs-Sera, D.; Hatfull, G.; Hansen, L. Unlocking the Potential of 46 New Bacteriophages for Biocontrol of Dickeya solani. Viruses 2018, 10, 621. [Google Scholar] [CrossRef]
- Czajkowski, R.; Ozymko, Z.; Lojkowska, E. Isolation and Characterization of Novel Soilborne Lytic Bacteriophages Infecting Dickeya Spp. Biovar 3 (‘D. solani’). Plant Pathol. 2014, 63, 758–772. [Google Scholar] [CrossRef]
- Matilla, M.A.; Fang, X.; Salmond, G.P. Viunalikeviruses Are Environmentally Common Agents of Horizontal Gene Transfer in Pathogens and Biocontrol Bacteria. ISME J. 2014, 8, 2143–2147. [Google Scholar] [CrossRef]
- Czajkowski, R.; Ozymko, Z.; De Jager, V.; Siwinska, J.; Smolarska, A.; Ossowicki, A.; Narajczyk, M.; Lojkowska, E. Genomic, Proteomic and Morphological Characterization of Two Novel Broad Host Lytic Bacteriophages ΦPD10.3 and ΦPD23.1 Infecting Pectinolytic Pectobacterium spp. and Dickeya spp. PLoS ONE 2015, 10, e0119812. [Google Scholar] [CrossRef]
- Muturi, P.; Yu, J.; Maina, A.N.; Kariuki, S.; Mwaura, F.B.; Wei, H. Bacteriophages Isolated in China for the Control of Pectobacterium carotovorum Causing Potato Soft Rot in Kenya. Virol. Sin. 2019, 34, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Day, A.; Ahn, J.; Salmond, G.P.C. Jumbo Bacteriophages Are Represented Within an Increasing Diversity of Environmental Viruses Infecting the Emerging Phytopathogen, Dickeya solani. Front. Microbiol. 2018, 9, 2169. [Google Scholar] [CrossRef]
- Petrzik, K.; Vacek, J.; Brázdová, S.; Ševčík, R.; Koloniuk, I. Diversity of Limestone Bacteriophages Infecting Dickeya solani Isolated in the Czech Republic. Arch. Virol. 2021, 166, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Lebecka, R.; Śliwka, J.; Grupa-Urbańska, A.; Szajko, K.; Marczewski, W. QTLs for Potato Tuber Resistance to Dickeya solani Are Located on Chromosomes II and IV. Plant Pathol. 2021, 70, 1745–1756. [Google Scholar] [CrossRef]
- Zadina, J.; Jermoljev, E. Šlechtění Bramboru, 1st ed.; Academia: Praha, Czech Republic, 1976. [Google Scholar]
- Petrzik, K.; Vacek, J.; Kmoch, M.; Binderová, D.; Brázdová, S.; Lenz, O.; Ševčík, R. Field Use of Protective Bacteriophages against Pectinolytic Bacteria of Potato. Microorganisms 2023, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Gross, D.C. A Bacteriophage-Typing System for Surveying the Diversity and Distribution of Strains of Erwinia carotovora in Potato Fields. Phytopathology 1991, 81, 220. [Google Scholar] [CrossRef]
- Koskella, B.; Meaden, S. Understanding Bacteriophage Specificity in Natural Microbial Communities. Viruses 2013, 5, 806–823. [Google Scholar] [CrossRef]
- Glasner, J.D.; Marquez-Villavicencio, M.; Kim, H.-S.; Jahn, C.E.; Ma, B.; Biehl, B.S.; Rissman, A.I.; Mole, B.; Yi, X.; Yang, C.-H.; et al. Niche-Specificity and the Variable Fraction of the Pectobacterium Pan-Genome. MPMI 2008, 21, 1549–1560. [Google Scholar] [CrossRef] [PubMed]
- Ravensdale, M.; Blom, T.J.; Gracia-Garza, J.A.; Svircev, A.M.; Smith, R.J. Bacteriophages and the Control of Erwinia carotovora subsp. carotovora. Can. J. Plant Pathol. 2007, 29, 121–130. [Google Scholar] [CrossRef]
- Fister, S.; Robben, C.; Witte, A.K.; Schoder, D.; Wagner, M.; Rossmanith, P. Influence of Environmental Factors on Phage–Bacteria Interaction and on the Efficacy and Infectivity of Phage P100. Front. Microbiol. 2016, 7, 197487. [Google Scholar] [CrossRef]
- Czajkowski, R.; Smolarska, A.; Ozymko, Z. The Viability of Lytic Bacteriophage ΦD5 in Potato-Associated Environments and Its Effect on Dickeya solani in Potato (Solanum tuberosum L.) Plants. PLoS ONE 2017, 12, e0183200. [Google Scholar] [CrossRef]
- Vreugdenhil, D.; Brandshaw, J. (Eds.) Potato Biology and Biotechnology: Advances and Perspectives, 1st ed.; Elsevier: Oxford, UK; San Diego, CA, USA, 2007; ISBN 978-0-444-51018-1. [Google Scholar]
- Williamson, K.E.; Radosevich, M.; Wommack, K.E. Abundance and diversity of viruses in six Delaware soils. Appl. Environ. Microbiol. 2005, 71, 3119–3125. [Google Scholar] [CrossRef] [PubMed]




| Variety | Maturity | Utilization |
|---|---|---|
| ‘Alice’ | early | fresh consumption |
| ‘Bella’ | medium-early | fresh consumption, French fries |
| ‘Bohemia’ | early | fresh consumption |
| ‘David’ | medium-early to medium-late | starch |
| ‘Dominika’ | medium-early | fresh consumption |
| ‘Jasmina’ | early | fresh consumption |
| ‘Jindra’ | medium-late to late | fresh consumption |
| ‘Katy’ | very early | fresh consumption |
| ‘Magda’ | very early | fresh consumption |
| ‘Mariannka’ | very early | fresh consumption |
| ‘Monika’ | early | fresh consumption |
| ‘Primarosa’ | very early | fresh consumption |
| ‘Red Anna’ | medium-early | fresh consumption |
| ‘Suzan’ | very early | fresh consumption |
| ‘Verne’ | medium-early to medium-late | starch |
| ‘Vysočina’ | medium-early | fresh consumption |
| ‘Westamyl’ | medium-late | starch |
| Degree of Infection | Symptoms on Plants |
|---|---|
| 0 | uninfected plant |
| 1 | stem blackening in the extent of 1–25% |
| 2 | stem blackening in the extent of 26–50% |
| 3 | stem blackening in the extent of 51–75% |
| 4 | stem blackening in the extent of 76–99% |
| 5 | the whole plant is affected by stem blackening and dies |
| Sensitivity of Genotypes | Mean Degree of Infection | Dickeya solani |
|---|---|---|
| relatively resistant | 8.99–7.50 | - |
| partially resistant (tolerant) | 7.49–6.00 | ‘Bella’, ‘Vysočina’, ‘Katy’ |
| susceptible | 5.99–4.50 | ‘Verne’, ‘Monika’, Suzan’, ‘Dominika’, ‘Mariannka’, ‘David, ‘Primarosa’, ‘Magda’ |
| highly susceptible | 4.49–3.00 | ‘Jindra’, ‘Jasmína’, ‘Red Anna’, ‘Bohemia’, ‘Westamyl’, ‘Alice’ |
| very highly susceptible | 2.99–1.00 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmoch, M.; Vacek, J.; Loubová, V.; Petrzik, K.; Brázdová, S.; Ševčík, R. Potential of Limestonevirus Bacteriophages for Ecological Control of Dickeya solani Causing Bacterial Potato Blackleg. Agriculture 2024, 14, 497. https://doi.org/10.3390/agriculture14030497
Kmoch M, Vacek J, Loubová V, Petrzik K, Brázdová S, Ševčík R. Potential of Limestonevirus Bacteriophages for Ecological Control of Dickeya solani Causing Bacterial Potato Blackleg. Agriculture. 2024; 14(3):497. https://doi.org/10.3390/agriculture14030497
Chicago/Turabian StyleKmoch, Martin, Josef Vacek, Věra Loubová, Karel Petrzik, Sára Brázdová, and Rudolf Ševčík. 2024. "Potential of Limestonevirus Bacteriophages for Ecological Control of Dickeya solani Causing Bacterial Potato Blackleg" Agriculture 14, no. 3: 497. https://doi.org/10.3390/agriculture14030497





