Assessment of the Carbon Footprint of Large Yellow Croaker Farming on the Aquaculture Vessel in Deep Sea in China
Abstract
:1. Introduction
2. Data and Methods
2.1. Research Methods
2.2. Research Object and Scope of Measurement
2.2.1. Research Object
2.2.2. Scope of Assessment
2.3. Data Sources and Processing
2.3.1. Data Sources
2.3.2. Data Processing
2.4. Calculation Methods of Each Process
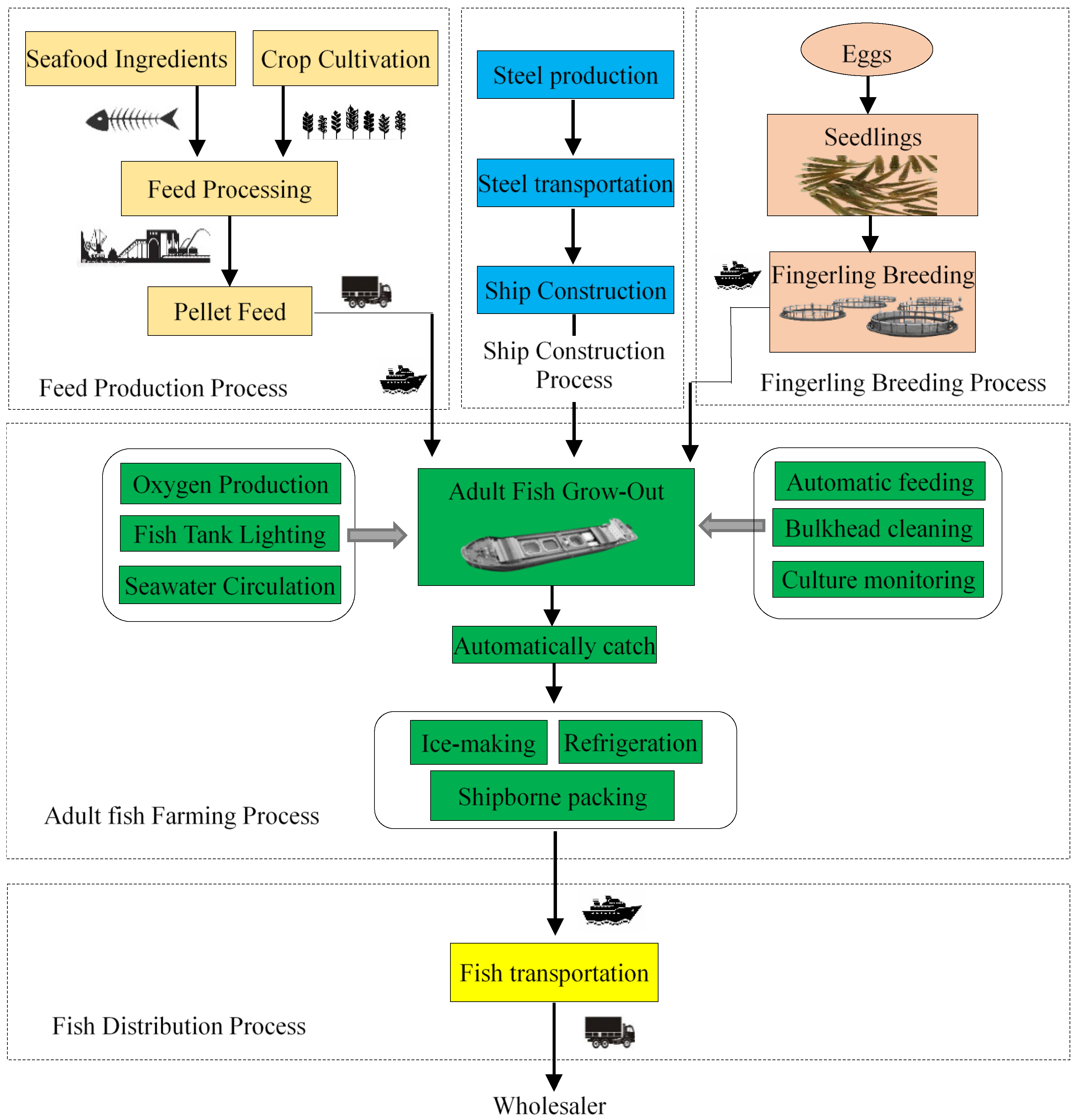
2.4.1. Process of Feed Production
2.4.2. Process of Ship Construction
2.4.3. Process of Fingerling Breeding
2.4.4. Process of Adult Fish Farming
- Aquaculture energy consumption
- Shipborne processing energy consumption
- Shipborne refrigeration
- Shipborne packaging
- Load of aquaculture workers’ consumption
2.4.5. Process of Fish Distribution
2.5. Measurement Method of the Carbon Footprint for a Unit Commodity Price
3. Results
3.1. Carbon Footprint and Contribution Rate of Each Process
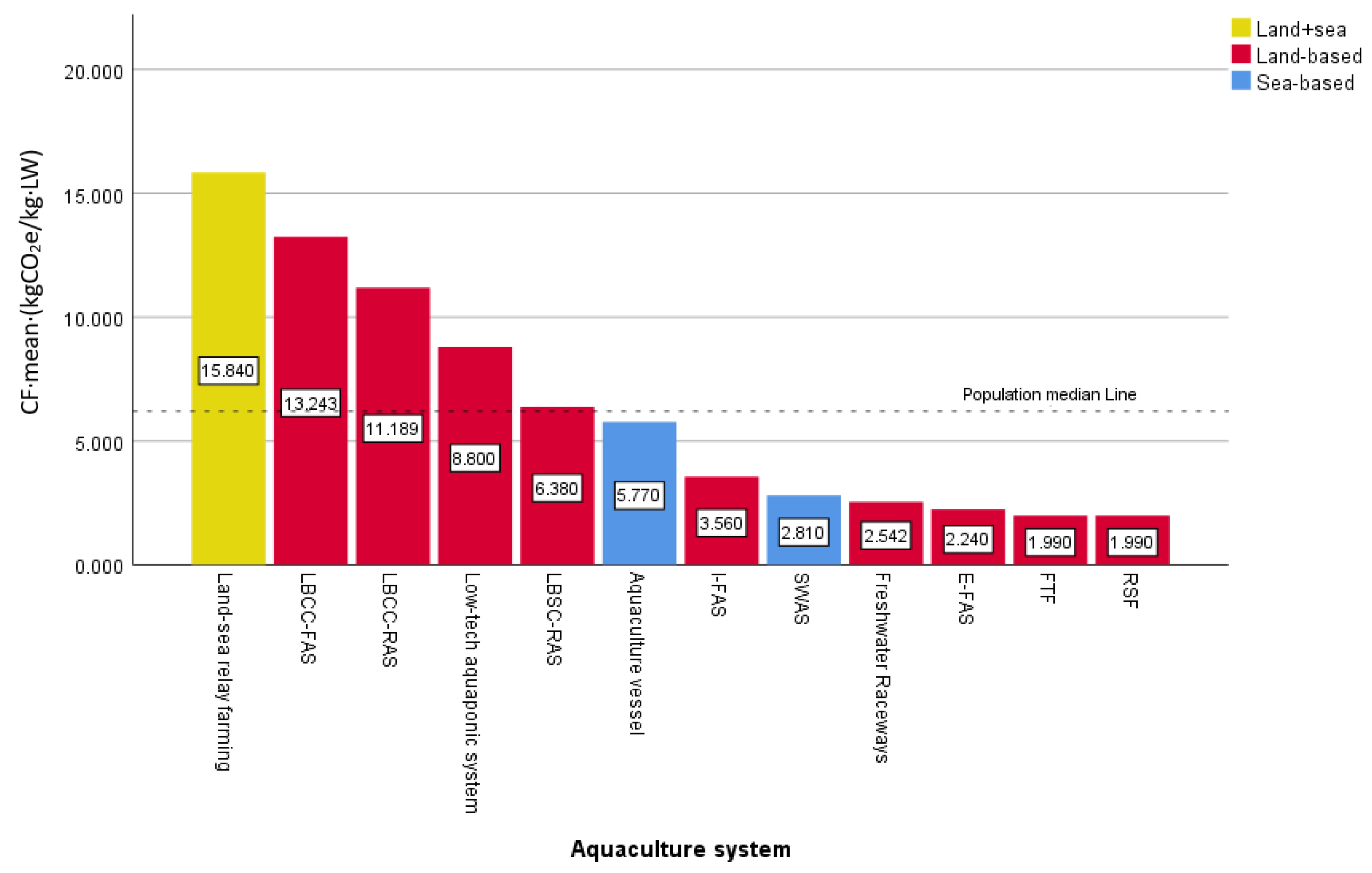
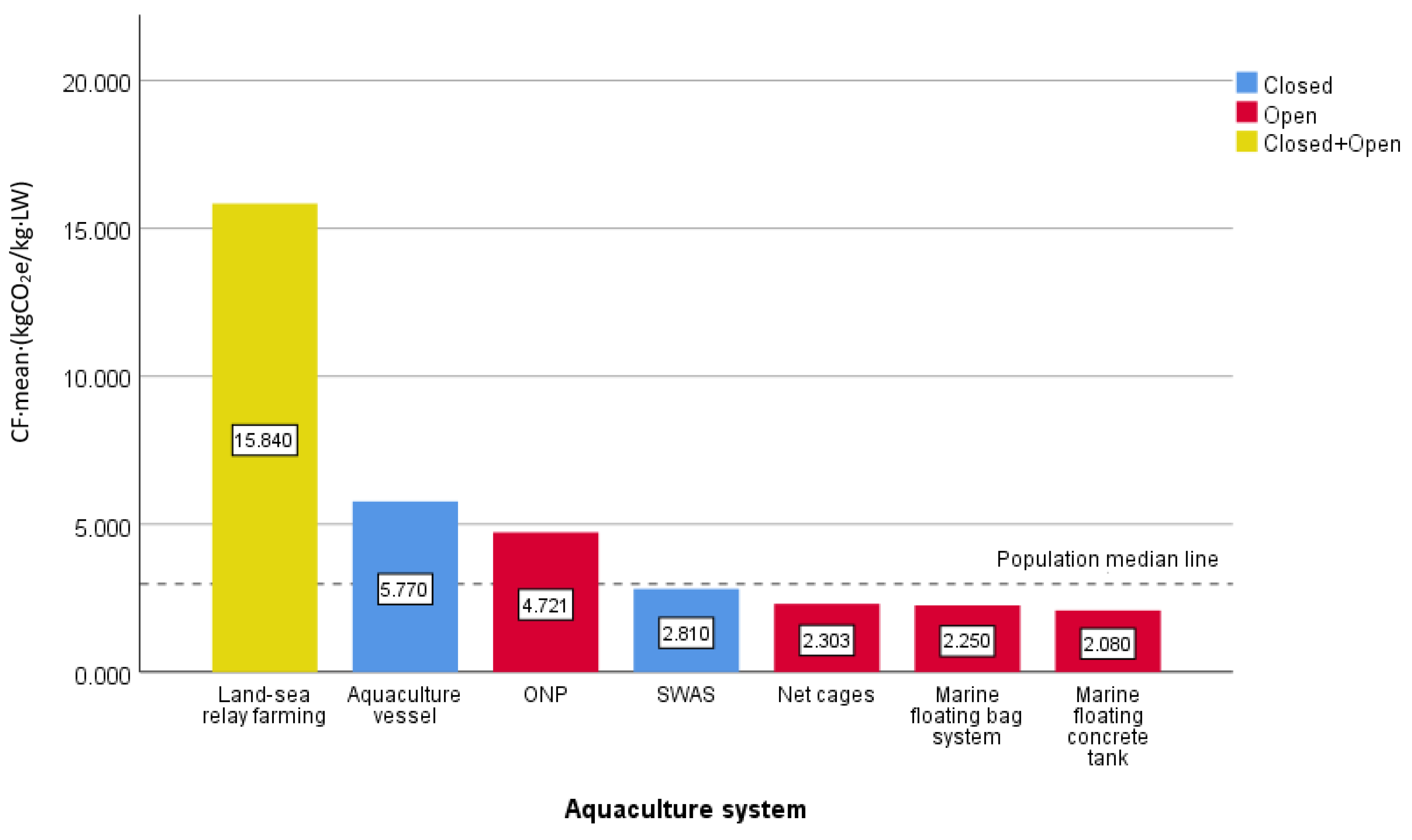
3.2. Comparison of the Carbon Footprint of Marine Fish Farming in Different Systems
4. Discussion and Suggestions
4.1. Discussion
- The intensive and closed aquaculture system relying on modern industry would promote aquaculture from small-scale and low-input farm systems to high-intensity intensive farm systems. The processes adopt mechanization or automation operation, and the CF level will be increased with the increase of external energy [46]. However, aquaculture vessels enable the transfer of traditional industrial closed systems to far-reaching seas while achieving intensive and efficient cultivation in deep-sea environments. With a controllable aquaculture environment, the fish stocking density and output were higher. Additionally, the effect of management, harvest, quality and safety were easy to control and the products were able to be balanced and listed. Therefore, the intensive and closed aquaculture mode would be the inevitable transformation and development direction of the aquaculture industry to a low-carbon economy [46,47], which was consistent with Dalia M. M. Yacout et al. [48], Wang X.H. et al. [46] and Wang H.H. et al. [47]. On the aquaculture vessel, the fish stocking density could reach 4 to 6 times that of traditional cages with a survival rate exceeding 90%. The higher stock density of the aquaculture system enables a reduction in the environmental impact of the unit aquaculture body [48]. Studies have found that the carbon footprint level of fish farming on aquaculture vessels was lower than those observed in overall average closed systems. Given the excessive use of offshore aquaculture areas, promoting deep-sea aquaculture becomes imperative. As a new type of deep-sea aquaculture system, it is expected to promote sustainable development within aquaculture.
- Through a comparative analysis of the literature on the carbon footprint of marine fish culturing systems, it was found that closed aquaculture vessels exhibited higher carbon footprint levels compared to open and semi-closed systems. Large-scale intensive closed aquaculture vessels effectively isolate the aquaculture system from the surrounding ecosystem, reducing direct ecological impacts faced by open systems and enhancing their resistance to environmental disturbances. To simulate an ecologically sustainable deep-sea aquaculture environment and create a stable growth environment for cultured organisms with high production quality and increased stock density, additional technical measures were required to mimic natural conditions in the deep sea. The incorporation of a 24 h uninterrupted seawater circulation system in the aquaculture tanks ensures full integration of tank water with deep-sea water; however, this also contributes significantly to carbon emissions due to the large electricity consumption of these equipment operations. Therefore, it is imperative to reduce energy consumption by optimizing energy utilization efficiency and adjusting energy utilization structures within the aquaculture vessel. Furthermore, from the environmental point of view, a high fish-stocking density determined a lower impact per kg increase of fish produced, especially in terms of global warming and cumulative energy demand [48]. Research focusing on feed formulation optimization and feeding methods would be beneficial in improving fish stocking densities.
- “Conson No. 1” is the world’s first 100,000-ton aquaculture vessel, and currently the only large-scale deep-sea aquaculture vessel in operation in China. To enhance the sustainable development capacity of this deep-sea marine aquaculture system and further facilitate the transition of the marine aquaculture industry towards low-carbon practices, we will leverage extensive long-term operational data from “Conson No. 1” to explore viable alternative energy sources for deep-sea aquaculture production on aquaculture vessels. Additionally, the systemic impact of various carbon reduction measures on aquaculture systems should be considered comprehensively so that a sustainable carbon reduction path will be found for deep-sea aquaculture systems.
4.2. Suggestions
- Adjust the structure of energy utilization. The adoption of green and low-carbon alternative fuels is imperative, along with the promotion of clean energy sources such as offshore wind energy, photovoltaic energy, biodiesel, methanol, “green ammonia”, and “green hydrogen” for application in aquaculture vessels. Extensive research should be conducted on hybrid power combination systems of far-reaching sea vessels, including combinations such as diesel engine and sail, sail and solar power, diesel–methanol dual fuel power, and sail and solar power. Substituting the common grid mix with renewable sources like photovoltaic systems could significantly mitigate the environmental impact associated with electricity generation, particularly in terms of global warming [48].
- Enhance energy utilization efficiency. Rationally set the water exchange cycle rate; effectively recover and utilize the potential energy and part of the kinetic energy in the seawater exchange system of the aquaculture tank; explore and apply energy efficiency technical measures with high maturity such as profile optimization, coating drag reduction and energy-saving appendage in ship design and construction; and enhance ship operational management through information and intelligent technology to improve overall energy efficiency.
- Optimize feed formula and improve feeding method. The theory and technology of nutrition regulation for large yellow croakers should be flexibly applied to fully meet the nutritional requirements of the species during different growth stages, farming methods, seasons, and regions. While the digestibility of raw materials and the processing requirements of various raw materials should be fully considered, feed raw materials with low EF value should be selected. The match between feed N content and fish needs should be studied in order to determine it accurately while improving feed N efficiency through phytase supplementation. Leveraging the controllable conditions advantage of aquaculture tanks, combined with specifications, feeding conditions, feed size, and feeding rate specific to large yellow croakers, promoting their growth could be achieved by increasing feeding frequency and rate.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Symbols | |
| EF | Emission factor (e.g., kgCO2e/kg DM/km) |
| EI | Emission intensity, i.e., the emissions per unit of output, e.g., kgCO2e/kg LW |
| Qa | Annual catch production (t) |
| W | Weight (kg) |
| TUL | Total unit load of equipment (kwh) |
| T | Running time (h) |
| SFC | Diesel generator unit fuel consumption (g/kwh) |
| F | Diesel oil loss rate (%) |
| Qnetderv | Average low calorific value of diesel oil (TJ/104 t) |
| EFderv | Diesel emission factor (kg/TJ) |
| R | Refrigerant loss rate (%) |
| GWP | Global Warming Potential |
| GWP100R404 | Greenhouse effect of R404 over 100 years(kg CO2e) |
| K | Productive GHG emission coefficient of aquaculture workers |
| N | Number of aquaculture workers |
| EIf | Emission intensity of unit commodity price (g CO2e/CNY) |
| Mf | Commodity price (CNY/kg) |
| Acronym | |
| CF | Carbon footprint expressed in terms of CO2 equivalent |
| LCA | Life cycle assessment |
| LW | Live weight |
| DM | Dry matter |
| FCR | Feed conversion rate |
| FAO | Food and Agriculture Organization of the United Nations |
| GHG | Greenhouse Gas |
| CF | Carbon footprint of per unit of product weight |
| FP | Feed production process |
| SC | Ship construction process |
| FB | Fingerling breeding process |
| AF | Adult fish farming process |
| FD | Fish distribution process |
| FPP | Feed production sub-process of FP |
| FPT | Feed transportation sub-process of FP |
| FBB | Fingerling breeding sub-process of FB |
| FBT | Fingerling transportation sub-process of FB |
| AFA | Aquaculture energy consumption sub-process of AF |
| AFP | Shipborne processing energy consumption sub-process of AF |
| AFR | Shipborne refrigeration sub-process of AF |
| AFB | Shipborne packaging sub-process of AF |
| AFW | Load of aquaculture workers sub-process of AF |
| LBCC-FAS | Land-based industrial flow-through system |
| LBCC-RAS | Land-based closed recirculating system |
| ONP | Open net pen |
| LBSC-RAS | Semi-closed recirculating system |
| I-FAS | Intensive recirculating aquaculture systems |
| SWAS | Floating, flow-through, solid-walled aquaculture system |
| E-FAS | Extensive flow-through aquaculture system |
| RSF | Recirculation system farm |
| FTF | Flow-through system |
| IPCC | Intergovernmental Panel on Climate Change |
| CNY | Chinese Yuan |
References
- Zheng, Y.Y.; Yu, F.W. Low-carbon agricultural development in the context of climate change: International experiences and China’s strategies. Chin. J. Eco-Agric. 2024, 32, 183–195. (In Chinese) [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Zhou, S.H.; Yuan, N.F. Developing low-carbon economy to cope with climate change-Low-carbon economy and its evaluation indicators. China Natl. Cond. Strength 2009, 12, 4–6. (In Chinese) [Google Scholar]
- Jin, S.Q.; Chen, J. A Study on Energy Consumption and Carbon Emission of China’s Aquaculture. China Fish. Econ. 2012, 30, 73–82. [Google Scholar]
- Ayer, N.W.; Tyedmers, P.H. Assessing alternative aquaculture technologies: Life cycle assessment of salmonid culture systems in Canada. J. Clean. Prod. 2009, 17, 362–373. [Google Scholar] [CrossRef]
- Chen, Z.X.; Cao, G.B.; Han, S.C. Life cycle assessment of rainbow trout aquaculture models in China. J. Agro-Environ. Sci. 2011, 30, 2113–2118. (In Chinese) [Google Scholar]
- Samuel-Fitwi, B.; Nagel, F.; Meyer, S.; Schroeder, J.P.; Schulz, C. Comparative life cycle assessment (LCA) of raising rainbow trout (Oncorhynchus mykiss) in different production systems. Aquac. Eng. 2013, 54, 85–92. [Google Scholar] [CrossRef]
- Liu, Y.; Rosten, T.W.; Henriksen, K.; Hognes, E.S.; Summerfelt, S.; Vinci, B. Comparative economic performance and carbon footprint of two farming models for producing Atlantic salmon (Salmo salar): Land-based closed containment system in freshwater and open net pen in seawater. Aquac. Eng. 2016, 71, 1–12. [Google Scholar] [CrossRef]
- Fu, X.Y. Environmental impact analysis of Larimichthys crocea cage culture based on life cycle assessment. Zhejiang Ocean. Univ. 2016, 5, 27. (In Chinese) [Google Scholar]
- Fu, X.Y.; Zhao, S.; Zhu, A.; Wu, C. Carbon footprint of Larimichthys crocea cage farm system based on life cycle assessment. China Water Transp. 2016, 16, 136–139. [Google Scholar]
- Johansen, U.; Nistad, A.A.; Ziegler, F.; Mehta, S.; Wocken, Y.; Hognes, E.S. Greenhouse Gas Emissions of Norwegian Salmon Products; Report No. 202:01198, Project No. 302006529 Version 1; SINTEF Ocean AS: Trondheim, Norway, 2022. [Google Scholar]
- FAO. Greenhouse Gas Emissions from Aquaculture: A Life Cycle Assessment of Three Asian Systems; Fisheries and Aquaculture Technical Paper 609; FAO: Rome, Italy, 2017. [Google Scholar]
- Dong, Y.; Li, B.; Jia, R. Life cycle environmental impact assessment on two aquaculture models in the Yangtze River basin. Adv. Fish. Sci. 2023, 44, 1–10. (In Chinese) [Google Scholar] [CrossRef]
- Philis, G.; Ziegler, F.; Gansel, L.C.; Jansen, M.D.; Gracey, E.O.; Stene, A. Comparing Life Cycle Assessment (LCA) of Salmonid Aquaculture Production Systems: Status and Perspectives. Sustainability 2019, 11, 2517. [Google Scholar] [CrossRef]
- Liu, H.; Xu, H.; Zhuang, Z.M. Review of floating closed aquaculture vessel development. Fish. Mod. 2022, 49, 1–7. (In Chinese) [Google Scholar]
- Tang, Q.H. Environmentally Friendly Aquaculture Development Strategy: New Ideas, New Tasks and New Approaches; Science Publishing House: Beijing, China, 2017. (In Chinese) [Google Scholar]
- GB/T24044-2008; Requirements and Guidelines for Environmental Management Life Cycle Assessment. Standardization Administration of the State: Beijing, China, 2008. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=329770D2F0539B875B094A56C308EC4E (accessed on 1 February 2023).
- National Fisheries Technology Extension Center. Technical Model of Far-Reaching Marine Aquaculture Facilities; China Agriculture Press: Beijing, China, 2021; p. 160. (In Chinese) [Google Scholar]
- Li, B.Y. The calculation of ship carbon footprint. China Shipp. Surv. 2010, 10, 48–51. (In Chinese) [Google Scholar]
- Fan, A.; Xiong, Y.; Yang, L.; Zhang, H.; He, Y. Carbon footprint model and low–carbon pathway of inland shipping based on micro–macro analysis. Energy 2023, 263, 126150. [Google Scholar] [CrossRef]
- Ko, N.; Gantner, J. Local added value and environmental impacts of ship scrapping in thecontext of a ship’s life cycle. Ocean. Eng. 2016, 122, 317–321. [Google Scholar] [CrossRef]
- Pelletier, N.; Tyedmers, P. Feeding farmed salmon: Is organic better? Aquaculture 2007, 272, 399–416. [Google Scholar] [CrossRef]
- FAO. Quantifying and Mitigating Greenhouse Gas Emissions from Global Aquaculture; Fisheries and Aquaculture Technical Paper 626; FAO: Rome, Italy, 2019. [Google Scholar]
- Kjær, L.L.; Pagoropoulos, A.; Hauschild, M.; Birkved, M.; Schmidt, J.H.; McAloone, T.C. From LCC to LCA using a hybrid input output model—A maritime case study. Procedia CIRP 2015, 29, 474–479. Available online: http://creativecommons.org/licenses/by-nc-nd/4.0/ (accessed on 21 April 2023). [CrossRef]
- Quang, P.K.; Dong, D.T.; Hai, P.T. Evaluating environmental impacts of an oil tanker using life cycle assessment method. J. Eng. Marit. Environ. 2021, 235, 705–717. [Google Scholar] [CrossRef]
- IPCC. 2019 Refinement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventory; IGES: Kyoto, Japan, 2019. [Google Scholar]
- Office of the National Climate Change Response Coordination Group. Study on Greenhouse Gas Inventories in China; China Environmental Science Press: Beijing, China, 2007. (In Chinese) [Google Scholar]
- ASHRAE. 2017 Ashrae Handbook—Fundamentals (SI Edition); American Society of Heating, Refrigerating and Air-Conditioning Engineers, Inc.: Peachtree Corners, GA, USA, 2017. [Google Scholar]
- Sun, C.H.; Xu, K.L.; Guo, A.J. Research on the Generation Mode and Carbon Emission of Express Packaging Waste in a University in Wuhan. Mod. Chem. Res. 2023, 8, 65–67. (In Chinese) [Google Scholar] [CrossRef]
- Su, Y.; Duan, H.; Wang, Z. Characterizing the environmental impact of packaging materials for express delivery via life cycle assessment. J. Clean. Prod. 2020, 274, 122961. [Google Scholar] [CrossRef]
- Yang, J.P.; Wang, Z.; Guo, L.M. Preliminary evaluation of the environment impact of carbon, nitrogen and phosphorus emissions from Marine fish farming—Take Atlantic salmon (Salmo salar) farming as an example. Fish. Sci. Technol. Inf. 2022, 49, 350–358. (In Chinese) [Google Scholar] [CrossRef]
- Hou, H.C.; Zhang, Y.; Ma, Z. Life cycle assessment of tiger puffer (Takifugu rubripes) farming: A case study in Dalian, China. Sci. Total Environ. 2022, 823, 153522. [Google Scholar] [CrossRef]
- Parker, R. Implications of high animal by-product feed inputs in life cycle assessments of farmed Atlantic salmon. Int. J. Life Cycle Assess. 2018, 23, 982–994. [Google Scholar] [CrossRef]
- Bordignon, F.; Sturaro, E.; Trocino, A.; Birolo, M.; Xiccato, G.; Berton, M. Comparative life cycle assessment of rainbow trout (Oncorhynchus mykiss) farming at two stocking densities in a low-tech aquaponics system. Aquaculture 2022, 556, 738264. [Google Scholar] [CrossRef]
- White, A. A Comprehensive Analysis of Efficiency in the Tasmanian Salmon Industry. Ph.D. Thesis, Bond University, Gold Coast, Australia, 2013. [Google Scholar]
- Dekamin, M.; Veisi, H.; Safari, E.; Liaghati, H.; Khoshbakht, K.; Dekamin, M.G. Life cycle assessment for rainbow trout (Oncorhynchus mykiss) production systems: A case study for Iran. J. Clean. Prod. 2015, 91, 43–55. [Google Scholar] [CrossRef]
- Aubin, J.; Papatryphon, E.; Van der Werf, H.M.G.; Chatzifotis, S. Assessment of the environmental impact of carnivorous finfish production systems using life cycle assessment. J. Clean. Prod. 2009, 17, 354–361. [Google Scholar] [CrossRef]
- Wilfart, A.; Prudhomme, J.; Blancheton, J.P.; Aubin, J. LCA and energy accounting of aquaculture systems: Towards ecological intensification. J. Environ. Manag. 2013, 121, 96–109. [Google Scholar] [CrossRef]
- Ytrestøyl, T.; Aas, T.S.; Berge, G.M.; Hatlen, B.; Sørensen, M.; Ruyter, B.; Thomassen, M.S.; Hognes, E.S.; Ziegler, F.; Sund, V.; et al. Resource Utilization and Eco-Efficiency of Norwegian Salmon Farming in 2010; Nofima: Tromsø, Norway, 2011. [Google Scholar]
- McGrath, K.P.; Pelletier, N.L.; Tyedmers, P.H. Tyedmers. Life Cycle Assessment of a Novel Closed-Containment Salmon Aquaculture Technology. Environ. Sci. Technol. 2015, 49, 5628–5636. [Google Scholar] [CrossRef]
- Boissy, J.; Aubin, J.; Drissi, A.; van der Werf, H.M.; Bell, G.J.; Kaushik, S.J. Environmental impacts of plant-based salmonid diets at feed and farm scales. Aquaculture 2011, 321, 61–70. [Google Scholar] [CrossRef]
- Ayer, N.; Martin, S.; Dwyer, R.L.; Laurin, L. Environmental performance of copper-alloy Net-pens: Life cycle assessment of Atlantic salmon grow-out in copper-alloy and nylon net-pens. Aquaculture 2016, 453, 93–103. [Google Scholar] [CrossRef]
- Ellingsen, H.; Aanondsen, S.A. Environmental Impacts of Wild Caught Cod and Farmed Salmon—A Comparison with Chicken. Int. J. Life Cycle Assess. 2006, 1, 60–65. [Google Scholar] [CrossRef]
- Abdou, K.; Aubin, J.; Romdhane, M.S.; Le Loc’h, F.; Lasram, F.B.R. Environmental assessment of seabass (Dicentrarchus labrax) and seabream (Sparus aurata) farming from a life cycle perspective: A case study of a Tunisian aquaculture farm. Aquaculture 2017, 471, 204–212. [Google Scholar] [CrossRef]
- Ziegler, F.; Winther, U.; Hognes, E.S.; Emanuelsson, A.; Sund, V.; Ellingsen, H. The Carbon Footprint of Norwegian Seafood Products on the global seafood market. J. Ind. Ecol. 2013, 17, 103–116. [Google Scholar] [CrossRef]
- d’Orbcastel, E.R.; Blancheton, J.P.; Aubin, J. Towards environmentally sustainable aquaculture: Comparison between two trout farming systems using Life Cycle Assessment. Aquac. Eng. 2009, 40, 113–119. [Google Scholar] [CrossRef]
- Wang, X.H. The Concept and Development of healthy Aquaculture (Part II). Fish. Guide Be Rich 2021, 22, 16–22. (In Chinese) [Google Scholar]
- Wang, H.H.; Hou, H.C.; Liu, Y. Research progress and development trend in recirculating aquaculture system. Fish. Sci. 2023, 42, 735–741. (In Chinese) [Google Scholar] [CrossRef]
- Yacout, D.M.; Soliman, N.F.; Yacout, M.M. Comparative life cycle assessment (LCA) of Tilapia in two production systems: Semi-intensive and intensive. Int. J. Life Cycle Assess. 2016, 21, 806–819. [Google Scholar] [CrossRef]


| Ingredients | Content (%) |
|---|---|
| Fish meal | 40–65 |
| Soybean meal | 5–25 |
| Wheat | 10–25 |
| Fish oil | 2–5 |
| Yeast | 2–4 |
| Shrimp meal | 2–5 |
| Corn gluten meal | 1–5 |
| Complex minerals | 1–2 |
| Choline chloride | 0.2–0.5 |
| Multivitamin | 0.2 |
| Process | Formula and Definition and Interpretation | |
|---|---|---|
| FP | (1) EIFP = (EFFPP + EFL1 × SL1 + EFS1 × SS1) × FCR | |
| where EIFP is the CF of the FD process, kgCO2e/kg LW, and EFFPP is the carbon emission factor of the production activity in the FD process, kgCO2e/kg DM(feed dry matter). EFL1 and EFS1 are used to represent the carbon emission factor of the feed transportation by land and sea, respectively, with the values of 0.164 × 10−3 kg CO2e/kg DM/km and 0.0068 × 10−3 kg CO2e/kg DM/km. SL1 and SS1 indicate distances of feed transportation by land and sea, respectively, which were 200 km and 20 km; FCR was set a value of 1.30. | ||
| SC | (2) EISC = (EIAF + EISC) × RSC | |
| where EISC is the CF of the SC process, kgCO2e/kg LW, and RSC is the ratio of the CF of the SC process to the whole life cycle of the ship (excluding ship dismantling), 4.7%. | ||
| FB | (3) EIFB = EIFBB + EFS2×SS2 ×(Wwater + Wfingerling) | |
| where EIFB is the CF of the FB process, kgCO2e/kg LW; EFFBB is the carbon emission factor of the breeding activity in FB process, kgCO2e/kg LW; EFS2 is the carbon emission factor of fingerling shipping, 0.06 × 10−3 kgCO2e/kg/km (including refrigeration); and SS2 is the shipping distance of fingerling ships, 20 km. Wfingerling is the weight of the fingerling required for 1 kg of large yellow croaker breeding, 0.44 kg. Wwater is the weight of the storage water required for fingerling storage and transportation, 17.76 kg. | ||
| AF | AFA | (4) EIAFA = TULA × T × SFC ÷ (1 – F) × Qnetderv × EFderv ÷ Qa × 10−7 |
| where EIAFA is the CF of energy consumption from aquaculture equipment, kgCO2e/kg LW. TULA is the total unit load of aquaculture equipment, 2810.4881 kwh; T is the running time, h, per year. SFC is the diesel generator unit fuel consumption, 180 g/kwh; F is the rate of diesel oil loss, 5%; Qnetderv is the average low calorific value of diesel oil, 433.3 TJ/104 t; EFderv is diesel emission factor, 74,100 kg/TJ. Qa is the annual catch production, 3700 t. | ||
| AFP | (5) EIAFP = TULP × T × SFC ÷ (1 – F) × Qnetderv × EFderv ÷ Qa × 10−7 | |
| where EIAFP is the CF of energy consumption from shipborne processing equipment, kgCO2e/kg LW. TULP is the total unit load of shipborne processing equipment, 298.6015 kwh. | ||
| AFR | (6) EIAFR = Wice × 0.1% × Rloss × GWP100R404 | |
| where EIAFR is the CF of shipborne refrigeration, kgCO2e/kg LW. Wice is the amount of ice needed by large yellow croakers, 0.25 kg. 0.1% is the proportion of refrigerant added to produce ice. Rloss is the refrigerant loss rate, 10%. GWP100R404 = 3940 kg CO2e [27]. | ||
| AFB | (7) EIAFB = ∑EFAFBj × (Wbox ÷ Wfish) | |
| where EIAFB is the LCA carbon footprint of shipboard packaging per 1 kg fish; EFbox is the life cycle carbon emission factor of the foam box; and EFAFBj is the carbon emission factors of the foam box in each stage of the life cycle, including raw material processing stage, production stage, landfill disposal stage. Wbox is the weight of the foam box, 0.2 kg. Wfish is the net weight of large yellow croakers in each package, 6 kg. | ||
| AFW | (8) EIAFW = ((K × N) ÷ Qa) × 10−3 | |
| where K is the productive GHG emission coefficient of aquaculture workers, and the GHG emission coefficient of workers is 10.5 kg/d/person, of which 25% is emitted due to aquaculture activity [3], K = 10.5 × 365 × 0.25. N is the number of aquaculture workers, N = 16. | ||
| FD | (9) EIFD = (EFS3 × SS3 + EFL3 × SL3) × WT × 10−3 | |
| where EIFD is the CF of the fish distribution process. EFS3 and EFL3 are the carbon emission factors of shipping and land transportation of fish distribution, respectively: 0.042 kgCO2e/t km (including refrigeration) and 0.101 kgCO2e/t km (including refrigeration). SS3 and SL3 are the shipping and land transportation distances of marketable large yellow croaker, respectively: 20 km and 50 km. WT is the total weight of an average 1 kg packaged marketable large yellow croaker, 1.33 kg. | ||
| TOTAL | (10) EI = ∑EIj | |
| where EI is the CF of the whole life cycle of large yellow croaker farming on the aquaculture vessel; EIj is each process’s CF throughout the whole life cycle; and EIj includes EIFP, EISC, EIFB, EIAF, and EIFD. The unit is kgCO2e/kg LW. | ||
| Life Cycle Stage | EFbox of Foam Box (kgCO2e/kg) | Data Source |
|---|---|---|
| Raw material processing | 3.040 | China Postal Industry Report (2014) [28] |
| Production | 0.758 | Su Y, et al. 2020 [29] |
| Landfill disposal | 0.117 | Commercial database GaBi 8.0 [28] |
| Process | CF and Contribution Rate | Sub-Processes | CF and Contribution Rate | ||||
|---|---|---|---|---|---|---|---|
| kgCO2e/kg LW | % | kgCO2e/kg LW | % | ||||
| FP | EIFP | 1.5456 | 24.86 | FPP | EIFPP | 1.5028 | 24.17 |
| FPT | EIFPT | 0.0428 | 0.69 | ||||
| SC | EISC | 0.1795 | 2.89 | ||||
| FB | EIFB | 0.1758 | 2.83 | FBB | EIFBB | 0.1540 | 2.48 |
| FBT | EIFBT | 0.0218 | 0.35 | ||||
| AF | EIAF | 4.3083 | 69.30 | AFA | EIAFA | 4.048 | 65.11 |
| AFP | EIAFP | 0.0291 | 0.47 | ||||
| AFR | EIAFR | 0.0985 | 1.58 | ||||
| AFB | EIAFB | 0.1286 | 2.07 | ||||
| AFW | EIAFW | 0.0041 | 0.07 | ||||
| FD | EIFD | 0.0078 | 0.13 | ||||
| Total | EI | 6.2170 | |||||
| Aquaculture System | Area, Open Degree | CF j | Sources of Literature/Variety/Nation |
|---|---|---|---|
| Aquaculture vessel | Sea-based, closed | 5.77 | this study, 2023; Large yellow croaker; China |
| LBCC-RAS a | Land-based, closed | 38.09 | Chen Z.X. et al. [5], 2011; Rainbow trout; China |
| LBCC-FAS b | Land-based, closed | 33.16 | Chen Z. X. et al. [5], 2011; Rainbow trout; China |
| Land-sea relay farming | Land-sea relay, closed-open relay | 15.84 | Hou H. C. [31], 2022; Tiger puffer; China |
| LBCC-RAS c | Land-based, closed | 13.62 | B. Samuel-Fitwi [6], 2013; Rainbow trout; Denmark |
| ONP c | Sea-based, open | 12.80 | Robert Parker [32], 2018; Salmon; Australia |
| LBCC-RAS a | Land-based, closed | 10.30 | Nathan W. Ayer [4], 2009; Salmon; Canada |
| Low-tech aquaponic | Land-based, closed | 8.80 | Francesco Bordignon [33], 2022; Rainbow trout; Italy |
| ONP c | Sea-based, open | 8.59 | White A [34], 2013; Salmon; Australia |
| LBCC-RAS a | Land-based, closed | 7.01 | Yajie Liu et al. [7], 2016; Salmon; USA |
| LBSC-RAS d | Land-based, semi-closed | 6.38 | Majid Dekamin [35], 2015; Rainbow trout; Iran |
| LBCC-RAS a | Land-based, closed | 6.10 | Majid Dekamin [35], 2015; Rainbow trout; Iran |
| LBCC-RAS a | Land-based, closed | 5.79 | J. Aubin [36], 2009; Turbot; France |
| LBCC-RAS a | Land-based, closed | 5.62 | Aurelie Wilfart [37], 2013; Rainbow trout; France |
| LBCC-FAS b | Land-based, closed | 5.41 | Nathan W. Ayer [4], 2009; Salmon; Canada |
| ONP c | Sea-based, open | 3.80 | Ulf Johansen et al. [10], 2022; Salmon; Norway |
| I-FAS e | Land-based, closed | 3.56 | B. Samuel-Fitwi [6], 2013; Rainbow trout; Germany |
| ONP c | Sea-based, open | 3.39 | Yajie Liu et al. [7], 2016; Salmon; Nordic Region |
| Net cages | Sea-based, open | 3.32 | J. Aubin [36], 2009; Perch; Greece |
| LBCC-FAS b | Land-based, closed | 2.99 | Aurelie Wilfart [37], 2013; Salmon; France |
| ONP c | Sea-based, open | 2.82 | Ytrestoyl T [38], 2011; Salmon; Norway |
| SWAS f | Sea-based, semi-closed | 2.81 | McGrath KP [39], 2015; Salmon; Canada |
| Freshwater raceways | Land-based, closed | 2.54 | J. Aubin [36], 2009; Rainbow trout; France |
| Net cages | Sea-based, open | 2.32 | Joachim Boissy [40], 2011; Salmon; Scotland |
| ONP c | Sea-based, open | 2.31 | Nathan.Ayer [41], 2016; Salmon; Chile |
| ONP c | Sea-based, open | 2.30 | Ellingsen H [42], 2006; Salmon; Norway |
| Marine floating bag | Sea-based, open | 2.25 | Nathan W. Ayer [4], 2009; Salmon; Canada |
| E-FAS g | Land-based, closed | 2.24 | B. Samuel-Fitwi [6], 2013; Rainbow trout; Germany |
| Net cages | Sea-based, open | 2.22 | Joachim Boissy [40], 2011; Rainbow trout; France |
| Marine floating concrete tank | Sea-based, open | 2.08 | Nathan.Ayer [41], 2016; Salmon; Chile |
| Net cage | Sea-based, open | 2.07 | Khaled Abdou [43], 2017; Sea bream;Tunisia |
| ONP c | Sea-based, open | 2.07 | Nathan W. Ayer [4], 2009; Salmon; Canada |
| ONP c | Sea-based, open | 2.00 | Winther U [44], 2009; Salmon; Norway |
| RSF h | land-based, closed | 1.99 | Emmanuelle Roque d O‘rbcastel [45], 2009; Rainbow trout; Denmark |
| FTF i | Land-based, closed | 1.99 | Emmanuelle Roque d O‘rbcastel [45], 2009; Rainbow trout; France |
| Net cages | Sea-based, open | 1.58 | Khaled Abdou [43], 2017; Sea bass; Tunisia |
| LBCC-FAS b | Land-based, closed | 1.16 | Majid Dekamin [35], 2015; Rainbow trout; Iran |
| Variable | N | Mean | Median | Interquartile Range | Test | Wilcoxon Test Statistic | p |
|---|---|---|---|---|---|---|---|
| CF | 21 | 8.627 | 5.770 | 6.874 | 6.2 | 109 | 0.821 |
| Variable | N | Mean | Median | Interquartile Range | Test | Wilcoxon Test Statistic | p |
|---|---|---|---|---|---|---|---|
| CF | 19 | 4.228 | 2.320 | 1.72 | 2.90 | 90.5 | 0.856 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, F.; Zheng, J.; Liu, H.; Cui, M. Assessment of the Carbon Footprint of Large Yellow Croaker Farming on the Aquaculture Vessel in Deep Sea in China. J. Mar. Sci. Eng. 2024, 12, 693. https://doi.org/10.3390/jmse12050693
Fan F, Zheng J, Liu H, Cui M. Assessment of the Carbon Footprint of Large Yellow Croaker Farming on the Aquaculture Vessel in Deep Sea in China. Journal of Marine Science and Engineering. 2024; 12(5):693. https://doi.org/10.3390/jmse12050693
Chicago/Turabian StyleFan, Fei, Jianli Zheng, Huang Liu, and Mingchao Cui. 2024. "Assessment of the Carbon Footprint of Large Yellow Croaker Farming on the Aquaculture Vessel in Deep Sea in China" Journal of Marine Science and Engineering 12, no. 5: 693. https://doi.org/10.3390/jmse12050693





