Field Assessment of Lamium album in Reducing Mycotoxin Biosynthesis in Winter Wheat Infected by Fusarium culmorum
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Studied Material
2.3. Fusarium Strain and Inoculum Preparation
2.4. Chemicals
2.5. Experimental Design and Procedure
2.6. Weight of 1000 Grains Evaluation
2.7. Chemical Analyses
2.7.1. ERG Content in the Harvested Wheat Grains
2.7.2. Mycotoxins in the Harvested Winter Wheat Grain
2.8. Statistical Analysis
3. Results
3.1. Comparative Effect of L. album Flower Extract on the Grain Weight of the Harvested Winter Wheat Cultivars
3.2. ERG Level in the Harvested Wheat Grains
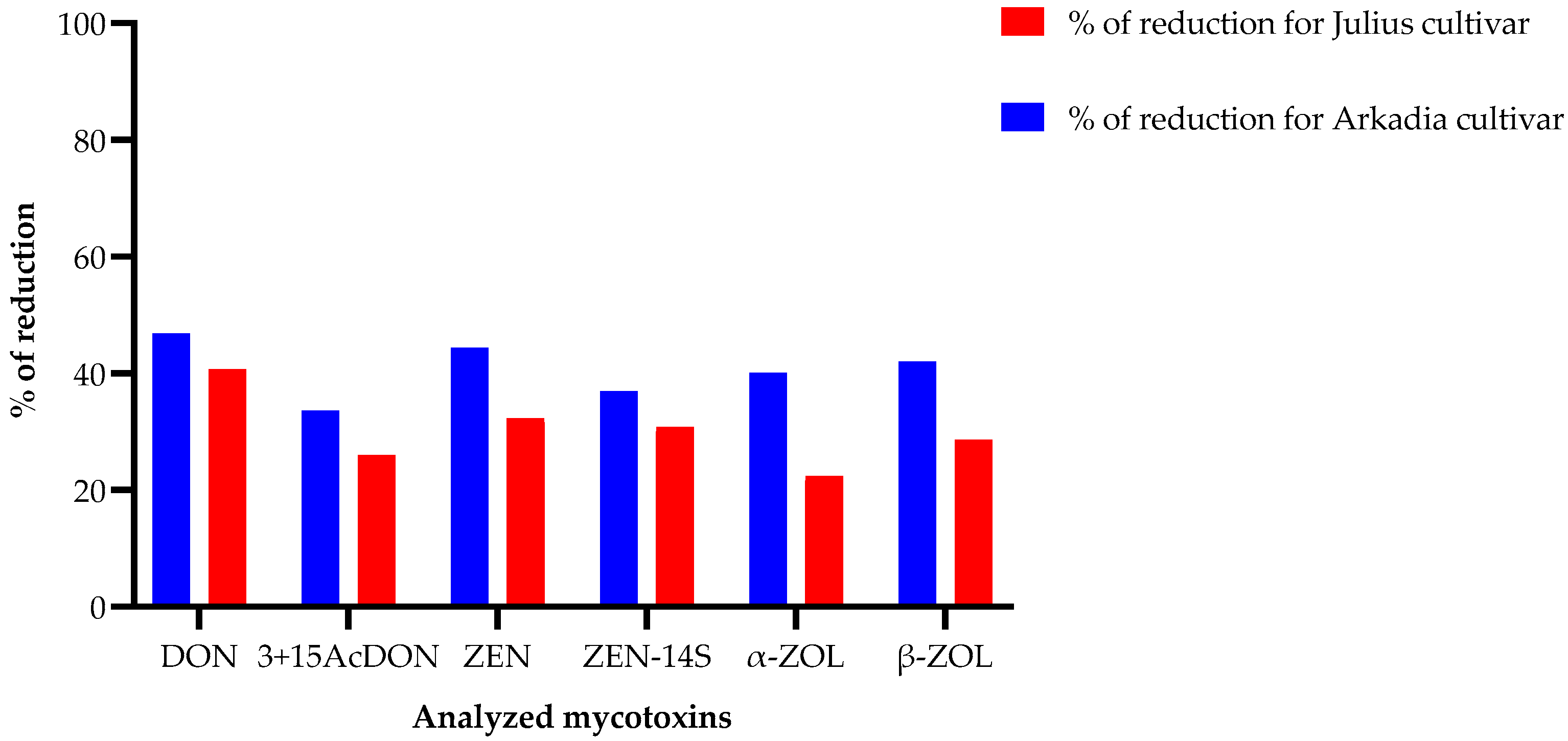
3.3. The Inhibitory Impact of L. album Flower Extracts on Mycotoxin Biosynthesis in the Investigated Winter Wheat Cultivars
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Awika, J.M. Major Cereal Grains Production and Use around the World. ACS Symp. Ser. 2011, 1089, 1–13. [Google Scholar] [CrossRef]
- FAO. FAOSTAT: Production: Crops and livestock products; FAO: Rome, Italy, 2022; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 27 September 2023).
- Petronaitis, T.; Simpfendorfer, S.; Hüberli, D. Importance of Fusarium Spp. in Wheat to Food Security: A Global Perspective. In Plant Diseases and Food Security in the 21st Century. Plant Pathology in the 21st Century; Springer: Cham, Switzerland, 2021; Volume 10, pp. 121–157. [Google Scholar] [CrossRef]
- Singh, R.P.; Singh, P.K.; Rutkoski, J.; Hodson, D.P.; He, X.; Jørgensen, L.N.; Hovmøller, M.S.; Huerta-Espino, J. Disease Impact on Wheat Yield Potential and Prospects of Genetic Control. Annu. Rev. Phytopathol. 2016, 54, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Scherm, B.; Balmas, V.; Spanu, F.; Pani, G.; Delogu, G.; Pasquali, M.; Migheli, Q. Fusarium culmorum: Causal Agent of Foot and Root Rot and Head Blight on Wheat. Mol. Plant Pathol. 2013, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Alisaac, E.; Mahlein, A.-K.; Alisaac, E.; Mahlein, A.-K. Fusarium Head Blight on Wheat: Biology, Modern Detection and Diagnosis and Integrated Disease Management. Toxins 2023, 15, 192. [Google Scholar] [CrossRef] [PubMed]
- Pierzgalski, A.; Bryła, M.; Kanabus, J.; Modrzewska, M.; Podolska, G. Updated Review of the Toxicity of Selected Fusarium Toxins and Their Modified Forms. Toxins 2021, 13, 768. [Google Scholar] [CrossRef]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as Human Carcinogens—The IARC Monographs Classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef]
- Cárdenas-Laverde, D.; Barbosa-Cornelio, R.; Coy-Barrera, E. Antifungal Activity against Fusarium oxysporum of Botanical End-Products: An Integration of Chemical Composition and Antifungal Activity Datasets to Identify Antifungal Bioactives. Plants 2021, 10, 2563. [Google Scholar] [CrossRef]
- Makhuvele, R.; Naidu, K.; Gbashi, S.; Thipe, V.C.; Adebo, O.A.; Njobeh, P.B. The Use of Plant Extracts and Their Phytochemicals for Control of Toxigenic Fungi and Mycotoxins. Heliyon 2020, 6, e05291. [Google Scholar] [CrossRef] [PubMed]
- Hollaway, G.J.; Industries, P. Yield Loss in Cereals, Caused by Fusarium culmorum and F. pseudograminearum Is Related to Fungal DNA in Soil Prior to Planting, Rainfall, and Cereal Type. Plant Dis. 2013, 97, 977–982. [Google Scholar] [CrossRef]
- Zubrod, J.P.; Bundschuh, M.; Arts, G.; Bru, C.A.; Kna, A.; Payraudeau, S.; Rasmussen, J.J.; Rohr, J.; Scharmu, A.; Smalling, K.; et al. Fungicides: An Overlooked Pesticide Class? Environ. Sci. Technol 2019, 53, 54. [Google Scholar] [CrossRef]
- Castro, J.C.; Pante, G.C.; Centenaro, B.M.; Almeida, R.T.R.D.; Pilau, E.J.; Dias Filho, B.P.; Mossini, S.A.G.; Abreu Filho, B.A.D.; Matioli, G.; Machinski Junior, M. Antifungal and Antimycotoxigenic Effects of Zingiber officinale, Cinnamomum zeylanicum and Cymbopogon Martinii Essential Oils against Fusarium verticillioides. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2020, 37, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- Seepe, H.A.; Nxumalo, W.; Amoo, S.O. Natural Products from Medicinal Plants against Phytopathogenic Fusarium Species: Current Research Endeavours, Challenges and Prospects. Molecules 2021, 26, 6539. [Google Scholar] [CrossRef] [PubMed]
- Acheuk, F.; Basiouni, S.; Shehata, A.A.; Dick, K.; Hajri, H.; Lasram, S.; Yilmaz, M.; Emekci, M.; Tsiamis, G.; Spona-Friedl, M.; et al. Status and Prospects of Botanical Biopesticides in Europe and Mediterranean Countries. Biomolecules 2022, 12, 311. [Google Scholar] [CrossRef] [PubMed]
- Kursa, W.; Jamiołkowska, A.; Wyrostek, J.; Kowalski, R. Antifungal Effect of Plant Extracts on the Growth of the Cereal Pathogen Fusarium Spp.—An In Vitro Study. Agronomy 2022, 12, 3204. [Google Scholar] [CrossRef]
- Jafarzadeh, S.; Abdolmaleki, K.; Javanmardi, F.; Hadidi, M.; Mousavi Khaneghah, A. Recent Advances in Plant-Based Compounds for Mitigation of Mycotoxin Contamination in Food Products: Current Status, Challenges and Perspectives. Int. J. Food Sci. Technol. 2022, 57, 2159–2170. [Google Scholar] [CrossRef]
- Perczak, A.; Gwiazdowska, D.; Marchwińska, K.; Juś, K.; Gwiazdowski, R.; Waśkiewicz, A. Antifungal Activity of Selected Essential Oils against Fusarium culmorum and F. graminearum and Their Secondary Metabolites in Wheat Seeds. Arch. Microbiol. 2019, 201, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, P.A.; Urbaniak, M.; Bryła, M.; Stepien, Ł.; Modrzewska, M.; Waśkiewicz, A. In Vitro Effects of Lemon Balm Extracts in Reducing the Growth and Mycotoxins Biosynthesis of Fusarium culmorum and F. proliferatum. Toxins 2022, 14, 355. [Google Scholar] [CrossRef] [PubMed]
- Uwineza, P.A.; Urbaniak, M.; Stępień, Ł.; Gramza-Michałowska, A.; Waśkiewicz, A. Lamium album Flower Extracts: A Novel Approach for Controlling Fusarium Growth and Mycotoxin Biosynthesis. Toxins 2023, 15, 651. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Vaquero, L.; Bueno, M.; Ventura-Aguilar, R.I.; Aguilar-Guadarrama, A.B.; Robledo, N.; Sepúlveda-Jiménez, G.; Vanegas-Espinoza, P.E.; Ibáñez, E.; Del Villar-Martínez, A.A. Seasonal Variation of Chemical Profile of Ruta graveolens Extracts and Biological Activity against Fusarium oxysporum, Fusarium proliferatum, and Stemphylium vesicarium. Biochem. Syst. Ecol. 2021, 95, 104223. [Google Scholar] [CrossRef]
- Seepe, H.A.; Lodama, K.E.; Sutherland, R.; Nxumalo, W.; Amoo, S.O. In Vivo Antifungal Activity of South African Medicinal Plant Extracts against Fusarium Pathogens and Their Phytotoxicity Evaluation. Plants 2020, 9, 1668. [Google Scholar] [CrossRef] [PubMed]
- Almasoodi, I.H.; Hussein, H.J.; Al-Rubaye, A.F. Antifungal Activity of the Two Medicinal Plants (Curcuma longa L and Boswellia carteri birdwood) against Fusarium Species Isolated from Maize Seeds. Int. J. Pharm. Res. 2020, 12, 408–414. [Google Scholar] [CrossRef]
- Rongai, D.; Pulcini, P.; Pesce, B.; Milano, F. Antifungal Activity of Pomegranate Peel Extract against fusarium Wilt of Tomato. Eur. J. Plant Pathol. 2017, 147, 229–238. [Google Scholar] [CrossRef]
- Suteu, D.; Rusu, L.; Zaharia, C.; Badeanu, M.; Daraban, G.M. Challenge of Utilization Vegetal Extracts as Natural Plant Protection Products. Appl. Sci. 2020, 10, 8913. [Google Scholar] [CrossRef]
- Duke, S.O.; B Powles, S. Glyphosate: A Once-in-a-Century Herbicide. Pest Manag. Sci. 2008, 63, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Deresa, E.M.; Diriba, T.F. Phytochemicals as Alternative Fungicides for Controlling Plant Diseases: A Comprehensive Review of Their Efficacy, Commercial Representatives, Advantages, Challenges for Adoption, and Possible Solutions. Heliyon 2023, 9, e13810. [Google Scholar] [CrossRef] [PubMed]
- Danga, S.P.Y.; Nukenine, E.N.; Fotso, G.T.; Adler, C. Use of NeemPro®, a Neem Product to Control Maize Weevil Sitophilus zeamais (Motsch.) (Coleoptera: Curculionidae) on Three Maize Varieties in Cameroon. Agric. Food Secur. 2015, 4, 1–7. [Google Scholar] [CrossRef]
- Nukenine, E.N.; Tofel, H.K.; Adler, C. Comparative Efficacy of NeemAzal and Local Botanicals Derived from Azadirachta indica and Plectranthus glandulosus against Sitophilus zeamais on Maize. J. Pest Sci. 2011, 84, 479–486. [Google Scholar] [CrossRef]
- Mahlo, S.M.; Chauke, H.R.; McGaw, L.; Eloff, J. Antioxidant and Antifungal Activity of Selected Medicinal Plant Extracts Against Phytopathogenic Fungi. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Cadena-Carrera, S.; Tramontin, D.P.; Bella Cruz, A.; Bella Cruz, R.C.; Müller, J.M.; Hense, H. Biological Activity of Extracts from Guayusa Leaves (Ilex guayusa loes.) Obtained by Supercritical CO2 and Ethanol as Cosolvent. J. Supercrit. Fluids 2019, 152, 104543. [Google Scholar] [CrossRef]
- Goyeneche, R.; Di Scala, K.; Ramirez, C.L.; Fanovich, M.A. Recovery of Bioactive Compounds from Beetroot Leaves by Supercritical CO2 Extraction as a Promising Bioresource. J. Supercrit. Fluids 2020, 155, 104658. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Vidović, S.; Radojčić Redovniković, I.; Jokić, S. New Perspective in Extraction of Plant Biologically Active Compounds by Green Solvents. Food Bioprod. Process. 2018, 109, 52–73. [Google Scholar] [CrossRef]
- Schoss, K.; Glavač, N.K.; Koce, J.D.; Anžlovar, S. Supercritical CO2 Plant Extracts Show Antifungal Activities against Crop-Borne Fungi. Molecules 2022, 27, 1132. [Google Scholar] [CrossRef] [PubMed]
- Waśkiewicz, A.; Morkunas, I.; Bednarski, W.; Mai, V.C.; Formela, M.; Beszterda, M.; Wiśniewska, H.; Goliński, P. Deoxynivalenol and Oxidative Stress Indicators in Winter Wheat Inoculated with Fusarium graminearum. Toxins 2014, 6, 575–591. [Google Scholar] [CrossRef] [PubMed]
- Iwanska, M.; Paderewski, J.; Stepien, M.; Rodrigues, P.C. Adaptation of Winter Wheat Cultivars to Different Environments: A Case Study in Poland. Agronomy 2020, 10, 632. [Google Scholar] [CrossRef]
- Akanmu, A.O.; Abiala, M.A.; Akanmu, A.M.; Adedeji, A.D.; Mudiaga, P.M.; Odebode, A.C. Plant Extracts Abated Pathogenic Fusarium Species of Millet Seedlings. Arch. Phytopathol. Plant Prot. 2013, 46, 1189–1205. [Google Scholar] [CrossRef]
- Heidtmann-Bemvenuti, R.; Tralamazza, S.M.; Jorge Ferreira, C.F.; Corrêa, B.; Badiale-Furlong, E. Effect of Natural Compounds on Fusarium graminearum Complex. J. Sci. Food Agric. 2016, 96, 3998–4008. [Google Scholar] [CrossRef] [PubMed]
- Mitura, K.; Cacak-Pietrzak, G.; Feledyn-Szewczyk, B.; Szablewski, T.; Studnicki, M. Yield and Grain Quality of Common Wheat (Triticum aestivum L.) Depending on the Different Farming Systems (Organic vs. Integrated vs. Conventional). Plants 2023, 12, 1022. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Urbaniak, M.; Stępień, Ł.; Gramza-Michałowska, A.; Waśkiewicz, A. Efficacy of Lamium album as a Natural Fungicide: Impact on Seed Germination, Ergosterol, and Mycotoxins in Fusarium culmorum -Infected Wheat Seedlings. Front. Microbiol. 2024, 15, 1363204. [Google Scholar] [CrossRef] [PubMed]
- Jończyk, K.; Feledyn-Szewczyk, B.; Stalenga, J. Assessment of the Usefulness of New Winter Wheat Varieties (Triticum aestivum L.) for Cultivation in Organic Farming. J. Res. Appl. Agric. Eng. 2018, 63, 43–49. [Google Scholar]
- Pinto, J.G.C.P.; Munaro, L.B.; Jaenisch, B.R.; Nagaoka, A.K.; Lollato, R.P. Wheat Variety Response to Seed Cleaning and Treatment after Fusarium Head Blight Infection. Agrosystems Geosci. Environ. 2019, 2, 1–8. [Google Scholar] [CrossRef]
- Pereira, O.R.; Domingues, M.R.M.; Silva, A.M.S.; Cardoso, S.M. Phenolic Constituents of Lamium album: Focus on Isoscutellarein Derivatives. FRIN 2012, 48, 330–335. [Google Scholar] [CrossRef]
- Pourmirzaee, T.; Kelayeh, S.; Abedinzade, M.; Ghorbani, A. A Review on Biological Effects of Lamium album (White Dead Nettle) and Its Components. J. Herbmed. Pharmacol. 2019, 8, 185–193. [Google Scholar] [CrossRef]
- Sulborska, A.; Konarska, A.; Matysik-Woźniak, A.; Dmitruk, M.; Weryszko-Chmielewska, E.; Skalska-Kamińska, A.; Rejdak, R. Phenolic Constituents of Lamium album L. Subsp. Album Flowers: Anatomical, Histochemical, and Phytochemical Study. Molecules 2020, 25, 6025. [Google Scholar] [CrossRef]
- Uwineza, P.A.; Gramza-Michałowska, A.; Bryła, M.; Waskiewicz, A. Antioxidant Activity and Bioactive Compounds of Lamium album Flower Extracts Obtained by Supercritical Fluid Extraction. Appl. Sci. 2021, 11, 7419. [Google Scholar] [CrossRef]
- Paduch, R.; Magdalena, W. Investigation of Biological Activity of Lamii albi flos Extracts. J. Ethnopharmacol. 2007, 110, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, R.U.; Thippeswamy, S.; Manjunath, K.; Mohana, D.C. Antifungal and Antimycotoxigenic Potency of Solanum torvum swartz. Leaf Extract: Isolation and Identification of Compound Active against Mycotoxigenic Strains of Aspergillus flavus and Fusarium verticillioides. J. Appl. Microbiol. 2015, 119, 1624–1636. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; Zhou, H.; Zhou, W.; Hu, L.; Chen, J.; Shi, Z. The Fungicidal Activity of Thymol against Fusarium Graminearum via Inducing Lipid Peroxidation and Disrupting Ergosterol Biosynthesis. Molecules 2016, 21, 770. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Baka, Z.A. Plant Extract Control of the Fungi Associated with Different Egyptian Wheat Cultivars Grains. J. Plant Prot. Res. 2014, 54, 231–237. [Google Scholar] [CrossRef]
- Abbas, A.; Yli-mattila, T. Biocontrol of Fusarium graminearum, a Causal Agent of Fusarium Head Blight of Wheat, and Deoxynivalenol Accumulation: From In Vitro to In Planta. Toxins 2022, 14, 299. [Google Scholar] [CrossRef]
- Drakopoulos, D.; Meca, G.; Torrijos, R.; Marty, A.; Kägi, A.; Jenny, E.; Forrer, H.R.; Six, J.; Vogelgsang, S. Control of Fusarium graminearum in Wheat with Mustard-Based Botanicals: From in Vitro to in Planta. Front. Microbiol. 2020, 11, 1595. [Google Scholar] [CrossRef] [PubMed]
- Chilaka, C.A.; De Boevre, M.; Atanda, O.O.; De Saeger, S. The Status of Fusarium Mycotoxins in Sub-Saharan Africa: A Review of Emerging Trends and Post-Harvest Mitigation Strategies towards Food Control. Toxins 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Veršilovskis, A.; Peters, J.; Van, D.R.; Zomer, P.; Mol, H.; De Nijs, M. Biological Synthesis and Semi-Preparative Purification of Zearalenone and α-Zearalenol Conjugates; EURL-MP-report_003; EURL Mycotoxins and Plant Toxins, RIKILT Wageningen University & Research: Wageningen, The Netherlands, 2018. [Google Scholar]
- Ksieniewicz-Woźniak, E.; Bryła, M.; Michałowska, D.; Waśkiewicz, A.; Yoshinari, T. Transformation of Selected Fusarium Toxins and Their Masked Forms during Malting of Various Cultivars of Wheat. Toxins 2021, 13, 866. [Google Scholar] [CrossRef] [PubMed]
- Ayed, Y.; Ayed-boussema, I.; Ouanes, Z.; Bacha, H. In Vitro and in Vivo Induction of Chromosome Aberrations by Alpha- and Beta-Zearalenols: Comparison with Zearalenone. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2011, 726, 42–46. [Google Scholar] [CrossRef]
- Sunic, K.; Kovac, T.; Loncaric, A.; Babic, J.; Sulyok, M.; Krska, R.; Drezner, G.; Spanic, V. Fusarium Secondary Metabolite Content in Naturally Produced and Artificially Provoked Fhb Pressure in Winter Wheat. Agronomy 2021, 11, 2239. [Google Scholar] [CrossRef]
- Golinski, P.; Waskiewicz, A.; Wisniewska, H.; Kiecana, I.; Mielniczuk, E.; Gromadzka, K.; Kostecki, M.; Bocianowski, J.; Rymaniak, E. Reaction of Winter Wheat (Triticum aestivum L.) Cultivars to Infection with Fusarium Spp.: Mycotoxin Contamination in Grain and Chaff. Food Addit. Contam. Part A 2010, 27, 1015–1024. [Google Scholar] [CrossRef]
| Plot * | ARK_C | ARK_E | JUL_C | JUL_E |
|---|---|---|---|---|
| FUS 1A-405 | 43.30 a,* ± 0.18 | 43.65 a ± 0.09 | 38.65 c ± 0.10 | 41.45 b ± 0.17 |
| FUS 1A-302 | 43.25 a ± 0.15 | 44.10 a ± 0.04 | 38.30 c ± 0.06 | 39.15 c ± 0.19 |
| FUS 1A-204 | 44.00 a ± 0.21 | 44.40 a ± 0.03 | 37.80 c ± 0.19 | 38.2 c ± 0.31 |
| FUS 1A-103 | 44.05 a ± 0.08 | 43.70 a ± 0.26 | 35.80 d ± 0.03 | 36.05 d ± 0.01 |
| Plot * | ARK_C | ARK_E | JUL_C | JUL_E |
|---|---|---|---|---|
| FUS 1A-405 | 28.50 f,* ± 0.76 | 5.63 i ± 0.62 | 53.54 c ± 2.25 | 35.62 e ± 3.69 |
| FUS 1A-302 | 21.25 g ± 0.97 | 13.01 h ± 2.67 | 46.61 d ± 2.76 | 29.26 f ± 1.17 |
| FUS 1A-204 | 18.64 g ± 1.16 | 7.40 i ± 1.66 | 63.75 b ± 3.83 | 20.88 g ± 0.97 |
| FUS 1A-103 | 35.92 e ± 4.35 | 9.60 hi ± 1.25 | 116.17 a ± 6.62 | 35.02 e ± 1.98 |
| Mean | 26.07 | 8.91 | 70.02 | 30.20 |
| ARK_C | ||||||
| Plot * | DON | 3- and 15-AcDON | ZEN | ZEN-14S | α-ZOL | β-ZOL |
| FUS 1A-405 | 68.54 f,* ± 1.45 | 106.56 e ± 5.93 | 175.74 c ± 3.80 | 550.30 d ± 22.29 | 0.57 g ± 0.03 | 1.75 ef ± 0.04 |
| FUS 1A-302 | 77.61 e ± 2.93 | 104.20 e ± 10.78 | 143.78 e ± 4.52 | 400.46 e ± 17.49 | 0.54 g ± 0.04 | 1.86 de ± 0.04 |
| FUS 1A-204 | 45.91 hi ± 4.39 | 82.96 f ± 5.29 | 105.06 i ± 8.54 | 338.01 f ± 43.19 | 0.27 g ± 0.02 | 1.03 gh ± 0.19 |
| FUS 1A-103 | 52.04 h ± 7.81 | 111.64 e ± 17.29 | 134.92 fg ± 1.80 | 524.45 d ± 24.89 | 0.46 g ± 0.03 | 2.84 bc ± 0.18 |
| Mean | 61.02 | 101.34 | 139.88 | 453.30 | 0.46 | 1.87 |
| ARK_E | ||||||
| Plot * | DON | 3- and 15-AcDON | ZEN | ZEN-14S | α-ZOL | β-ZOL |
| FUS 1A-405a | 33.14 j ± 3.77 | 55.41 g ± 3.99 | 65.38 l ± 4.42 | 392.24 e ± 22.95 | 0.42 g ± 0.05 | 0.39 i ± 0.05 |
| FUS 1A-302a | 43.40 i ± 3.11 | 79.04 f ± 1.49 | 97.52 ij ± 4.05 | 215.79 g ± 7.60 | 0.24 g ± 0.03 | 1.10 gh ± 0.12 |
| FUS 1A-204a | 29.83 jk ± 2.44 | 54.86 g ± 4.40 | 67.48 l ± 4.61 | 131.50 h ± 26.86 | 0.14 g ± 0.03 | 0.89 hi ± 0.23 |
| FUS 1A-103a | 23.45 k ± 2.09 | 79.90 f ± 3.89 | 80.87 k ± 1.39 | 403.30 e ± 11.98 | 0.30 g ± 0.06 | 1.96 de ± 0.29 |
| Mean | 32.46 | 67.30 | 77.81 | 285.71 | 0.27 | 1.09 |
| JUL_C | ||||||
| Plot * | DON | 3- and 15-AcDON | ZEN | ZEN-14S | α-ZOL | β-ZOL |
| FUS 2A-405 | 98.37 bc ± 3.41 | 161.44 b ± 9.82 | 186.91 b ± 9.72 | 733.00 b ± 11.18 | 2.16 e ± 0.17 | 1.85 de ± 0.35 |
| FUS 2A-302 | 84.77 d ± 4.45 | 128.91 d ± 3.06 | 142.19 ef ± 5.26 | 510.65 d ± 58.76 | 4.04 b ± 0.53 | 2.90 b ± 0.13 |
| FUS 2A-204 | 104.84 b ± 3.87 | 145.63 c ± 5.43 | 188.80 b ± 3.50 | 805.38 a ± 9.01 | 5.24 a ± 0.70 | 2.86 bc ± 0.63 |
| FUS 2A-103 | 135.04 a ± 3.22 | 195.30 a ± 6.88 | 222.23 a ± 6.45 | 626.84 c ± 48.48 | 2.67 d ± 0.24 | 4.15 a ± 0.53 |
| Mean | 105.76 | 157.82 | 185.03 | 668.97 | 3.53 | 2.94 |
| JUL_E | ||||||
| Plot * | DON | 3- and 15-AcDON | ZEN | ZEN-14S | α-ZOL | β-ZOL |
| FUS 2A-405a | 45.31 hi ± 3.91 | 127.82 d ± 2.49 | 128.90 g ± 2.69 | 520.88 d ± 13.73 | 1.16 f ± 0.38 | 1.29 fgh ± 0.20 |
| FUS 2A-302a | 61.88 g ± 5.42 | 78.89 f ± 2.94 | 94.02 j ± 3.80 | 340.10 f ± 40.91 | 3.36 c ± 0.49 | 2.33 cd ± 0.37 |
| FUS 2A-204a | 49.14 hi ± 4.03 | 106.04 e ± 5.93 | 118.23 h ± 3.07 | 381.46 ef ± 32.81 | 4.35 b ± 0.19 | 1.48 efg ± 0.43 |
| FUS 2A-103a | 94.68 c ± 3.59 | 154.44 bc ± 5.59 | 160.01 d ± 2.46 | 609.29 c ± 12.52 | 1.64 f ± 0.26 | 3.28 b ± 0.33 |
| Mean | 62.75 | 116.80 | 125.29 | 462.93 | 2.62 | 2.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uwineza, P.A.; Kwiatkowska, M.; Gwiazdowski, R.; Stępień, Ł.; Bryła, M.; Waśkiewicz, A. Field Assessment of Lamium album in Reducing Mycotoxin Biosynthesis in Winter Wheat Infected by Fusarium culmorum. Agriculture 2024, 14, 647. https://doi.org/10.3390/agriculture14050647
Uwineza PA, Kwiatkowska M, Gwiazdowski R, Stępień Ł, Bryła M, Waśkiewicz A. Field Assessment of Lamium album in Reducing Mycotoxin Biosynthesis in Winter Wheat Infected by Fusarium culmorum. Agriculture. 2024; 14(5):647. https://doi.org/10.3390/agriculture14050647
Chicago/Turabian StyleUwineza, Pascaline Aimee, Maria Kwiatkowska, Romuald Gwiazdowski, Łukasz Stępień, Marcin Bryła, and Agnieszka Waśkiewicz. 2024. "Field Assessment of Lamium album in Reducing Mycotoxin Biosynthesis in Winter Wheat Infected by Fusarium culmorum" Agriculture 14, no. 5: 647. https://doi.org/10.3390/agriculture14050647








