Structural Features and Healthy Properties of Polysaccharides Occurring in Mushrooms
Abstract
:1. Introduction
2. Extraction of Polysaccharides
2.1. Conventional Extraction
2.2. Pressurized Water Extraction
2.3. Supercritical Fluid Extraction
2.4. Ultrasound-Assisted Extraction
2.5. Microwave-Assisted Extraction
3. Purification
4. Available Techniques for Structural Elucidation
4.1. Chain Composition of Monosaccharides
4.2. Presence and Position of Branches
4.3. Configuration of the Glycosidic Bond
4.4. Conformational Arrangement
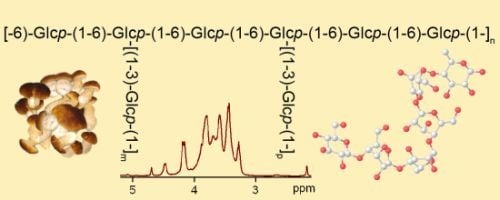
5. Structural Features of Polysaccharides Occurring in Mushrooms
| Mushroom | Type of polysaccharide | Structural features | Ref. |
|---|---|---|---|
| Agaricus bisporus | Heteropolysaccharide | Mucilage composed of glucose and galactose | [32] |
| Agaricus bisporus | Heteropolysaccharide | Xylomannan | [32] |
| Agaricus bitorquis | Homopolysaccharide | β-(1→3)-linked glucan | [58] |
| Agaricus blazei | Glucan-Protein complex | αβ-glucan | [59] |
| Auricularia auricula-judae | Homopolysaccharide | β-(1→3)- D-glucan with branches at (1→6) | [57] |
| Boletus erythropus | Homopolysaccharide | (1→3)-linked glucose with branches at O-6 | [73] |
| Calocybe indica | Homopolysaccharide | αβ-(1→4),(1→6)-glucan | [70] |
| Calocybe indica | Homopolysaccharide | β-(1→3),(1→4)-glucan | [71] |
| Calocybe indica | Heteropolysaccharide | α-(1→3)-linked galactose, with β-(1→4),(1→6)-glucose and fucose branches | [72] |
| Flammulina velutipes | Heteropolysaccharide | 6- O-galactopyranoses substituted at O-2 by 3-O-D-mannopyranosyl-L-fucopyranosyl, α-D-mannopyranosyl, and α-L-fucopyranosyl | [47] |
| Ganoderma atrum | Protein-glucan complex | β-linked mannose, galactose and glucose | [56] |
| Ganoderma lucidum | Homopolysaccharide | β-(1→3)-linked D-glucan | [53] |
| Ganoderma lucidum | Homopolysaccharide | (1→6)-glucan with (1→4) branches at O-4 | [54] |
| Ganoderma lucidum | Heterolpolysaccharide | α-(1→4)-D-glucopyranosyl and β-(1→6)-D-galactopyranosyl with branches at O-6 of glucose and O-2 of galactose | [55] |
| Geastrum saccatum | Glucan-Protein complex | β-linked glucan | [46] |
| Hericium erinaceus | Homopolysaccharide | β-(1→3)-D-glucan with branches at O-6 | [51] |
| Hericium erinaceus | Heterolpolysaccharide | α-(1→6)-D-galactopyranosyl with rhamnose and glucose branches at O-2 | [52] |
| Lentinus edodes | Homopolysaccharide | (1→3),(1→6)-D-polysaccharide | [60] |
| Lentinus edodes | Heteropolysaccharide | Fucomannogalactan of (1→6)-linked α-D-galactopyranoses branched at O-2 | [61] |
| Phellinus linteus | Homopolysaccharide | β-(1→3)-linked D-glucan | [25] |
| Pleurotus eryngii | Homopolysaccharide | α-(1→3)-linked D-glucan | [64] |
| Pleurotus florida | Homopolysaccharide | α-(1→3)-D-glucan branched at O-3 and O-6 by β-D-glucose | [66] |
| Pleurotus florida | Homopolysaccharide | β-(1→3),(1→6)-D-glucan | [67] |
| Pleurotus ostreatus | Homopolysaccharide | (1→3),(1→6)-D-polysaccharide | [63] |
| Pleurotus ostreatus | Homopolysaccharide | α-(1→3)-D-glucan | [64] |
| Schizophyllum commune | Homopolysaccharide | (1→3),(1→6)-D-glucan | [48] |
| Sparasis crispa | Homopolysaccharide | β-(1→3)-D-glucan | [25] |
| Termitomyces eurhizus | Homopolysaccharide | β-(1→3)-D-glucan | [49] |
| Termitomyces microcapus | Homopolysaccharide | β-D-glucan | [50] |
6. Healthy Properties
6.1. Tumor Therapy
6.2. Cardiovascular Disease
6.4. Antimicrobial Activity
6.5. Antioxidant Activity
6.6. Other Effects
7. Conclusions
Acknowledgments
References
- Chiron, N.; Michelot, D. Odeurs des champignons: Chimie et rôle dans les interactions biotiques—Une revue. Cryptogam. Mycol. 2005, 26, 299–364. [Google Scholar]
- Maga, J.A. Mushroom flavor. J. Agric. Food Chem. 1981, 29, 1–4. [Google Scholar] [CrossRef]
- Tsai, S.Y.; Tsai, H.L.; Mau, J.L. Non-volatile taste components of Agaricus blazei, Agrocybe cylindracea and Boletus edulis. Food Chem. 2008, 107, 977–983. [Google Scholar] [CrossRef]
- Beluhan, S.; Ranogajec, A. Chemical composition and non-volatile components of croatian wild edible mushrooms. Food Chem. 2011, 124, 1076–1082. [Google Scholar] [CrossRef]
- Manzi, P.; Aguzzi, A.; Pizzoferrato, L. Nutritional value of mushrooms widely consumed in Italy. Food Chem. 2001, 73, 321–325. [Google Scholar] [CrossRef]
- Manzi, P.; Marconi, S.; Aguzzi, A.; Pizzoferrato, L. Commercial mushrooms: Nutritional quality and effect of cooking. Food Chem. 2004, 84, 201–206. [Google Scholar] [CrossRef]
- Chang, S.T.; Wasser, S.P. The role of culinary-medicinal mushrooms on human welfare with a pyramid model for human health. Int. J. Med. Mushrooms 2012, 14, 95–134. [Google Scholar] [CrossRef]
- Bohn, J.A.; BeMiller, J.N. (1→3)-β-D-glucans as biological response modifiers: A review of structure-functional activity relationships. Carbohydr. Polym. 1995, 28, 3–14. [Google Scholar] [CrossRef]
- Da Silva, M.D.C.; Fukuda, E.K.; Vasconcelos, A.F.D.; Dekker, R.F.H.; Matias, A.C.; Monteiro, N.K.; Cardoso, M.S.; Barbosa, A.M.; Silveira, J.L.M.; Sassaki, G.L.; et al. Structural characterization of the cell wall D-glucans isolated from the mycelium of Botryosphaeria rhodina MAMB-05. Carbohydr. Res. 2008, 343, 793–798. [Google Scholar]
- Muller, A.; Ensley, H.; Pretus, H.; McNamee, R.; Jones, E.; McLaughlin, E.; Chandley, W.; Browder, W.; Lowman, D.; Williams, D. The application of various protic acids in the extraction of (1→3)-β-D-glucan from Saccharomyces cerevisiae. Carbohydr. Res. 1997, 299, 203–208. [Google Scholar] [CrossRef]
- Lo, T.C.T.; Tsao, H.H.; Wang, A.Y.; Chang, C.A. Pressurized water extraction of polysaccharides as secondary metabolites from Lentinula edodes. J. Agric. Food Chem. 2007, 55, 4196–4201. [Google Scholar] [CrossRef]
- Santoyo, S.; Plaza, M.; Jaime, L.; Ibanez, E.; Reglero, G.; Senorans, F.J. Pressurized liquid extraction as an alternative process to obtain antiviral agents from the edible microalga Chlorella vulgaris. J. Agric. Food Chem. 2010, 58, 8522–8527. [Google Scholar]
- Pereira, C.G.; Meireles, M.A.A. Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food Bioprocess Technol. 2010, 3, 340–372. [Google Scholar] [CrossRef]
- Fu, Y.-J.; Liu, W.; Zu, Y.-G.; Shi, X.-G.; Liu, Z.-G.; Schwarz, G.; Efferth, T. Breaking the spores of the fungus Ganoderma lucidum by supercritical CO2. Food Chem. 2009, 112, 71–76. [Google Scholar] [CrossRef]
- Riera, E.; Blanco, A.; Garcia, J.; Benedito, J.; Mulet, A.; Gallego-Juarez, J.A.; Blasco, M. High-power ultrasonic system for the enhancement of mass transfer in supercritical CO2 extraction processes. Ultrasonics 2010, 50, 306–309. [Google Scholar] [CrossRef]
- Ma, J.; Qiao, Z.; Xiang, X. Optimisation of extraction procedure for black fungus polysaccharides and effect of the polysaccharides on blood lipid and myocardium antioxidant enzymes activities. Carbohydr. Polym. 2011, 84, 1061–1068. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, H.; Xu, Z.; Zheng, B.; Lin, Y.; Gan, C.; Lo, Y.M. Ultrasonic-assisted extraction and antioxidant activity of polysaccharides recovered from white button mushroom (Agaricus bisporus). Carbohydr. Polym. 2012, 88, 522–529. [Google Scholar] [CrossRef]
- Zhang, Z.; Lv, G.; Pan, H.; Shi, L.; Fan, L. Optimization of the microwave-assisted extraction process for polysaccharides in himematsutake (Agaricus blazei Murrill) and evaluation of their antioxidant activities. Food Sci. Technol. Res. 2011, 17, 461–470. [Google Scholar] [CrossRef]
- Song, J.-F.; Li, D.-J.; Liu, C.-Q. Response surface analysis of microwave-assisted extraction of polysaccharides from cultured Cordyceps militaris. J. Chem. Technol. Biotechnol. 2009, 84, 1669–1673. [Google Scholar] [CrossRef]
- Ru, Q.M.; Zhang, L.R.; Chen, J.D.; Pei, Z.M.; Zheng, H.L. Microwave-assisted extraction and identification of polysaccharide from Lycoris aurea. Chem. Nat. Compd. 2009, 45, 474–477. [Google Scholar] [CrossRef]
- Dong, J.-Z.; Wang, Z.-C.; Wang, Y. Rapid extraction of polysaccharides from fruits of Lycium barbarum L. J. Food Biochem. 2011, 35, 1047–1057. [Google Scholar] [CrossRef]
- Huang, S.-Q.; Ning, Z.-X. Extraction of polysaccharide from Ganoderma lucidum and its immune enhancement activity. Int. J. Biol. Macromol. 2010, 47, 336–341. [Google Scholar] [CrossRef]
- Chen, Y.; Gu, X.; Huang, S.-Q.; Li, J.; Wang, X.; Tang, J. Optimization of ultrasonic/microwave assisted extraction (UMAE) of polysaccharides from Inonotus obliquus and evaluation of its anti-tumor activities. Int. J. Biol. Macromol. 2010, 46, 429–435. [Google Scholar] [CrossRef]
- Palacios, I.; Lozano, M.; Moro, C.; D’Arrigo, M.; Rostagno, M.A.; Martínez, J.A.; García-Lafuente, A.; Guillamón, E.; Villares, A. Antioxidant properties of phenolic compounds occurring in edible mushrooms. Food Chem. 2011, 128, 674–678. [Google Scholar]
- Park, H.G.; Shim, Y.Y.; Choi, S.O.; Park, W.M. New method development for nanoparticle extraction of water-soluble β-(1→3)-D-glucan from edible mushrooms, Sparassis crispa and Phellinus linteus. J. Agric. Food Chem. 2009, 57, 2147–2154. [Google Scholar] [CrossRef]
- Ragaee, S.M.; Wood, P.J.; Wang, Q.; Tosh, S.M.; Brummer, Y.; Huang, X. Isolation, fractionation, and structural characteristics of alkali-extractable beta-glucan from rye whole meal. Cereal Chem. 2008, 85, 289–294. [Google Scholar] [CrossRef]
- Li, W.; Cui, S.W.; Kakuda, Y. Extraction, fractionation, structural and physical characterization of wheat β-D-glucans. Carbohydr. Polym. 2006, 63, 408–416. [Google Scholar] [CrossRef]
- Bao, X.F.; Fang, J.N.; Li, X.Y. Structural characterization and immunomodulating activity of a complex glucan from spores of Ganoderma lucidum. Biosci. Biotechnol. Biochem. 2001, 65, 2384–2391. [Google Scholar] [CrossRef]
- Ye, L.B.; Zhang, J.S.; Yang, Y.; Zhou, S.A.; Liu, Y.F.; Tang, Q.L.; Du, X.J.; Chen, H.; Pan, Y.J. Structural characterisation of a heteropolysaccharide by NMR spectra. Food Chem. 2009, 112, 962–966. [Google Scholar] [CrossRef]
- Johansson, L.; Virkki, L.; Anttila, H.; Esselstrom, H.; Tuomainen, P.; Sontag-Strohm, T. Hydrolysis of beta-glucan. Food Chem. 2006, 97, 71–79. [Google Scholar] [CrossRef]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A simple and rapid preparation of alditol acetates for monosaccharide analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Bernardo, D.; Mendoza, C.G.; Calonje, M.; Novaes-Ledieu, M. Chemical analysis of the lamella walls of Agaricus bisporus fruit bodies. Curr. Microbiol. 1999, 38, 364–367. [Google Scholar] [CrossRef]
- Ciucanu, I. Per-O-methylation reaction for structural analysis of carbohydrates by mass spectrometry. Anal. Chim. Acta 2006, 576, 147–155. [Google Scholar] [CrossRef]
- Agrawal, P.K. NMR spectroscopy in the structural elucidation of oligosaccharides and glycosides. Phytochemistry 1992, 31, 3307–3330. [Google Scholar] [CrossRef]
- Laws, A.P.; Chadha, M.J.; Chacon-Romero, M.; Marshall, V.M.; Maqsood, M. Determination of the structure and molecular weights of the exopolysaccharide produced by Lactobacillus acidophilus 5e2 when grown on different carbon feeds. Carbohydr. Res. 2008, 343, 301–307. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, H.B.; Zhang, X.Y.; Chen, Z.X. Purification and identification of a novel heteropolysaccharide RBPS2a with anti-complementary activity from defatted rice bran. Food Chem. 2008, 110, 150–155. [Google Scholar] [CrossRef]
- Calonje, M.; García Mendoza, C.; Perez Cabo, A.; Novaes-Ledieu, M. New contributions to the wall polysaccharide structure of the vegetative mycelium and fruit body cell walls of Agaricus bisporus. Microbiología 1996, 12, 599–606. [Google Scholar]
- Lee, J.S.; Kwon, J.S.; Won, D.P.; Lee, K.E.; Shin, W.C.; Hong, E.K. Study on macrophage activation and structural characteristics of purified polysaccharide from the liquid culture broth of Cordyceps militaris. Carbohydr. Polym. 2010, 82, 982–988. [Google Scholar] [CrossRef]
- Yoshioka, Y.; Uehara, N.; Saito, H. Conformation-dependent change in antitumor-activity of linear and branched (1→3)-beta-D-glucans on the basis of conformational elucidation by C-13 Nuclear Magnetic Resonance spectroscopy. Chem. Pharm. Bull. 1992, 40, 1221–1226. [Google Scholar] [CrossRef]
- Kulicke, W.M.; Lettau, A.I.; Thielking, H. Correlation between immunological activity, molar mass, and molecular structure of different (1→3)-beta-D-glucans. Carbohydr. Res. 1997, 297, 135–143. [Google Scholar] [CrossRef]
- Jelsma, J.; Kreger, D.R. Ultrastructural observations on (1→3)-beta-D-glucan from fungal cell-walls. Carbohydr. Res. 1975, 43, 200–203. [Google Scholar] [CrossRef]
- Sweeley, C.C.; Nunez, H.A. Structural analysis of glycoconjugates by mass spectrometry and nuclear magnetic resonance spectroscopy. Annu. Rev. Biochem. 1985, 54, 765–801. [Google Scholar] [CrossRef]
- Young, S.H.; Jacobs, R.R. Sodium hydroxide-induced conformational change in schizophyllan detected by the fluorescence dye, aniline blue. Carbohydr. Res. 1998, 310, 91–99. [Google Scholar] [CrossRef]
- Ko, Y.T.; Lin, Y.L. 1,3-beta-glucan quantification by a fluorescence microassay and analysis of its distribution in foods. J. Agric. Food Chem. 2004, 52, 3313–3318. [Google Scholar] [CrossRef]
- Ogawa, K.; Dohmaru, T.; Yui, T. Dependence of complex-formation of (1→3)-beta-D-glucan with congo red on temperature in alkaline-solutions. Biosci. Biotechnol. Biochem. 1994, 58, 1870–1872. [Google Scholar] [CrossRef]
- Dore, C.; Azevedo, T.C.G.; de Souza, M.C.R.; Rego, L.A.; de Dantas, J.C.M.; Silva, F.R.F.; Rocha, H.A.O.; Basela, I.G.; Leite, E.L. Antiinflammatory, antioxidant and cytotoxic actions of beta-glucan-rich extract from Geastrum saecatum mushroom. Int. Immunopharmacol. 2007, 7, 1160–1169. [Google Scholar] [CrossRef]
- Smiderle, F.R.; Carbonero, E.R.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Characterization of a heterogalactan: Some nutritional values of the edible mushroom Flammulina velutipes. Food Chem. 2008, 108, 329–333. [Google Scholar] [CrossRef]
- Numata, M.; Tamesue, S.; Fujisawa, T.; Haraguchi, S.; Hasegawa, T.; Bae, A.H.; Li, C.; Sakurai, K.; Shinkai, S. Beta-1,3-glucan polysaccharide (schizophyllan) acting as a one-dimensional host for creating supramolecular dye assemblies. Org. Lett. 2006, 8, 5533–5536. [Google Scholar] [CrossRef]
- Chakraborty, I.; Mondal, S.; Rout, D.; Islam, S.S. A water-insoluble (1→3)-beta-D-glucan from the alkaline extract of an edible mushroom Termitomyces eurhizus. Carbohydr. Res. 2006, 341, 2990–2993. [Google Scholar] [CrossRef]
- Chandra, K.; Ghosh, K.; Roy, S.K.; Mondal, S.; Maiti, D.; Ojha, A.K.; Das, D.; Islam, S.S. A water-soluble glucan isolated from an edible mushroom Termitomyces microcapus. Carbohydr. Res. 2007, 342, 2484–2489. [Google Scholar] [CrossRef]
- Dong, Q.; Jia, L.M.; Fang, J.N. A beta-D-glucan isolated from the fruiting bodies of Hericium erinaceus and its aqueous conformation. Carbohydr. Res. 2006, 341, 791–795. [Google Scholar] [CrossRef]
- Jia, L.M.; Liu, L.; Dong, Q.; Fang, J.N. Structural investigation of a novel rhamnoglucocalactan isolated from the fruiting bodies of the fungus Hericium erinaceus. Carbohydr. Res. 2004, 339, 2667–2671. [Google Scholar] [CrossRef]
- Wang, J.G.; Zhang, L. Structure and chain conformation of five water-soluble derivatives of a beta-D-glucan isolated from Ganoderma lucidum. Carbohydr. Res. 2009, 344, 105–112. [Google Scholar] [CrossRef]
- Dong, Q.; Wang, Y.; Shi, L.; Yao, J.; Li, J.; Ma, F.; Ding, K. A novel water-soluble beta-D-glucan isolated from the spores of Ganoderma lucidum. Carbohydr. Res. 2012, 353, 100–105. [Google Scholar] [CrossRef]
- Bao, X.F.; Wang, X.S.; Dong, Q.; Fang, J.N.; Li, X.Y. Structural features of immunologically active polysaccharides from Ganoderma lucidum. Phytochemistry 2002, 59, 175–181. [Google Scholar]
- Chen, Y.; Xie, M.Y.; Nie, S.P.; Li, C.; Wang, Y.X. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008, 107, 231–241. [Google Scholar] [CrossRef]
- Xu, S.; Xu, X.; Zhang, L. Branching structure and chain conformation of water-soluble glucan extracted from Auricularia auricula-judae. J. Agric. Food Chem. 2012, 60, 3498–3506. [Google Scholar] [CrossRef]
- Nandan, C.K.; Patra, P.; Bhanja, S.K.; Adhikari, B.; Sarkar, R.; Mandal, S.; Islam, S.S. Structural characterization of a water-soluble beta-(1→6)-linked D-glucan isolated from the hot water extract of an edible mushroom, Agaricus bitorquis. Carbohydr. Res. 2008, 343, 3120–3122. [Google Scholar] [CrossRef]
- Gonzaga, M.L.C.; Ricardo, N.; Heatley, F.; Soares, S.D. Isolation and characterization of polysaccharides from Agaricus blazei Murill. Carbohydr. Polym. 2005, 60, 43–49. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Li, S.; Wang, X.H.; Zhang, L.N.; Cheung, P.C.K. Advances in lentinan: Isolation, structure, chain conformation and bioactivities. Food Hydrocoll. 2011, 25, 196–206. [Google Scholar] [CrossRef]
- Carbonero, E.R.; Gracher, A.H.P.; Komura, D.L.; Marcon, R.; Freitas, C.S.; Baggio, C.H.; Santos, A.R.S.; Torri, G.; Gorin, P.A.J.; Iacomini, M. Lentinus edodes heterogalactan: Antinociceptive and anti-inflammatory effects. Food Chem. 2008, 111, 531–537. [Google Scholar] [CrossRef]
- Palacios, I.; Guillamón, E.; García-Lafuente, A.; Villares, A. Structural characterization of water-soluble polysaccharides from the fruiting bodies of Lentinus edodes mushrooms. Curr. Nutr. Food Sci. 2012, 8, 234–241. [Google Scholar]
- Karacsonyi, S.; Kuniak, L. Polysaccharides of Pleurotus ostreatus—Isolation and structure of pleuran, an alkali-insoluble β-D-glucan. Carbohydr. Polym. 1994, 24, 107–111. [Google Scholar] [CrossRef]
- Synytsya, A.; Mickova, K.; Jablonsky, I.; Spevacek, J.; Erban, V.; Kovarikova, E.; Copikova, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohydr. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Palacios, I.; García-Lafuente, A.; Guillamón, E.; Villares, A. Novel isolation of water-soluble polysaccharides from the fruiting bodies of Pleurotus ostreatus mushrooms. Carbohydr. Res. 2012, 358, 72–77. [Google Scholar] [CrossRef]
- Santos-Neves, J.C.; Pereira, M.I.; Carbonero, E.R.; Gracher, A.H.P.; Alquini, G.; Gorin, P.A.J.; Sassaki, G.L.; Iacomini, M. A novel branched alpha beta-glucan isolated from the basidiocarps of the edible mushroom Pleurotus florida. Carbohydr. Polym. 2008, 73, 309–314. [Google Scholar] [CrossRef]
- Rout, D.; Mondal, S.; Chakraborty, I.; Islam, S.S. The structure and conformation of a water-insoluble (1→3),(1→6)-beta-D-glucan from the fruiting bodies of Pleurotus florida. Carbohydr. Res. 2008, 343, 982–987. [Google Scholar] [CrossRef]
- Carbonero, E.R.; Gracher, A.H.P.; Rosa, M.C.C.; Torri, G.; Sassaki, G.L.; Gorin, P.A.J.; Iacomini, M. Unusual partially 3-O-methylated alpha-galactan from mushrooms of the genus Pleurotus. Phytochemistry 2008, 69, 252–257. [Google Scholar] [CrossRef]
- Rosado, F.R.; Carbonero, E.R.; Kemmelmeier, C.; Tischer, C.A.; Gorin, P.A.J.; Iacomini, M. A partially 3-O-methylated (1→4)-linked α-D-galactan and α-D-mannan from Pleurotus ostreatoroseus Sing. FEMS Microbiol. Lett. 2002, 212, 261–265. [Google Scholar]
- Mandal, E.K.; Maity, K.; Maity, S.; Gantait, S.K.; Behera, B.; Maiti, T.K.; Sikdar, S.R.; Islam, S.S. Chemical analysis of an immunostimulating (1→4)-, (1→6)-branched glucan from an edible mushroom, Calocybe indica. Carbohydr. Res. 2012, 347, 172–177. [Google Scholar] [CrossRef]
- Mandal, S.; Maity, K.K.; Bhunia, S.K.; Dey, B.; Patra, S.; Sikdar, S.R.; Islam, S.S. Chemical analysis of new water-soluble (1→6)-, (1→4)-α, β-glucan and water-insoluble (1→3)-, (1→4)-β-glucan (calocyban) from alkaline extract of an edible mushroom, Calocybe indica (Dudh Chattu). Carbohydr. Res. 2010, 345, 2657–2663. [Google Scholar] [CrossRef]
- Mandal, E.K.; Maity, K.; Maity, S.; Gantait, S.K.; Maiti, S.; Maiti, T.K.; Sikdar, S.R.; Islam, S.S. Structural characterization of an immunoenhancing cytotoxic heteroglycan isolated from an edible mushroom Calocybe indica var. APK2. Carbohydr. Res. 2011, 346, 2237–2243. [Google Scholar] [CrossRef]
- Chauveau, C.; Talaga, P.; Wieruszeski, J.M.; Strecker, G.; Chavant, L. A water-soluble β-D-glucan from Boletus erythropus. Phytochemistry 1996, 43, 413–415. [Google Scholar] [CrossRef]
- Yue, L.; Cui, H.; Li, C.; Lin, Y.; Sun, Y.; Niu, Y.; Wen, X.; Liu, J. A polysaccharide from Agaricus blazei attenuates tumor cell adhesion via inhibiting E-selectin expression. Carbohydr. Polym. 2012, 88, 1326–1333. [Google Scholar] [CrossRef]
- Hsieh, T.-C.; Wu, J.M. Suppression of proliferation and oxidative stress by extracts of Ganoderma lucidum in the ovarian cancer cell line OVCAR-3. Int. J. Mol. Med. 2011, 28, 1065–1069. [Google Scholar]
- Lee, K.H.; Cho, C.H.; Rhee, K.-H. Synergic anti-tumor activity of gamma-irradiated exo-polysaccharide from submerged culture of Grifola frondosa. J. Med. Plants Res. 2011, 5, 2378–2386. [Google Scholar]
- Selvi, S.; Umadevi, P.; Murugan, S.; Senapathy, G.J. Anticancer potential evoked by Pleurotus florida and Calocybe indica using T24 urinary bladder cancer cell line. Afr. J. Biotechnol. 2011, 10, 7279–7285. [Google Scholar]
- Zhang, L.; Fan, C.; Liu, S.; Zang, Z.; Jiao, L.; Zhang, L. Chemical composition and antitumor activity of polysaccharide from Inonotus obliquus. J. Med. Plants Res. 2011, 5, 1251–1260. [Google Scholar]
- Lo, T.C.-T.; Hsu, F.-M.; Chang, C.A.; Cheng, J.C.-H. Branched alpha-(1,4) glucans from Lentinula edodes (L10) in combination with radiation enhance cytotoxic effect on human lung adenocarcinoma through the toll-like receptor 4 mediated induction of THP-1 differentiation/activation. J. Agric. Food Chem. 2011, 59, 11997–12005. [Google Scholar] [CrossRef]
- Wang, G.; Dong, L.; Zhang, Y.; Ji, Y.; Xiang, W.; Zhao, M. Polysaccharides from Phellinus linteus inhibit cell growth and invasion and induce apoptosis in HepG2 human hepatocellular carcinoma cells. Biologia 2012, 67, 247–254. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, H.; Ng, T.B. Isolation and purification of polysaccharides with anti-tumor activity from Pholiota adiposa (Batsch) P. Kumm. (higher Basidiomycetes). Int. J. Med. Mushrooms 2012, 14, 271–284. [Google Scholar] [CrossRef]
- Chihara, G.; Hamuro, J.; Maeda, Y.Y.; Shiio, T.; Suga, T.; Takasuka, N.; Sasaki, T. Antitumor and metastasis-inhibitory activities of lentinan as an immunomodulator: An overview. Cancer Detect. Prev. 1987, 1, 423–443. [Google Scholar]
- Ochiai, T.; Isono, K.; Suzuki, T.; Koide, Y.; Gunji, Y.; Nagata, M.; Ogawa, N. Effect of immunotherapy with lentinan on patients survival and immunological parameters in patients with advanced gastric-cancer—Results of a multicenter randomized controlled-study. Int. J. Immunother. 1992, 8, 161–169. [Google Scholar]
- Taguchi, T.; Abe, O.; Enomoto, K.; Kusama, S.; Tomiyama, J.; Tominaga, K.; Ogawa, N. Life-span prolongation effect of lentinan on patients with advanced or recurrent breast-cancer. Int. J. Immunopharmacol. 1982, 4, 271–271. [Google Scholar]
- Kosaka, A.; Suga, T.; Yamashita, A. Dose reductive effect of lentinan on the epirubicin therapy for breast cancer patients. Int. J. Immunother. 1995, 11, 143–151. [Google Scholar]
- Hamuro, J.; Takatsuki, F.; Suga, T.; Kikuchi, T.; Suzuki, M. Synergistic antimetastatic effects of lentinan and interleukin-2 with preoperative and postoperative treatments. Jpn. J. Cancer Res. 1994, 85, 1288–1297. [Google Scholar] [CrossRef]
- Shimizu, C.; Kihara, M.; Aoe, S.; Araki, S.; Ito, K.; Hayashi, K.; Watari, J.; Sakata, Y.; Ikegami, S. Effect of high beta-glucan barley on serum cholesterol concentrations and visceral fat area in japanese men—A randomized, double-blinded, placebo-controlled trial. Plant Food Hum. Nutr. 2008, 63, 21–25. [Google Scholar] [CrossRef]
- Naumann, E.; van Rees, A.B.; Onning, G.; Oste, R.; Wydra, M.; Mensink, R.P. Beta-glucan incorporated into a fruit drink effectively lowers serum LDL-cholesterol concentrations. Am. J. Clin. Nutr. 2006, 83, 601–605. [Google Scholar]
- Drozdowski, L.A.; Reimer, R.A.; Temelli, F.; Bell, R.C.; Vasanthan, T.; Thomson, A.B.R. β-glucan extracts inhibit the in vitro intestinal uptake of long-chain fatty acids and cholesterol and down-regulate genes involved in lipogenesis and lipid transport in rats. J. Nutr. Biochem. 2010, 21, 695–701. [Google Scholar] [CrossRef]
- Gordon, M.; Guralnik, M.; Kaneko, Y.; Mimura, T.; Goodgame, J.; DeMarzo, C.; Pierce, D.; Baker, M.; Lang, W. A phase II controlled study of a combination of the immune modulator, lentinan, with didanosine (ddi) in HIV patients with CD4 cells of 200–500/mm3. J. Med. 1995, 26, 193–207. [Google Scholar]
- Drandarska, I.; Kussovski, V.; Nikolaeva, S.; Markova, N. Combined immunomodulating effects of BCG and lentinan after intranasal application in guinea pigs. Int. Immunopharmacol. 2005, 5, 795–803. [Google Scholar] [CrossRef]
- Asatiani, M.D.; Elisashvili, V.I.; Wasser, S.P.; Reznick, A.Z.; Nevo, E. Free-radical scavenging activity of submerged mycelium extracts from higher basidiomycetes mushrooms. Biosci. Biotechnol. Biochem. 2007, 71, 3090–3092. [Google Scholar] [CrossRef]
- Asatiani, M.D.; Elisashvili, V.; Wasser, S.P.; Reznick, A.Z.; Nevo, E. Antioxidant activity of submerged cultured mycelium extracts of higher basidiomycetes mushrooms. Int. J. Med. Mushrooms 2007, 9, 151–158. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic, polysaccharidic, and lipidic fractions of mushrooms from northeastern Portugal: Chemical compounds with antioxidant properties. J. Agric. Food Chem. 2012, 60, 4634–4640. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Chen, Y.; Hu, M.; Ding, J.; Xu, C.; Wang, R. In vitro antioxidant activities of the polysaccharides from Tricholoma lobayense. Int. J. Biol. Macromol. 2012, 50, 534–539. [Google Scholar] [CrossRef]
- Kao, P.-F.; Wang, S.-H.; Hung, W.-T.; Liao, Y.-H.; Lin, C.-M.; Yang, W.-B. Structural characterization and antioxidative activity of low-molecular-weights β-1,3-glucan from the residue of extracted Ganoderma lucidum fruiting bodies. J. Biomed. Biotechnol. 2012. [Google Scholar] [CrossRef]
- Li, N.; Li, L.; Fang, J.C.; Wong, J.H.; Ng, T.B.; Jiang, Y.; Wang, C.R.; Zhang, N.Y.; Wen, T.Y.; Qu, L.Y.; et al. Isolation and identification of a novel polysaccharide-peptide complex with antioxidant, anti-proliferative and hypoglycaemic activities from the abalone mushroom. Biosci. Rep. 2012, 32, 221–228. [Google Scholar] [CrossRef]
- Liatis, S.; Tsapogas, P.; Chala, E.; Dimosthenopoulos, C.; Kyriakopoulos, K.; Kapantais, E.; Katsilambros, N. The consumption of bread enriched with betaglucan reduces LDL-cholesterol and improves insulin resistance in patients with type 2 diabetes. Diabetes Metab. 2009, 35, 115–120. [Google Scholar] [CrossRef]
- Barone Lumaga, R.; Azzali, D.; Fogliano, V.; Scalfi, L.; Vitaglione, P. Sugar and dietary fibre composition influence, by different hormonal response, the satiating capacity of a fruit-based and a β-glucan-enriched beverage. Food Funct. 2012, 3, 67–75. [Google Scholar] [CrossRef]
- Ye, M.; Chen, W.-X.; Qiu, T.; Yuan, R.-Y.; Ye, Y.-W.; Cai, J.-M. Structural characterisation and anti-ageing activity of extracellular polysaccharide from a strain of Lachnum sp. Food Chem. 2012, 132, 338–343. [Google Scholar] [CrossRef]
- Velebny, S.; Hrckova, G.; Kogan, G. Impact of treatment with praziquantel, silymarin and/or β-glucan on pathophysiological markers of liver damage and fibrosis in mice infected with Mesocestoides vogae (Cestoda) tetrathyridia. J. Helminthol. 2008, 82, 211–219. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Villares, A.; Mateo-Vivaracho, L.; Guillamón, E. Structural Features and Healthy Properties of Polysaccharides Occurring in Mushrooms. Agriculture 2012, 2, 452-471. https://doi.org/10.3390/agriculture2040452
Villares A, Mateo-Vivaracho L, Guillamón E. Structural Features and Healthy Properties of Polysaccharides Occurring in Mushrooms. Agriculture. 2012; 2(4):452-471. https://doi.org/10.3390/agriculture2040452
Chicago/Turabian StyleVillares, Ana, Laura Mateo-Vivaracho, and Eva Guillamón. 2012. "Structural Features and Healthy Properties of Polysaccharides Occurring in Mushrooms" Agriculture 2, no. 4: 452-471. https://doi.org/10.3390/agriculture2040452
APA StyleVillares, A., Mateo-Vivaracho, L., & Guillamón, E. (2012). Structural Features and Healthy Properties of Polysaccharides Occurring in Mushrooms. Agriculture, 2(4), 452-471. https://doi.org/10.3390/agriculture2040452