Advances in Eco-Efficient Agriculture: The Plant-Soil Mycobiome
Abstract
:1. Introduction
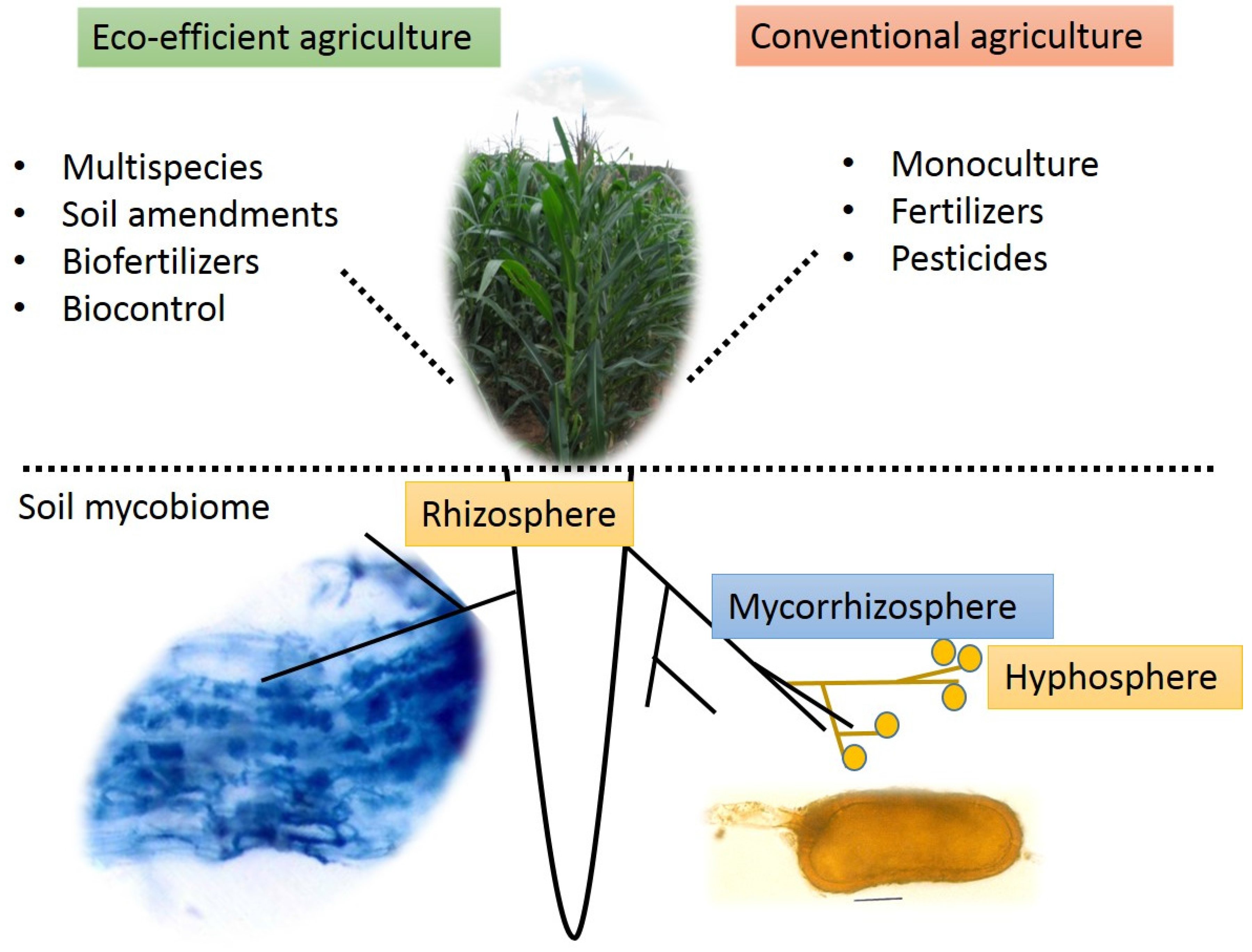
2. The Soil Mycobiome
3. The Plant-Soil Mycobiome
- Alnus (Betulaceae)/Myricaceae/Casuarinaceae group,
- Eleagnaceae/ Rhamnaceae group,
- Frankia symbiotic with actinorhizal plants (Coriariaceae, Datiscaceae, and Rosaceae) and the genus Ceanothus (Rhamnaceae)
4. The Mycobiome in Anthropogenic Soils
5. Latest Advancements in the Mycobiome of Tropical Agro-Ecosystems
6. Redesigning Agro-Ecosystems for Sustainability
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviation
| AM | Arbuscular mycorrhizas |
| AMF | Arbuscular mycorrhizal fungi |
| EMF | Ectomycorrhizal fungi |
| SOC | Soil organic carbon |
References
- Talbot, J.M.; Bruns, T.D.; Taylor, J.W.; Smith, D.P.; Branco, S.; Glassman, S.I.; Erlandson, S.; Vilgalys, R.; Liao, H.L.; Smith, M.E.; et al. Endemism and functional convergence across the North American soil mycobiome. Proc. Natl. Acad. Sci. USA 2014, 111, 6341–6346. [Google Scholar] [CrossRef] [PubMed]
- Azcón, R. Mycorrhizosphere: The role of PGPR. In Root Engineering; Morte, A., Varma, A., Eds.; Springer: Berlin, Germany, 2014; pp. 107–144. [Google Scholar]
- Specht, A.; Guru, S.; Houghton, L.; Keniger, L.; Driver, P.; Ritchie, E.G.; Lai, K.; Treloar, A. Data management challenges in analysis and synthesis in the ecosystem sciences. Sci. Total Environ. 2015, 534, 144–158. [Google Scholar] [CrossRef] [PubMed]
- Balestrini, R.; Lumini, E.; Borriello, R.; Bianciotto, V. Plant-Soil Biota Interactions. In Soil Microbiology, Ecology, and Biochemistry; Paul, E.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 311–329. [Google Scholar]
- Saleem, M.; Moe, L.A. Multitrophic microbial interactions for eco- and agro-biotechnological processes: Theory and practice. Trends Biotechnol. 2014, 32, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Jansa, J.; Gryndler, M. Biotic environment of the arbuscular mycorrhizal fungi in soil. In Arbuscular Mycorrhizas: Physiology and Function; Koltaiand, H., Kapulnik, Y., Eds.; Springer: Heidelberg, Germany, 2010; pp. 209–236. [Google Scholar]
- DeLonge, M.S.; Miles, A.; Car, L. Investing in the transition to sustainable agriculture. Environ. Sci. Policy 2016, 55, 266–273. [Google Scholar] [CrossRef]
- Pagano, M.C.; Dantas, B.D.; Weber, O.B.; Correa, E.A.; Tancredi, F.D.; Duarte, N.F.; Bago, A.; Cabello, M.N. Mycorrhizas in Agroecosystems. In Recent Advances on Mycorrhizal Fungi; Pagano, M.C., Ed.; Springer: Basel, Switzerland, 2016; pp. 91–100. [Google Scholar]
- Dantas, B.L.; Weber, O.B.; Neto, J.P.M.; Rossetti, A.G.; Pagano, M.C. Diversity of arbuscular mycorrhizal fungi in an organic orchard of semi-arid land of Ceará, Brazil. Cienc. Rural 2015, 45, 1480–1486. [Google Scholar] [CrossRef]
- Azevedo, E.J.A.; Duarte, N.F.; Parreira, A.G.; Pagano, M.C. Biodiversidade de fungos micorrizicos na fazenda experimental de Pitangui—Minas Gerais: Potencialidades de aplicação na agricultura e restauração de áreas. In Proceedings of the II Simpósio de Microbiologia da UFMG, Belo Horizonte, Brazil, 5–6 October 2015.
- Buscot, F. TrophinOak—Helmholtz Centre for Environmental Research UFZ in Halle. Available online: www.trophinoak.de (accessed on 23 January 2017).
- Johnson, N.C. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol. 2010, 185, 631–647. [Google Scholar] [CrossRef] [PubMed]
- Reich, P.B.; Hobbie, S.E. Decade-long soil nitrogen constraint on the CO2 fertilization of plant biomass. Nat. Clim. Chang. 2013, 3, 278–282. [Google Scholar] [CrossRef]
- Jansa, J.; Bukovská, P.; Gryndler, M. Mycorrhizal hyphae as ecological niche for highly specialized hypersymbionts—Or just soil free-riders? Front. Plant Sci. 2013, 4, 134. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 3rd ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Barto, E.K.; Hilker, M.; Muller, F.; Mohney, B.K.; Weidenhamer, J.D.; Rillig, M.C. The fungal fast lane: Common mycorrhizal networks extend bioactive zones of allelochemicals in soils. PLoS ONE 2011, 6, e27195. [Google Scholar] [CrossRef] [PubMed]
- Scheublin, T.R.; Sanders, I.R.; Keel, C.; VanDerMeer, J.R. Characterisation of microbial communities colonizing the hyphal surfaces of arbuscular mycorrhizal fungi. ISME J. 2010, 4, 752–763. [Google Scholar] [CrossRef] [PubMed]
- Oakley, B.; North, M.; Franklin, J.F.; Hedlund, B.P.; Staley, J.T. Diversity and Distribution of Frankia Strains Symbiotic with Ceanothus in California. Appl. Environ. Microbiol. 2004, 70, 11. [Google Scholar] [CrossRef] [PubMed]
- Judd, W.S.; Campbell, C.S.; Kellogg, E.A.; Stevens, P.F.; Donoghue, M.J. Plant Systematics: A Phylogenetic Approach; Sinauer Associates: Sunderland, MA, USA, 2009. [Google Scholar]
- Schwintzer, C.R.; Tjepkema, J.D. The Biology of Frankia and Actinorhizal Plants; Academic Press, Inc.: San Diego, CA, USA, 1990. [Google Scholar]
- Stacey, G.; Burris, R.H.; Evans, H.J. Biological Nitrogen Fixation; Chapman and Hall: New York, NY, USA, 1992. [Google Scholar]
- Babur, S.M.; Welsh, A.; Hahn, D. Saprophytic growth of inoculated Frankia sp. in soil microcosms. FEMS Microbiol. Ecol. 2007, 62, 280–289. [Google Scholar]
- Baker, D.D.; Mullin, B.C. Actinorhizal symbioses. In Biological Nitrogen Fixation; Stacey, G., Burris, R.H., Evans, H.J., Eds.; Chapman and Hall: New York, NY, USA, 1992; pp. 259–292. [Google Scholar]
- Wall, L.G. The Actinorhizal Symbiosis. J. Plant Growth Regul. 2000, 19, 167–182. [Google Scholar] [PubMed]
- Clawson, M.L.; Benson, D.R. Natural diversity of Frankia strains in actinorhizal root nodules from promiscuous hosts in the family Myricaceae. Appl. Environ. Microbiol. 1999, 65, 4521–4527. [Google Scholar] [PubMed]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Marra, R.; Woo, S.L.; Lorito, M. Trichoderma–plant–pathogen interactions. Soil Biol. Biochem. 2008, 40, 1–10. [Google Scholar] [CrossRef]
- Kredics, L.; Hatvani, L.; Naeimi, S.; Körmöczi, P.; Manczinger, L.; Vágvölgyi, C.; Druzhinina, I. Biodiversity of the Genus Hypocrea/Trichoderma in Different Habitats. In Biotechnology and Biology of Trichoderma; Gupta, V.G., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–24. [Google Scholar]
- Gupta, V.K.; Schmoll, M.; Herrera-Estrella, A.; Upadhyay, R.S.; Druzhinina, I.; Tuohy, M.G. Biotechnology and Biology of Trichoderma; Gupta, V.G., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Piccoli, I.; Chiarini, F.; Carletti, P.; Furlan, L.; Lazzaro, B.; Nardi, S.; Berti, A.; Sartori, L.; Dalconi, M.C.; Morari, F. Disentangling the effects of conservation agriculture practices on the vertical distribution of soil organic carbon. Evidence of poor carbon sequestration in North-Eastern Italy. Agric. Ecosyst. Environ. 2016, 230, 68–78. [Google Scholar] [CrossRef]
- Unterseher, M.; Siddique, A.B.; Brachmann, A.; Peršoh, D. Diversity and Composition of the Leaf Mycobiome of Beech (Fagus sylvatica) Are Affected by Local Habitat Conditions and Leaf Biochemistry. PLoS ONE 2016, 1, e0152878. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hidalgo, K.; Chin-Pampillo, J.S.; Masís-Mora, M.; Carazo-Rojas, E.; Rodríguez-Rodríguez, C.E. Optimization of a Fungally Bioaugmented Biomixture for Carbofuran Removal in On-Farm Biopurification Systems. Water Air Soil Pollut. 2016. [Google Scholar] [CrossRef]
- Douds, D.D., Jr.; Lee, J.; McKeever, L.; Ziegler-Ulsh, C.; Ganser, S. Utilization of inoculum of AM fungi produced on-farm increases the yield of Solanum lycopersicum: A summary of 7 years of field trials on a conventional vegetable farm with high soil phosphorus. Sci. Hortic. 2016, 207, 89–96. [Google Scholar] [CrossRef]
- Sniegowski, K.; Bers, K.; Ryckeboer, J.; Jaeken, P.; Spanoghe, P.; Springael, D. Robust Linuron Degradation in On-Farm Biopurification Systems Exposed to Sequential Environmental Changes. Appl. Environ. Microbiol. 2011, 77, 6614–6621. [Google Scholar] [CrossRef] [PubMed]
- Martini, M.C.; Albicoro, F.J.; Nour, E.; Schlüter, A.; van Elsas, J.D.; Springael, D.; Smalla, K.; Pistorio, M.; Lagares, A.; Del Papa, M.F. Characterization of a collection of plasmid-containing bacteria isolated from an on-farm biopurification system used for pesticide removal. Plasmid 2015, 80, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Siddique, T.; Saleem, M.; Arshad, M.; Khalid, A. Impact of pesticides on soil microbial diversity, enzymes, and biochemical reactions. Adv. Agron. 2009, 102, 159–200. [Google Scholar]
- Fließbach, A.; Oberholzer, H.-R.; Gunst, L.; Mäder, P. Soil organic matter and biological soil quality indicators after 21 years of organic and conventional farming. Agric. Ecosyst. Environ. 2007, 118, 273–284. [Google Scholar] [CrossRef]
- Araujo, A.S.F.; Monteiro, R.T.R.; Abarkeli, R.B. Effect of glyphosate on the microbial activity of two Brazilian soils. Chemosphere 2003, 52, 799–804. [Google Scholar] [CrossRef]
- Imfeld, G.; Vuilleumier, S. Measuring the effects of pesticides on bacterial communities in soil: A critical review. Eur. J. Soil Biol. 2012, 49, 22–30. [Google Scholar] [CrossRef]
- Altieri, M.A. Agroecological foundations of alternative agriculture in California. Agric. Ecosyst. Environ. 1992, 39, 23–53. [Google Scholar] [CrossRef]
- Viti, C.; Tatti, E.; Decorosi, F.; Lista, E.; Rea, E.; Tullio, M.; Sparvoli, E.; Giovannetti, L. Compost Effect on Plant Growth-Promoting Rhizobacteria and Mycorrhizal Fungi Population in Maize Cultivations. Compost Sci. Util. 2010, 4, 273–281. [Google Scholar] [CrossRef]
- Pagano, M.C.; Covacevich, F. Arbuscular Mycorrhizas in Agroecosystems. In Mycorrhizal Fungi: Soil, Agriculture and Environmental Implications; Fulton, S.M., Ed.; Nova Science Publishers: New York, NY, USA, 2011; pp. 35–65. [Google Scholar]
- Pagano, M.C.; Jorio, A. The contributions of mycorrhizal fungi. In Microbial Bioresources; Gupta, V.K., Sharma, G.D., Tuohy, M.G., Gaur, R., Eds.; CABI: London, UK, 2016; pp. 14–28. [Google Scholar]
- Kumar, V. Use of Integrated Nutrient Management to Enhance Soil Fertility and Crop Yield of Hybrid Cultivar of Brinjal (Solanum melongena L.) Under Field Conditions. Adv. Plants Agric. Res. 2016, 4, 00130. [Google Scholar] [CrossRef]
- Prieto, I.; Roldán, A.; Huygens, D.; Alguacil, M.M.; Navarro-Cano, J.A.; Querejeta, J.I. Species-specific roles of ectomycorrhizal fungi in facilitating interplant transfer of hydraulically redistributed water between Pinus halepensis saplings and seedlings. Plant Soil 2016, 406, 15–27. [Google Scholar] [CrossRef]
- Powlson, D.S.; Stirling, C.M.; Thierfelder, C.; White, R.P.; Jate, M.L. Does conservation agriculture deliver climate change mitigation through soil carbon sequestration in tropical agro-ecosystems? Agric. Ecosyst. Environ. 2016, 220, 164–174. [Google Scholar] [CrossRef]
- Amézquita, M.C.; Ibrahim, M.; Llanderal, T.; Buurman, P.; Amézquita, E. Carbon Sequestration in Pastures, Silvo-Pastoral Systems and Forests in Four Regions of the Latin American Tropics. J. Sustain. For. 2004, 21, 31–49. [Google Scholar] [CrossRef]
- Pagano, M.C.; Cabello, M.N. Mycorrhizal Interactions for Reforestation: Constraints to Dryland Agroforest in Brazil. ISRN Ecol. 2011, 2011, 890850. [Google Scholar] [CrossRef]
- Resende, A.S.; Xavier, R.P.; Quesada, D.M.; Urquiaga, S.; Alves, B.J.R.; Boddey, R.M. Use of green manures in increase inputs of biological nitrogen fixation to sugar cane. Biol. Fert. Soils 2003, 37, 215–220. [Google Scholar]
- Jank, L.; Barrios, S.C.; do Valle, C.B.; Simeão, R.M.; Alves, G.F. The value of improved pastures to Brazilian beef production. Crop Pasture Sci. 2014, 65, 1132–1137. [Google Scholar] [CrossRef]
- Alvarenga, R.C.; Noce, M.A. Integração Lavoura-Pecuária; Embrapa Milho e Sorgo: Sete Lagoas, Brazil, 2005; p. 16. [Google Scholar]
- Lemaire, G.; Franzluebbers, A.; Carvalho, P.C.F.; Dedieu, B. Integrated crop–livestock systems: Strategies to achieve synergy between agricultural production and environmental quality. Agric. Ecosyst. Environ. 2014, 190, 4–8. [Google Scholar] [CrossRef]
- Nelson, H.W.; Williamson, T.B.; Macaulay, C.; Mahony, C. Assessing the potential for forest management practitioner participation in climate change adaptation. For. Ecol. Manag. 2016, 360, 388–399. [Google Scholar] [CrossRef]
- Federici, S.; Tubiello, F.N.; Salvatore, M.; Jacobs, H.; Schmidhuber, J. New estimates of CO2 forest emissions and removals: 1990–2015. For. Ecol. Manag. 2015, 352, 89–98. [Google Scholar] [CrossRef]
- Scherr, S.J.; Sthapit, S. Mitigating climate change through food and land use. Worldwatch Pap. 2009, 179, 1–49. [Google Scholar]
- Ferreira, C.C.; Silva, H.D. Formação de Povoamentos Florestais; Embrapa Florestas: Colombo, Sri Lanka, 2008; p. 109. [Google Scholar]
- Melotto, A.M.; Laura, V.A. Sistemas Silvipastoris para Bovinos e Ovinos; Embrapa Gado de Corte: Campo Grande, Brazil, 2009; p. 36. [Google Scholar]
- IPCC. Climate Change 2001: The Science of Climate Change; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Loaiciga, H.A.; Valdes, J.B.; Vogel, R.; Garvey, J.; Schwarz, H. Global warming and the hydrologic cycle. J. Hydrol. 1996, 174, 83–127. [Google Scholar] [CrossRef]
- Booth, T.H.; Broadhurst, L.M.; Pinkard, E.; Prober, S.M.; Dillon, S.K.; Bush, D.; Pinyopusarerk, K.; Doran, J.C.; Ivkovich, M.; Young, A.G. Native forests and climate change: Lessons from eucalypts. For. Ecol. Manag. 2015, 347, 18–29. [Google Scholar] [CrossRef]
- Alvarenga, R.C.; Porfirio-da-Silua, V.; Gontijo Neto, M.M.; Viana, M.C.M.; Vilela, L. Sistema Integração lavoura-Pecuária-Floresta: Condicionamento do solo e intensificação da produção de lavouras. Informe Agropecu. Belo Horizonte 2010, 31, 59–67. [Google Scholar]
- Quijas, S.; Schmid, B.; Balvanera, P. Plant diversity enhances provision of ecosystem services: A new synthesis. Basic Appl. Ecol. 2010, 11, 582–593. [Google Scholar] [CrossRef] [Green Version]
- Pagano, M.C.; Cabello, M.N. Arbuscular Mycorrhizas alleviate plant stress: Analysis of studies from South America. In Biotechnological Techniques of Stress Tolerance in Plants; Miransari, M., Ed.; Studium Press LLC: Houston, TX, USA, 2013; pp. 131–150. [Google Scholar]
- Hirsch, P.R.; Mauchline, T.H. Who’s who in the plant root microbiome? Nat. Biotechnol. 2012, 30, 961–962. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tring, S.G.; Dangl, J.L.; Bucklera, E.S.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 16, 6548–6553. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.L.; Subramanian, S.; Lamont, J.R.; Bywater-Ekegärd, M. Signaling in the phytomicrobiome: Breadth and potential. Front. Plant Sci. 2015, 6, 709. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Qiu, Y.L. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 2006, 16, 299–363. [Google Scholar] [CrossRef] [PubMed]
- Kuyper, T.W.; Goede, R.G.M. Interaction between higher plants and soil-dwelling organisms. In Vegetation Ecology; der Maarel, E., Ed.; Blackwell: Oxford, UK, 2005; pp. 286–308. [Google Scholar]
- Pagano, M.C. (Ed.) Mycorrhiza: Occurrence and Role in Natural and Restored Environments; Nova Science Publishers: Hauppauge, NY, USA, 2012.
- Davinic, M.; Moore-Kuceraa, J.; Acosta-Martínez, V.; Zak, J.; Allen, V. Soil fungal distribution and functionality as affected by grazing and vegetation components of integrated crop–livestock agroecosystems. Appl. Soil Ecol. 2013, 66, 61–70. [Google Scholar] [CrossRef]
- Miranda, E.M.; Saggin Júnior, O.J.; da Silva, E.M.R. Selection of arbuscular mycorrhizal fungi for the forage peanut intercropped with signal grass. Pesq Agropec Bras 2008, 43, 9. [Google Scholar]
- Martha Júnior, G.B.; Alves, E.; Contini, E. Dimensão econômica de sistemas de integração lavoura-pecuária. Pesq. Agropec. Bras. 2011, 46, 1117–1126. [Google Scholar] [CrossRef]
- Balbino, L.C.; Cordeiro, L.A.M.; Oliveira, P.; Kluthcouski, J.; Galerani, P.R.; Vilela, L. Agricultura sustentável por meio da integração lavoura-pecuária-floresta. Inf. Agron. IPNI 2012, 138, 1–18. [Google Scholar]
- Dubeux, J.C.B., Jr.; Muir, J.P.; Ramachandran Nair, P.K.; Sollenberger, L.E.; Silva, H.M.S.; de Mello, A.C.L. The advantages and challenges of 3 integrating tree legumes into pastoral systems. In International Conference on Forages in Warm Climates, Proceedings of the 1st International Conference on Forages in Warm Climates, Lavras, Brazil, 1–3 June 2015; Evangelista, A.R., Avila, C.L.S., Casagrande, D.R., Lara, M.A.S., Bernardes, T.F., Eds.; University of Lavras: Lavras, Brazil, 2015; p. 393. [Google Scholar]
- Pacheco, A.R.; De Chaves, R.Q.; Nicoli, C.M.L. Integration of Crops, Livestock, and Forestry: A System of Production for the Brazilian Cerrados. In Eco-Efficiency: From Vision to Reality; Hershey, C.H., Neate, P., Eds.; CIAT: Cali, Colombia, 2013; pp. 51–61. [Google Scholar]
- Smith, D.L.; Zhou, X. An effective integrated research approach to study climate change in Canada. Can. J. Plant Sci. 2014, 94, 995–1008. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the core Arabidopsis thaliana root. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B. (Ed.) Principles of Plant-Microbe Interactions. Microbes for Sustainable Agriculture; Springer: Basel, Switzerland, 2015.
| Key Words | Total Number of Journal Articles | Number of Journal Articles/Period † |
|---|---|---|
| Plant Microbiome | 822 | 742 |
| Mycosphere | 37 | 23 |
| Plant + Mycobiome | 13 | 12 |
| Phytobiome | 14 | 14 |
| Soil + Mycobiome | 5 | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, M.C.; Correa, E.J.A.; Duarte, N.F.; Yelikbayev, B.; O’Donovan, A.; Gupta, V.K. Advances in Eco-Efficient Agriculture: The Plant-Soil Mycobiome. Agriculture 2017, 7, 14. https://doi.org/10.3390/agriculture7020014
Pagano MC, Correa EJA, Duarte NF, Yelikbayev B, O’Donovan A, Gupta VK. Advances in Eco-Efficient Agriculture: The Plant-Soil Mycobiome. Agriculture. 2017; 7(2):14. https://doi.org/10.3390/agriculture7020014
Chicago/Turabian StylePagano, Marcela Claudia, Eduardo J. Azevedo Correa, Neimar F. Duarte, Bakhytzhan Yelikbayev, Anthonia O’Donovan, and Vijai Kumar Gupta. 2017. "Advances in Eco-Efficient Agriculture: The Plant-Soil Mycobiome" Agriculture 7, no. 2: 14. https://doi.org/10.3390/agriculture7020014






