Vegetable Production in PFALs: Control of Micro-Environmental Factors, Principal Components and Automated Systems
Abstract
:1. Introduction
1.1. Review Objectives
1.2. The Emergence of PFALs
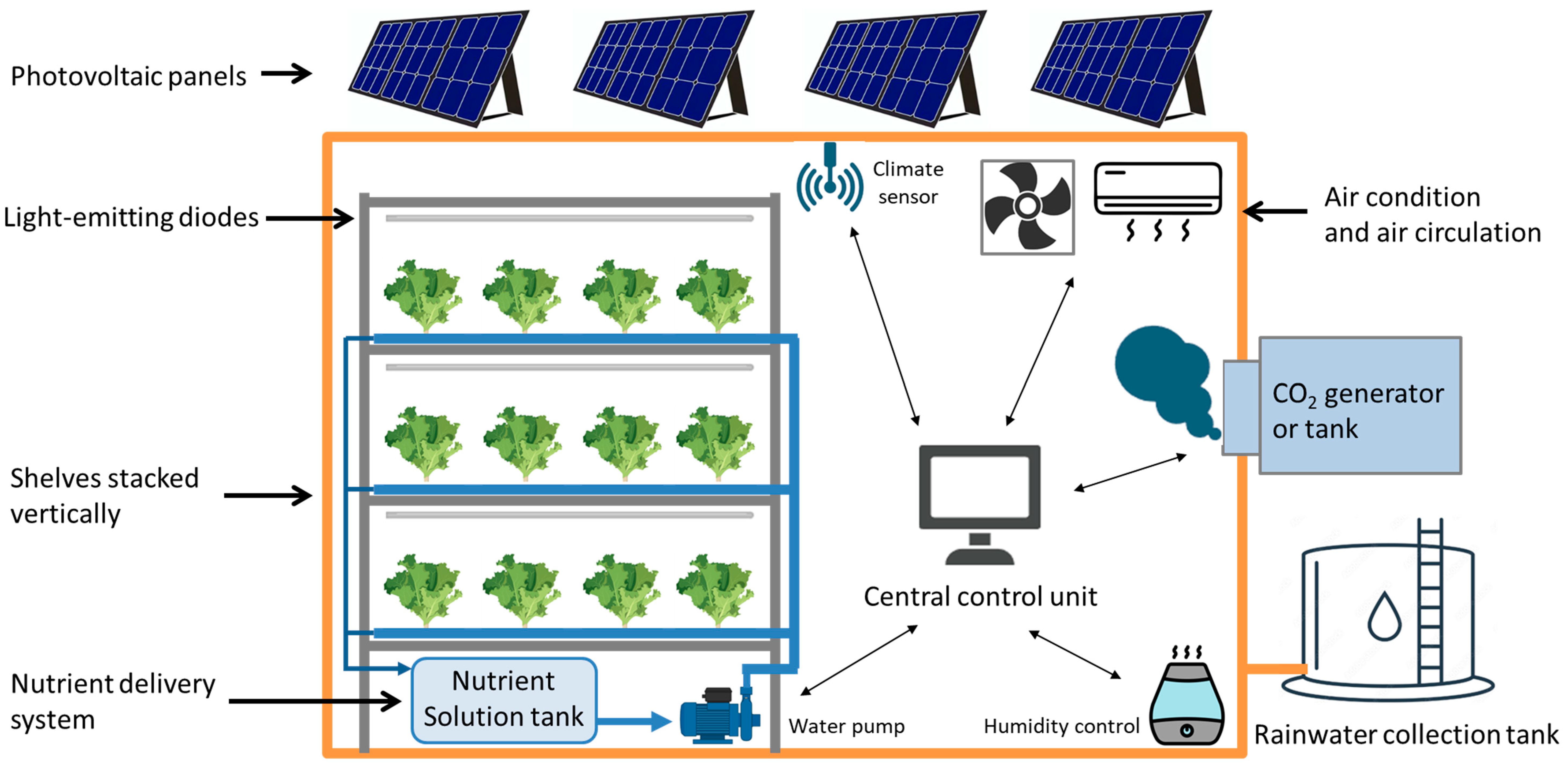
2. Principal Components and Automated Systems
2.1. Environmental Control Systems
2.2. Soilless Cultivation Systems
- NFT, wherein a thin film of nutrient solution flows (either continuously or intermittently) over the roots;
- DWC, which consists of crops grown with their roots continuously submerged in a nutrient solution;
- The aggregate culturing of growing crops in bagged substrates (e.g., rockwool or coconut coir slabs) or containers (e.g., Dutch/Bato buckets) with the nutrient solution applied via drip emitters.
2.3. Design of the Artificial Lighting System
2.4. Automation and IoT Systems
3. Control of Micro-Environmental Factors for Vegetable and Food Production
3.1. Control of the Nutrient Solution
3.2. Control of Light Properties
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Galanakis, C.M. The Food Systems in the Era of the Coronavirus (COVID-19) Pandemic Crisis. Foods 2020, 9, 523. [Google Scholar] [CrossRef] [PubMed]
- United Nations. United Nations. United Nations World Urbanization Prospects: The 204 Revision—Highlights. In Statistical Papers—United Nations (Ser. A), Population and Vital Statistics Report; United Nations: New York, NY, USA, 2014; ISBN 9789210568098. [Google Scholar]
- Foley, J.; Ramankutty, N.; Brauman, K.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Huff, A.G.; Beyeler, W.E.; Kelley, N.S.; McNitt, J.A. How Resilient Is the United States’ Food System to Pandemics? J. Environ. Sci. 2015, 5, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Rizou, M.; Galanakis, I.M.; Aldawoud, T.M.S.; Galanakis, C.M. Safety of Foods, Food Supply Chain and Environment within the COVID-19 Pandemic. Trends Food Sci. Technol. 2020, 102, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Ostan, R.; Lanzarini, C.; Pini, E.; Scurti, M.; Vianello, D.; Bertarelli, C.; Fabbri, C.; Izzi, M.; Palmas, G.; Biondi, F.; et al. Inflammaging and Cancer: A Challenge for the Mediterranean Diet. Nutrients 2015, 7, 2589–2621. [Google Scholar] [CrossRef] [PubMed]
- Dias, J.S. World Importance, Marketing and Trading of Vegetables. Acta Hortic. 2011, 921, 153–169. [Google Scholar] [CrossRef]
- Schreinemachers, P.; Simmons, E.B.; Wopereis, M.C.S. Tapping the Economic and Nutritional Power of Vegetables. Glob. Food Secur. 2018, 16, 36–45. [Google Scholar] [CrossRef]
- Eigenbrod, C.; Gruda, N. Urban Vegetable for Food Security in Cities. A Review. Agron. Sustain. Dev. 2015, 35, 483–498. [Google Scholar] [CrossRef]
- Walters, S.A.; Midden, K.S. Sustainability of Urban Agriculture: Vegetable Production on Green Roofs. Agriculture 2018, 8, 168. [Google Scholar] [CrossRef]
- Thomaier, S.; Specht, K.; Henckel, D.; Dierich, A.; Siebert, R.; Freisinger, U.B.; Sawicka, M. Farming in and on Urban Buildings: Present Practice and Specific Novelties of Zero-Acreage Farming (ZFarming). Renew. Agric. Food Syst. 2015, 30, 43–54. [Google Scholar] [CrossRef]
- Pennisi, G.; Sanyé-Mengual, E.; Orsini, F.; Crepaldi, A.; Nicola, S.; Ochoa, J.; Fernandez, J.A.; Gianquinto, G. Modelling Environmental Burdens of Indoor-Grown Vegetables and Herbs as Affected by Red and Blue LED Lighting. Sustainability 2019, 11, 4063. [Google Scholar] [CrossRef]
- Kozai, T. PFAL Business and R&D in the World: Current Status and Perspectives. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, MA, USA, 2019; pp. 35–68. [Google Scholar]
- Carotti, L.; Graamans, L.; Puksic, F.; Butturini, M.; Meinen, E.; Heuvelink, E.; Stanghellini, C. Plant Factories Are Heating Up: Hunting for the Best Combination of Light Intensity, Air Temperature and Root-Zone Temperature in Lettuce Production. Front. Plant Sci. 2021, 11, 592171. [Google Scholar] [CrossRef] [PubMed]
- Mainos, D.; Bantis, F.; Ntinas, G.K.; Koukounaras, A. Yield, Quality, and Resources Use Efficiency of Wild Rocket Baby Leaves Grown under Different Controlled Environment Systems and Various Growing Seasons. Horticulturae 2023, 9, 661. [Google Scholar] [CrossRef]
- SharathKumar, M.; Heuvelink, E.; Marcelis, L.F.M. Vertical Farming: Moving from Genetic to Environmental Modification. Trends Plant Sci. 2020, 25, 722–724. [Google Scholar] [CrossRef]
- Nicola, S.; Pignata, G.; Ferrante, A.; Bulgari, R.; Cocetta, G.; Ertani, A. Water Use Efficiency in Greenhouse Systems and Its Application in Horticulture. AgroLife Sci. J. 2020, 9, 248–261. [Google Scholar]
- Kozai, T.; Niu, G. Role of the Plant Factory with Artificial Lighting (PFAL) in Urban Areas. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 7–34. ISBN 9780128166918. [Google Scholar]
- Kozai, T. Smart Plant Factory: The Next Generation Indoor Vertical Farms; Springer: Singapore, 2018; ISBN 9789811310652. [Google Scholar]
- Tian, Z.; Ma, W.; Yang, Q.; Duan, F. Application status and challenges of machine vision in plant factory—A review. Inform. Proces. Agric. 2022, 9, 195–211. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tabuchi, T. Tomato Cultivation in a Plant Factory with Artificial Light: Effect of UV-A Irradiation During the Growing Period on Yield and Quality of Ripening Fruit. Hortic. J. 2022, 91, 16–23. [Google Scholar] [CrossRef]
- Takahashi, A.; Yasutake, D.; Hidaka, K.; Ono, S.; Kitano, M.; Hirota, T.; Yokohama, G.; Nakamura, T.; Toro, M. Yield and Photosynthesis Related to Growth Forms of Two Strawberry Cultivars in a Plant Factory with Artificial Lighting. HortScience 2024, 59, 394–399. [Google Scholar] [CrossRef]
- Gómez, C.; Currey, C.J.; Dickson, R.W.; Kim, H.J.; Hernández, R.; Sabeh, N.C.; Raudales, R.E.; Brumfield, R.G.; Laury-Shaw, A.; Wilke, A.K.; et al. Controlled Environment Food Production for Urban Agriculture. HortScience 2019, 54, 1448–1458. [Google Scholar] [CrossRef]
- Rabbi, B.; Chen, Z.H.; Sethuvenkatraman, S. Protected Cropping in Warm Climates: A Review of Humidity Control and Cooling Methods. Energies 2019, 12, 2737. [Google Scholar] [CrossRef]
- Von Caemmerer, S.; Baker, N. The Biology of Transpiration. From Guard Cells to Globe. Plant Physiol. 2007, 143, 3. [Google Scholar] [CrossRef]
- Kozai, T. Resource Use Efficiency of Closed Plant Production System with Artificial Light: Concept, Estimation and Application to Plant Factory. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2013, 89, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Both, A.-J.; Frantz, J.M.; Bugbee, B. Carbon Dioxide Enrichment in Controlled Environments. In Light Management in Controlled Environments; Meister Media Worldwide: Willoughby, OH, USA, 2017; pp. 82–86. [Google Scholar]
- Nelson, P.V. Greenhouse Operation and Management, 7th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2012. [Google Scholar]
- Wang, J.; Lu, W.; Tong, Y.; Yang, Q. Leaf Morphology, Photosynthetic Performance, Chlorophyll Fluorescence, Stomatal Development of Lettuce (Lactuca sativa L.) Exposed to Different Ratios of Red Light to Blue Light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef]
- Kozai, T.; Sakaguchi, S.; Akiyama, T.; Yamada, K.; Ohshima, K. Design and Management of PFAL. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 295–312. ISBN 9780128017753. [Google Scholar]
- Bantis, F.; Smirnakou, S.; Ouzounis, T.; Koukounaras, A.; Ntagkas, N.; Radoglou, K. Current Status and Recent Achievements in the Field of Horticulture with the Use of Light-Emitting Diodes (LEDs). Sci. Hortic. 2018, 235, 437–451. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of Different Ratios of Blue and Red Led Light on Brassicaceae Microgreens under a Controlled Environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A Review on the Effects of Light-Emitting Diode (LED) Light on the Nutrients of Sprouts and Microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Yokoyama, R. Energy Consumption and Heat Sources in Plant Factories. In Plant Factory Using Artificial Light: Adapting to Environmental Disruption and Clues to Agricultural Innovation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 177–184. ISBN 9780128139745. [Google Scholar]
- Chen, H.; Dong, X.; Lei, J.; Zhang, N.; Wang, Q.; Shi, Z.; Yang, J. Life Cycle Assessment of Carbon Capture by an Intelligent Vertical Plant Factory within an Industrial Park. Sustainability 2024, 16, 697. [Google Scholar] [CrossRef]
- Serrano, L.T.C.; Águila, M.V.G.; Paniagua, F.S.; Suárez, J.A.C.; Rodríguez, A.M. Software engineering for a mini-PFAL (plant factory with artificial lighting) with IoT interconnectivity. Rev. Cienc. Técnicas Agropecu. 2022, 31, 1–13. [Google Scholar]
- Zheng, S. IT networks and plant factories. In Plant Factory Using Artificial Light; Anpo, M., Fukuda, H., Wada, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–226. [Google Scholar]
- Chowdhury, M.E.H.; Khandakar, A.; Ahmed, S.; Al-Khuzaei, F.; Hamdalla, J.; Haque, F.; Reaz, M.B.I.; Al Shafei, A.; Al-Emadi, N. Design, Construction and Testing of Iot Based Automated Indoor Vertical Hydroponics Farming Test-Bed in Qatar. Sensors 2020, 20, 5637. [Google Scholar] [CrossRef]
- Jones, J.B. Complete Guide for Growing Plants Hydroponically; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Oxfordshire, UK, 2014; p. 206. [Google Scholar]
- Orsini, F.; Pennisi, G.; Zulfiqar, F.; Gianquinto, G. Sustainable Use of Resources in Plant Factories with Artificial Lighting (PFALs). Eur. J. Hortic. Sci. 2020, 85, 297–309. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Gao, J.; Syed, T.N.; Chandio, F.A.; Buttar, N.A. Modern Plant Cultivation Technologies in Agriculture under Controlled Environment: A Review on Aeroponics. J. Plant Interact. 2018, 13, 338–352. [Google Scholar] [CrossRef]
- Calone, R.; Pennisi, G.; Morgenstern, R.; Sanyé-Mengual, E.; Lorleberg, W.; Dapprich, P.; Winkler, P.; Orsini, F.; Gianquinto, G. Improving Water Management in European Catfish Recirculating Aquaculture Systems through Catfish-Lettuce Aquaponics. Sci. Total Environ. 2019, 687, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Kanematsu, Y.; Yoshikawa, N.; Okubo, T.; Takagaki, M. Environmental and Resource Use Analysis of Plant Factories with Energy Technology Options: A Case Study in Japan. J. Clean. Prod. 2018, 186, 703–717. [Google Scholar] [CrossRef]
- Zhang, X.; He, D.; Niu, G.; Yan, Z.; Song, J. Effects of Environment Lighting on the Growth, Photosynthesis, and Quality of Hydroponic Lettuce in a Plant Factory. Int. J. Agric. Biol. Eng. 2018, 11, 33–40. [Google Scholar] [CrossRef]
- Zou, T.; Huang, C.; Wu, P.; Ge, L.; Xu, Y. Optimization of Artificial Light for Spinach Growth in Plant Factory Based on Orthogonal Test. Plants 2020, 9, 490. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Niu, G.; Takagaki, M. Plant Factory An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Sugano, M. Elemental Technologies for Realizing a Fully-Controlled Artificial Light-Type Plant Factory. In Proceedings of the 12th International Conference & Expo on Emerging Technologies for a Smarter World (CEWIT), Melville, NY, USA, 19–20 October 2015. [Google Scholar]
- Graamans, L.; van den Dobbelsteen, A.; Meinen, E.; Stanghellini, C. Plant Factories; Crop Transpiration and Energy Balance. Agric. Syst. 2017, 153, 138–147. [Google Scholar] [CrossRef]
- Wu, B.S.; Hitti, Y.; MacPherson, S.; Orsat, V.; Lefsrud, M.G. Comparison and perspective of conventional and LED lighting for photobiology and industry applications. Environ. Exp. Bot. 2020, 171, 103953. [Google Scholar] [CrossRef]
- Nelson, J.A.; Bugbee, B. Economic Analysis of Greenhouse Lighting: Light Emitting Diodes vs. High Intensity Discharge Fixtures. PLoS ONE 2014, 9, e99010. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Kim, S.K.; Lee, S.G.; Lee, H.J.; Kwon, J.K. Application of plasma lighting for growth and flowering of tomato plants. Hortic. Environ. Biotechnol. 2018, 59, 827–833. [Google Scholar] [CrossRef]
- Kozai, T. Design and management of PFALs. In Plant Factory: An Indoor Vertical Farming System for Efficient Quality Food Production; Academic Press: Cambridge, MA, USA, 2019; pp. 35–68. [Google Scholar]
- Hiroki, R.; Shimizu, H.; Ito, A.; Nakashima, H.; Miyasaka, J.; Ohdoi, K. Identifying the Optimum Light Cycle for Lettuce Growth in a Plant Factory. Acta Hortic. 2014, 1037, 863–868. [Google Scholar] [CrossRef]
- Dorais, M. The Use of Supplemental Lighting for Vegetable Crop Production: Light Intensity, Crop Response, Nutrition, Crop Management, Cultural Practices. In Proceedings of the Canadian Greenhouse Conference, Edmonton, AB, Canada, 9–10 June 2003; Volume 9. [Google Scholar]
- Wittmann, S.; Jüttner, I.; Mempel, H. Indoor Farming Marjoram Production—Quality, Resource Efficiency, and Potential of Application. Agronomy 2020, 10, 769. [Google Scholar] [CrossRef]
- Ojha, T.; Misra, S.; Raghuwanshi, N.S. Wireless Sensor Networks for Agriculture: The State-of-the-Art in Practice and Future Challenges. Comput. Electron. Agric. 2015, 118, 66–84. [Google Scholar] [CrossRef]
- Rault, T.; Bouabdallah, A.; Challal, Y. Energy Efficiency in Wireless Sensor Networks: A Top-down Survey. Comput. Netw. 2014, 67, 104–122. [Google Scholar] [CrossRef]
- Hong, G.Z.; Hsieh, C.L. Application of Integrated Control Strategy and Bluetooth for Irrigating Romaine Lettuce in Greenhouse. IFAC-Pap. 2016, 49, 381–386. [Google Scholar] [CrossRef]
- Selmani, A.; Outanoute, M.; El Khayat, M.; Guerbaoui, M.; Ed-Dahhak, A.; Lachhab, A.; Bouchikhi, B. Towards Autonomous Greenhouses Solar-Powered. Procedia Comput. Sci. 2019, 148, 495–501. [Google Scholar] [CrossRef]
- Chen, W.T.; Yeh, Y.H.F.; Liu, T.Y.; Lin, T.T. An Automated and Continuous Plant Weight Measurement System for Plant Factory. Front. Plant Sci. 2016, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.S.; Kim, H.J.; Cho, W.J. On-the-Go Image Processing System for Spatial Mapping of Lettuce Fresh Weight in Plant Factory. IFAC-Pap. 2018, 51, 130–134. [Google Scholar] [CrossRef]
- Nagano, S.; Moriyuki, S.; Wakamori, K.; Mineno, H.; Fukuda, H. Leaf-Movement-Based Growth Prediction Model Using Optical Flow Analysis and Machine Learning in Plant Factory. Front. Plant Sci. 2019, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Montoya, A.P.; Obando, F.A.; Osorio, J.A.; Morales, J.G.; Kacira, M. Design and Implementation of a Low-Cost Sensor Network to Monitor Environmental and Agronomic Variables in a Plant Factory. Comput. Electron. Agric. 2020, 178, 105758. [Google Scholar] [CrossRef]
- Spinelli, G.M.; Gottesman, Z.L. A Low-Cost Arduino-Based Datalogger with Cellular Modem and FTP Communication for Irrigation Water Use Monitoring to Enable Access to CropManage. HardwareX 2019, 6, 66. [Google Scholar] [CrossRef]
- Cai, X.; Cui, S.; He, L.; Zhang, Y.; Wang, Y.; Lv, X. Research on Automatic pH Control System of Nutrient Solution in Smart Plant Factory Based on STM32. In Proceedings of the 2020 IEEE 9th Joint International Information Technology and Artificial Intelligence Conference (ITAIC), Chongqing, China, 11–13 December 2020; pp. 1837–1840. [Google Scholar]
- Ijaz, F.; Siddiqui, A.A.; Im, B.K.; Lee, C. Remote Management and Control System for LED Based Plant Factory Using ZigBee and Internet. In Proceedings of the 14th International Conference On Advanced Communication Technology (ICACT), Pyeonhchang, Republic of Korea, 19–22 February 2012. [Google Scholar]
- Ohara, H.; Hirai, T.; Kouno, K.; Nishiura, Y. Automatic Plant Cultivation System (Automated Plant Factory). Environ. Control Biol. 2015, 53, 93–99. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Choi, K.Y.; Lee, Y.B.; Son, J.E. Growth characteristics of sowthistle (Ixeris dentata Nakai) under different levels of light intensity, electrical conductivity of nutrient solution, and planting density in a plant factory. Hortic. Environ. Biotechnol. 2012, 53, 368–372. [Google Scholar] [CrossRef]
- Cho, Y.Y.; Cha, M.K.; Ku, Y.G.; Kim, H.C.; Cho, J.H.B. Effect of Different Culture Nutrient Solution EC on Carrot Top Growth and Nutritional Contents in a Closed–type Plant Factory System. Hortic. Sci. Technol. 2018, 36, 37–45. [Google Scholar] [CrossRef]
- Lam, V.P.; Kim, S.J.; Park, J.S. Optimizing the Electrical Conductivity of a Nutrient Solution for Plant Growth and Bioactive Compounds of Agastache rugosa in a Plant Factory. Agronomy 2020, 10, 76. [Google Scholar] [CrossRef]
- Bantis, F.; Fotelli, M.; Ilić, Z.S.; Koukounaras, A. Physiological and Phytochemical Responses of Spinach Baby Leaves Grown in a PFAL System with Leds and Saline Nutrient Solution. Agriculture 2020, 10, 574. [Google Scholar] [CrossRef]
- Sakamoto, K.; KoGI, M.; Yanagisawa, T. Effects of Salinity and Nutrients in Seawater on Hydroponic Culture of Red Leaf Lettuce. Environ. Control Biol. 2014, 52, 189–195. [Google Scholar] [CrossRef]
- Nakamura, S.; Senoh, M.; Nagahama, S.I.; Iwasa, N.; Matsushita, T.; Mukai, T. Blue InGaN-Based Laser Diodes with an Emission Wavelength of 450 Nm. Appl. Phys. Lett. 2000, 76, 22–24. [Google Scholar] [CrossRef]
- Pennisi, G.; Blasioli, S.; Cellini, A.; Maia, L.; Crepaldi, A.; Braschi, I.; Spinelli, F.; Nicola, S.; Fernandez, J.A.; Stanghellini, C.; et al. Unraveling the Role of Red:Blue LED Lights on Resource Use Efficiency and Nutritional Properties of Indoor Grown Sweet Basil. Front. Plant Sci. 2019, 10, 305. [Google Scholar] [CrossRef]
- Bantis, F. Light Spectrum Differentially Affects the Yield and Phytochemical Content of Microgreen Vegetables in a Plant Factory. Plants 2021, 10, 2182. [Google Scholar] [CrossRef]
- Smith, H.L.; Mcausland, L.; Murchie, E.H. Don’t Ignore the Green Light: Exploring Diverse Roles in Plant Processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Claypool, N.B.; Heinrich Lieth, J. Green Light Improves Photosystem Stoichiometry in Cucumber Seedlings (Cucumis sativus) Compared to Monochromatic Red Light. Plants 2021, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gao, M.; He, R.; Zhang, Y.; Song, S.; Su, W.; Liu, H. Far-Red Light Suppresses Glucosinolate Profiles of Chinese Kale through Inhibiting Genes Related to Glucosinolate Biosynthesis. Environ. Exp. Bot. 2021, 188, 104507. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR Irradiation Improves Growth and Nutritional Properties of Lettuce Grown in an Artificial Light Plant Factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Liu, X.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. Response of Growth, Photosynthetic Electron Transfer, and Chloroplast Ultrastructure to Different LED Light Combination in Green Onion (Allium fistulosum L.). Physiol. Plant 2021, 172, 1662–1672. [Google Scholar] [CrossRef] [PubMed]
- Tsaballa, A.; Xanthopoulou, A.; Sperdouli, I.; Bantis, F.; Boutsika, A.; Chatzigeorgiou, I.; Tsaliki, E.; Koukounaras, A.; Ntinas, G.K.; Ganopoulos, I. LED Omics in Rocket Salad (Diplotaxis tenuifolia): Comparative Analysis in Different Light-Emitting Diode (LED) Spectrum and Energy Consumption. Plants 2023, 12, 1203. [Google Scholar] [CrossRef] [PubMed]
- Spalholz, H.; Perkins-Veazie, P.; Hernández, R. Impact of Sun-Simulated White Light and Varied Blue: Red Spectrums on the Growth, Morphology, Development, and Phytochemical Content of Green- and Red-Leaf Lettuce at Different Growth Stages. Sci. Hortic. 2020, 264, 109195. [Google Scholar] [CrossRef]
- Bantis, F.; Koukounaras, A.; Siomos, A.S.; Radoglou, K.; Dangitsis, C. Optimal LED Wavelength Composition for the Production of High-Quality Watermelon and Interspecific Squash Seedlings Used for Grafting. Agronomy 2019, 9, 870. [Google Scholar] [CrossRef]
- Vaštakaite-Kairien, V.; Kelly, N.; Runkle, E.S. Regulation of the Photon Spectrum on Growth and Nutritional Attributes of Baby-Leaf Lettuce at Harvest and during Postharvest Storage. Plants 2021, 10, 549. [Google Scholar] [CrossRef]
- Prinzenberg, A.E.; van der Schoot, H.; van Deth, O.; Ouzounis, T.; Gabriëls, S.; Meijer-Dekens, F.; Marcelis, L.F.M.; Visser, R.G.F.; Heuvelink, E.; Schouten, H.J. Does Tomato Breeding for Improved Performance under LED Supplemental Lighting Make Sense? Euphytica 2022, 218, 30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bantis, F.; Chatzigeorgiou, I.; Sismanis, M.; Ntinas, G.K.; Koukounaras, A. Vegetable Production in PFALs: Control of Micro-Environmental Factors, Principal Components and Automated Systems. Agriculture 2024, 14, 642. https://doi.org/10.3390/agriculture14040642
Bantis F, Chatzigeorgiou I, Sismanis M, Ntinas GK, Koukounaras A. Vegetable Production in PFALs: Control of Micro-Environmental Factors, Principal Components and Automated Systems. Agriculture. 2024; 14(4):642. https://doi.org/10.3390/agriculture14040642
Chicago/Turabian StyleBantis, Filippos, Ioanna Chatzigeorgiou, Michail Sismanis, Georgios K. Ntinas, and Athanasios Koukounaras. 2024. "Vegetable Production in PFALs: Control of Micro-Environmental Factors, Principal Components and Automated Systems" Agriculture 14, no. 4: 642. https://doi.org/10.3390/agriculture14040642







