Novel Universal Bond Containing Bioactive Monomer Promotes Odontoblast Differentiation In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Aqueous Solutions of the Materials
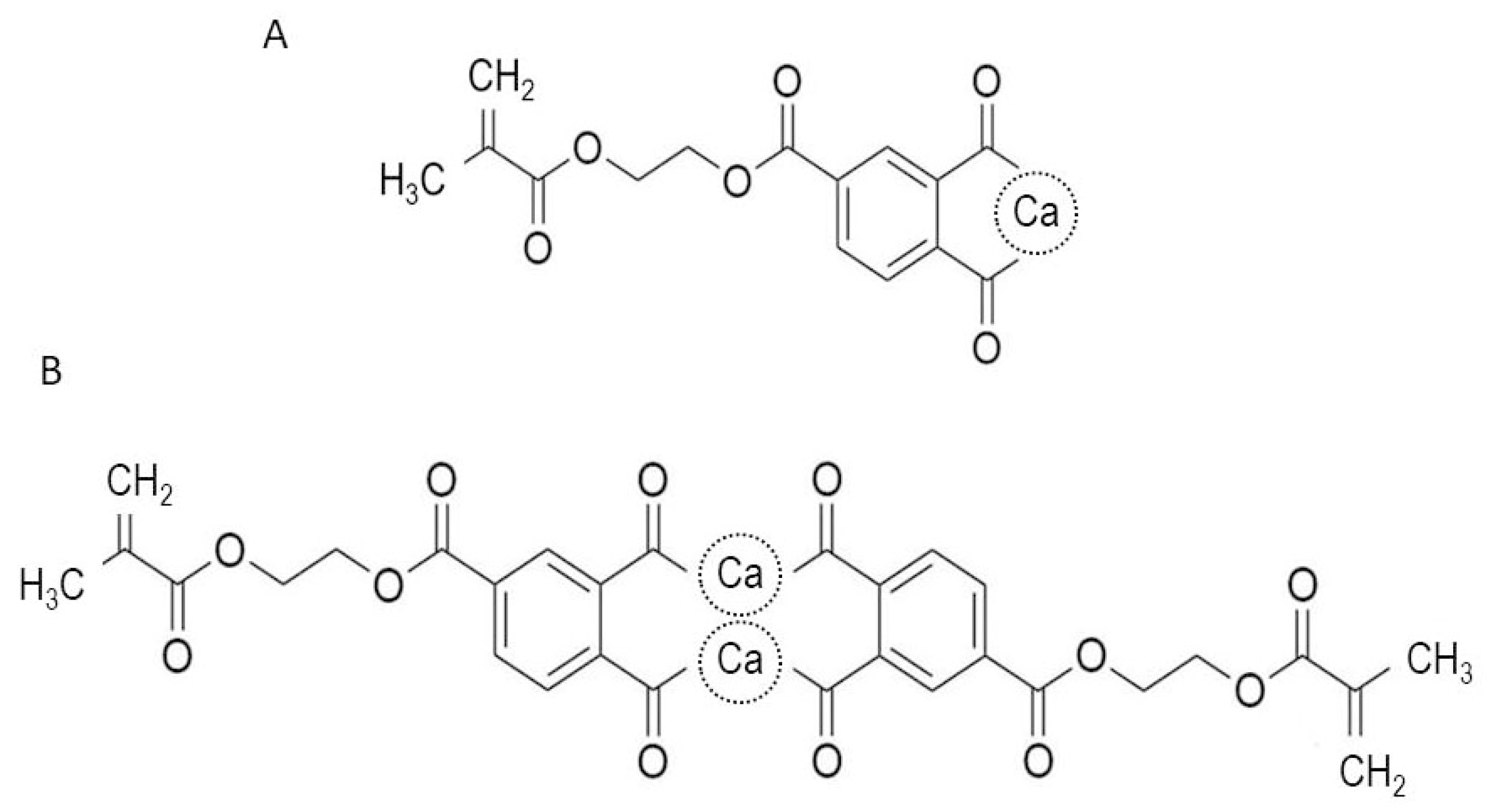
2.2. Cell Culture
2.3. Cell Proliferation
2.4. Alkaline Phosphatase Activity
2.5. Quantitative Real-Time Reverse-Transcription Polymerase Chain Reaction
2.6. Mineralization
2.7. Statistical Analyses
3. Results
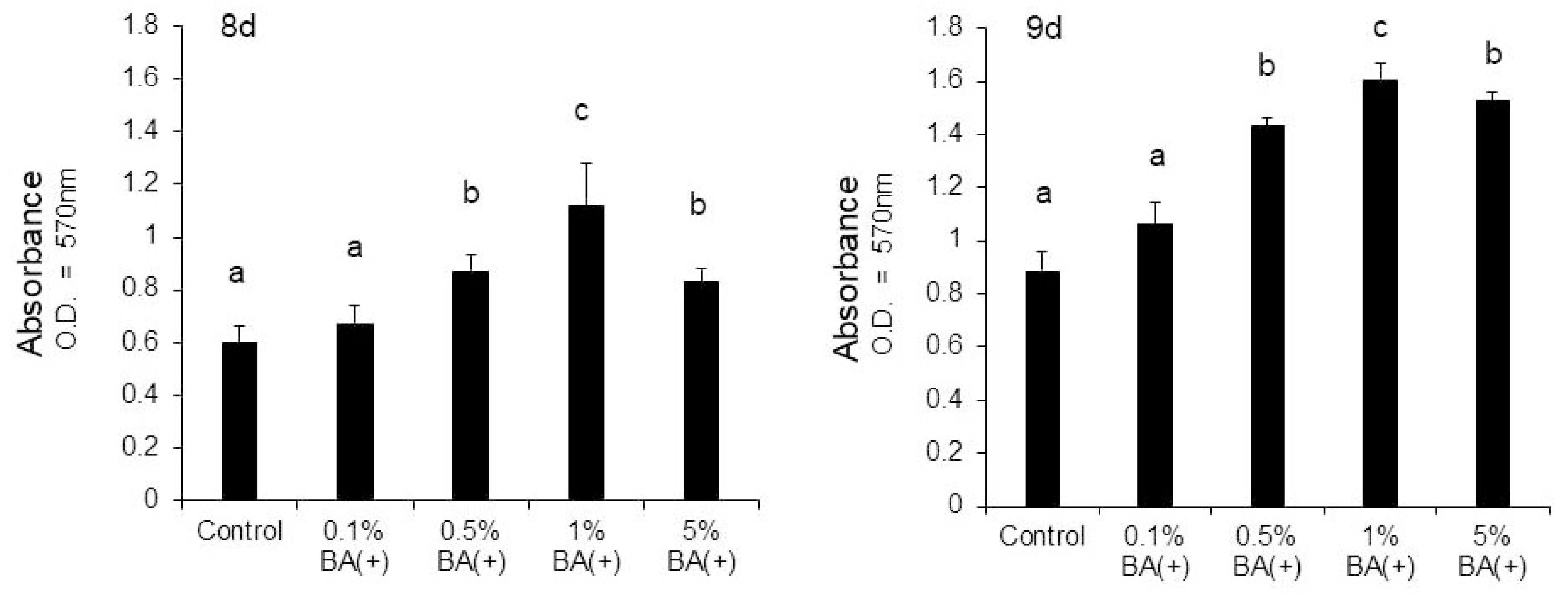
3.1. Cell Proliferation
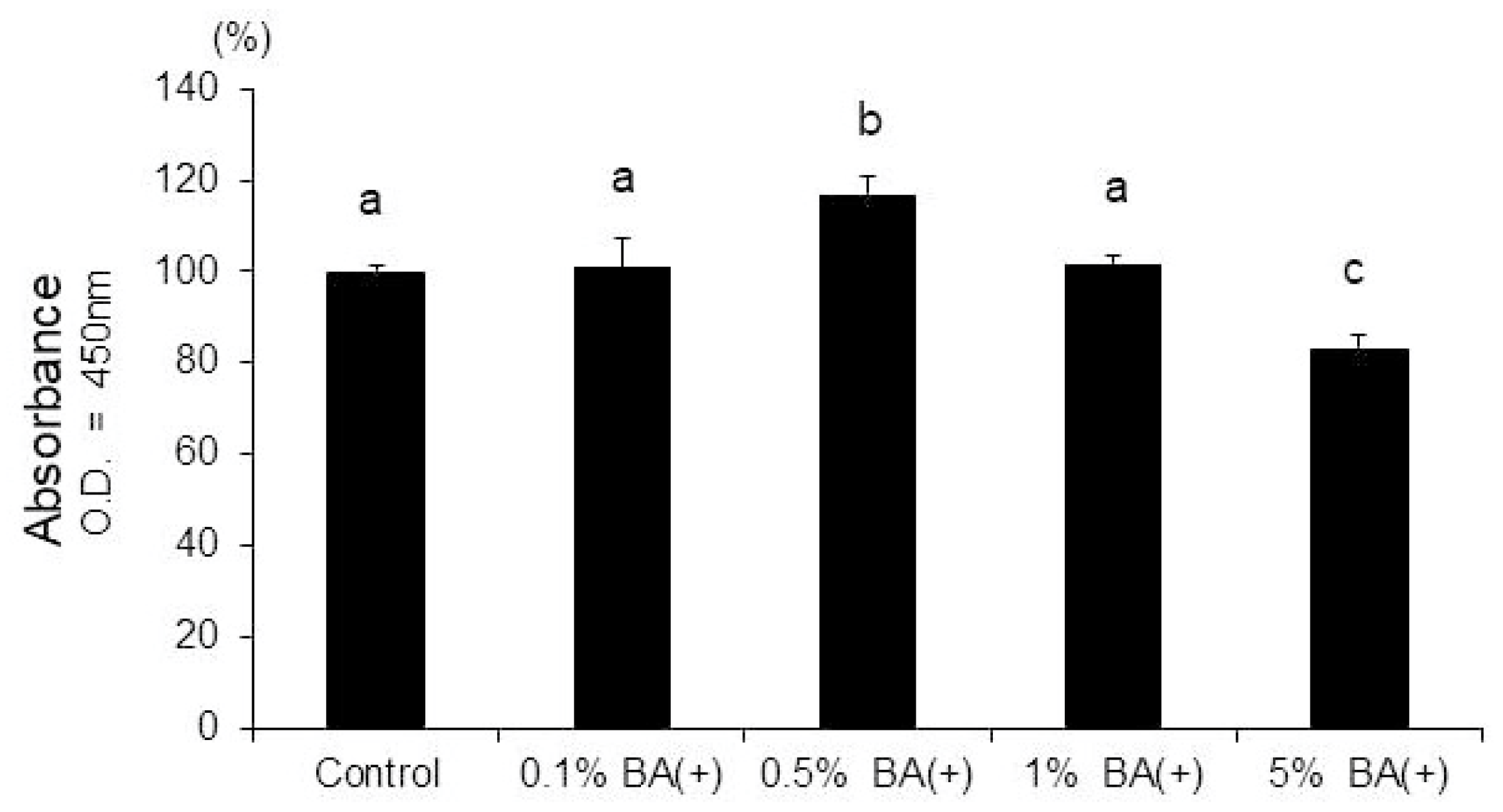
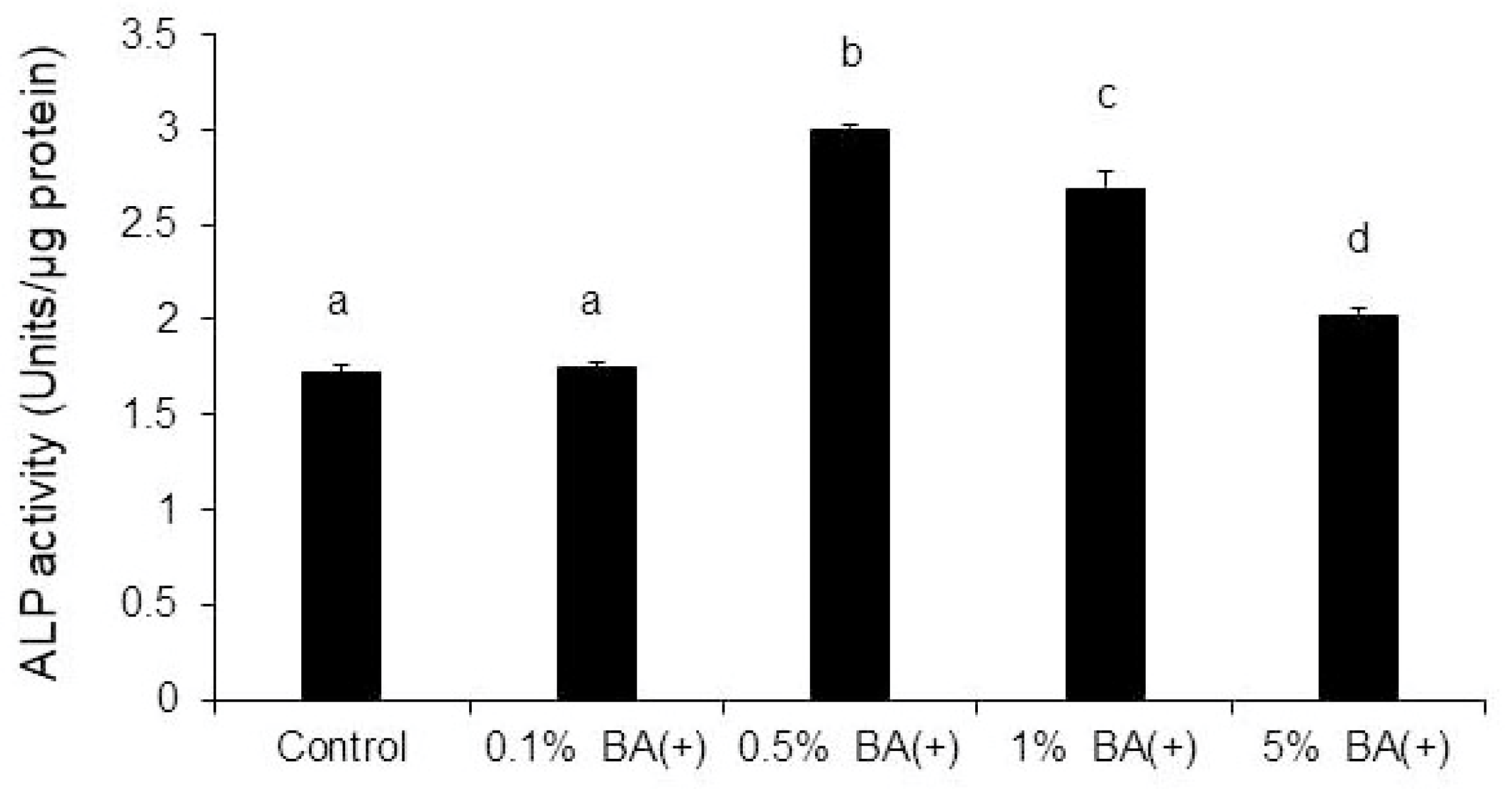
3.2. ALP Activity
3.3. Quantitative Real-Time RT-PCR
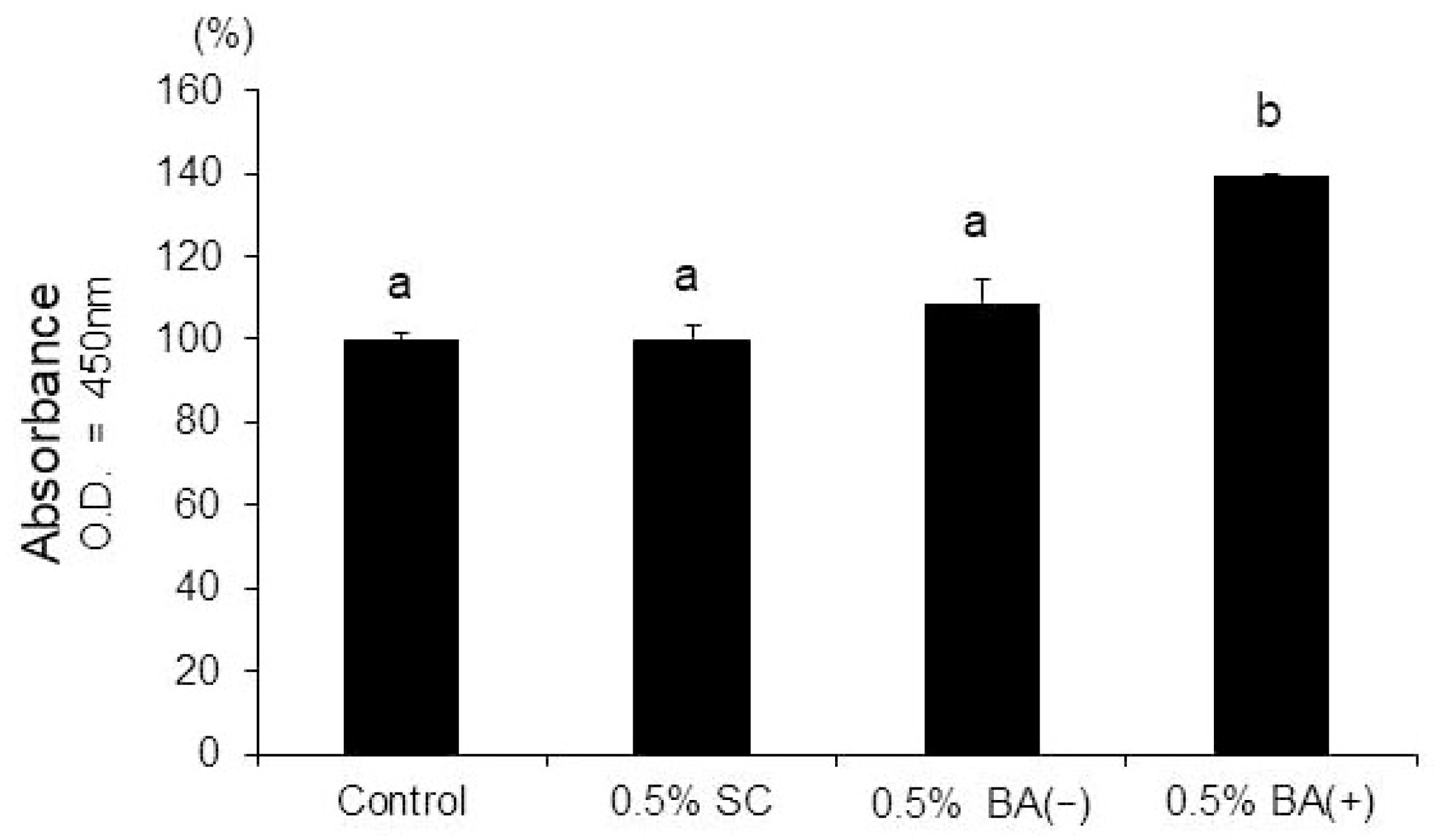
3.4. Mineralization
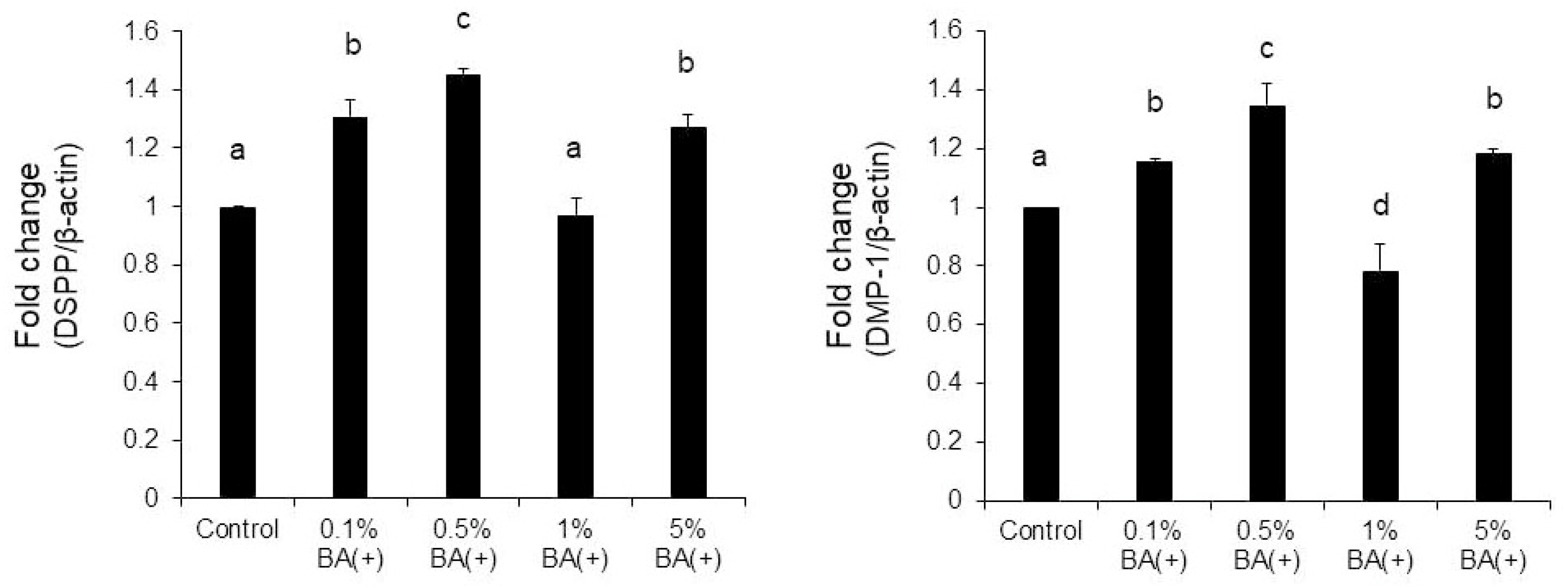
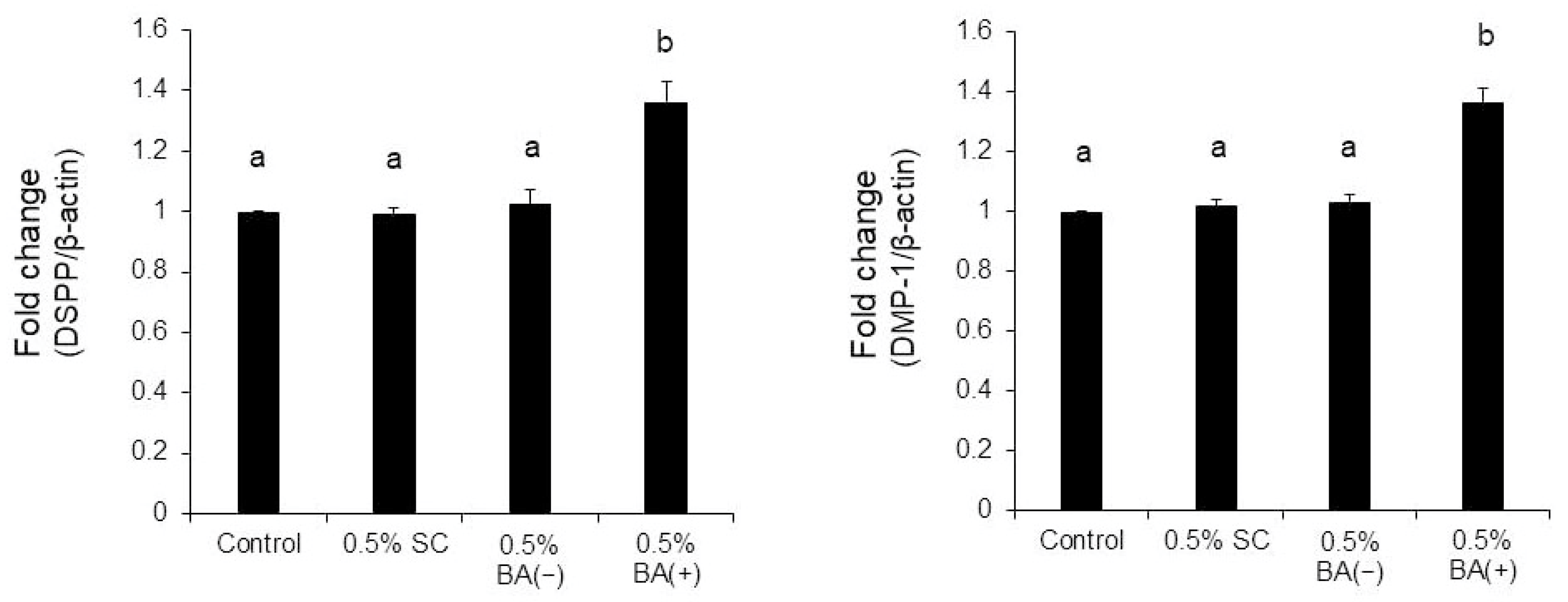
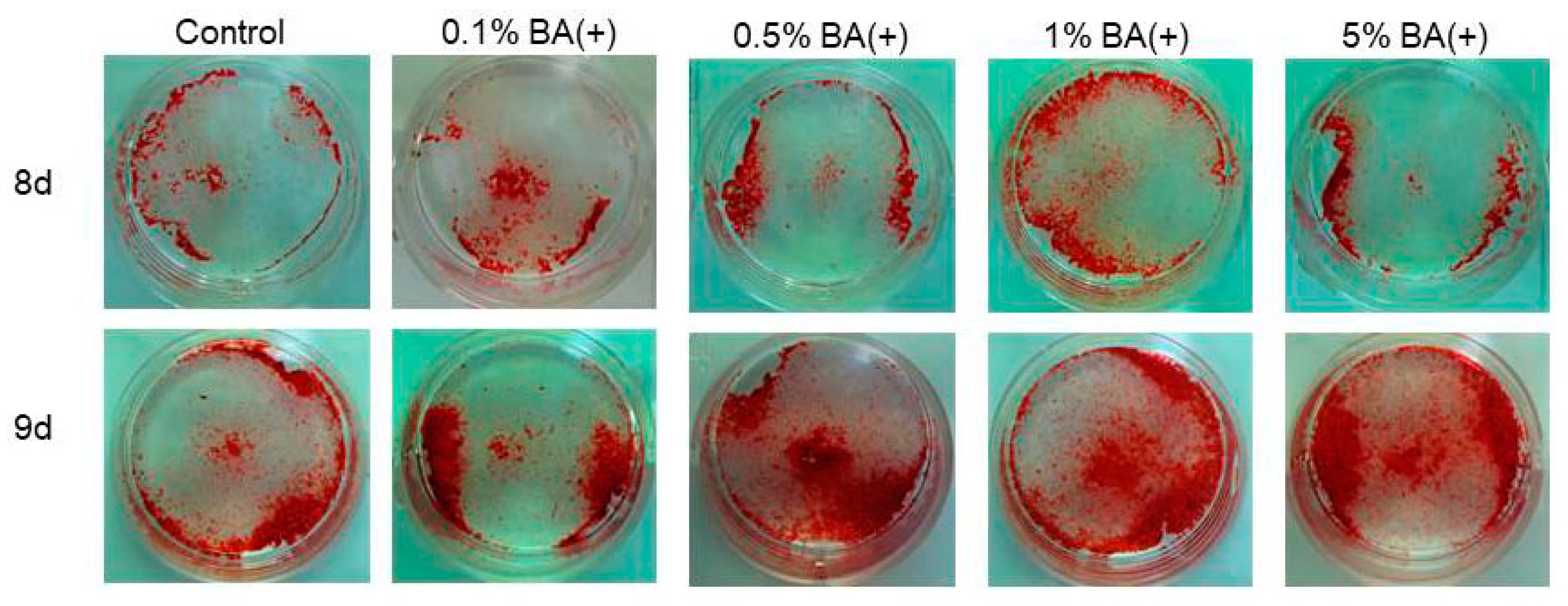


4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Selwitz, R.H.; Ismail, A.I.; Pitts, N.B. Dental caries. Lancet 2007, 369, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef]
- Stansbury, J.W. Curing dental resins and composites by photopolymerization. J. Esthet. Dent. 2000, 12, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Resin composite-state of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Tayas, M.J.; Anusavice, K.J.; Frencken, J.E.; Mount, G.J. Minimal intervention dentistry—A review. FDI commission project 1-97. Int. Dent. J. 2000, 50, 1–12. [Google Scholar] [CrossRef]
- Chalmers, J.M. Minimal intervention dentistry: Part 2. Strategies for addressing restorative challenges in older patients. J. Can. Dent. Assoc. 2006, 72, 435–440. [Google Scholar]
- Kunert, M.; Lukomska-Szymanska, M. Bio-inductive materials in direct and indirect pulp capping—A review article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef]
- Mente, J.; Hufnagel, S.; Leo, M.; Michel, A.; Gehrig, H.; Panagidis, D.; Saure, D.; Pfefferle, T. Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: Long-term results. J. Endod. 2014, 40, 1746–1751. [Google Scholar] [CrossRef]
- Poggio, C.; Ceci, M.; Dagna, A.; Beltrami, R.; Colombo, M.; Chiesa, M. In vitro cytotoxicity evaluation of different pulp capping materials: A comparative study. Arh. Hig. Rada. Toksikol. 2015, 66, 181–188. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Khademi, A. Outcomes of vital pulp therapy in permanent teeth with different medicaments based on review of the literature. Dent. Res. J. 2015, 12, 406–417. [Google Scholar]
- Qureshi, A.; Soujanya, E.; Nandakumar; Pratapkumar; Sambashivarao. Recent advances in pulp capping materials: An overview. J. Clin. Diagn. Res. 2014, 8, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Grewal, N.; Salhan, R.; Kaur, N.; Patel, H.B. Comparative evaluation of calcium silicate-based dentin substitute (Biodentine®) and calcium hydroxide (pulpdent) in the formation of reactive dentin bridge in regenerative pulpotomy of vital primary teeth: Triple blind, randomized clinical trial. Contemp. Clin. Dent. 2016, 7, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Dammaschke, T.; Stratmann, U.; Wolff, P.; Sagheri, D.; Schäfer, E. Direct pulp capping with mineral trioxide aggregate: An immunohistologic comparison with calcium hydroxide in rodents. J. Endod. 2010, 36, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, N.; Takigawa, T.; Horie, T.; Maeda, H.; Yamamoto, Y.; Momoi, Y.; Yamamoto, K.; Okiji, T. A review of the literature on the efficacy of mineral trioxide aggregate in conservative dentistry. Dent. Mater. J. 2019, 38, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, B.; Alaghehmand, H.; Kordafshari, T.; Daryakenari, G.; Ehsani, M.; Bijani, A. Coronal discoloration induced by calcium-enriched mixture, mineral trioxide aggregate and calcium hydroxide: A spectrophotometric analysis. Iran Endod. J. 2016, 11, 23–28. [Google Scholar]
- Shin, J.H.; Jang, J.H.; Park, S.H.; Kim, E. Effect of mineral trioxide aggregate surface treatments on morphology and bond strength to composite resin. J. Endod. 2014, 40, 1210–1216. [Google Scholar] [CrossRef]
- Savadi Oskoee, S.; Bahari, M.; Kimyai, S.; Motahhari, P.; Eghbal, M.J.; Asgary, S. Shear bond strength of calcium enriched mixture cement and mineral trioxide aggregate to composite resin with two different adhesive systems. J. Dent. 2014, 11, 665–671. [Google Scholar]
- Marconyak, L.J.; Kirkpatrick, T.C.; Roberts, H.W.; Roberts, M.D.; Aparicio, A.; Himel, V.T.; Sabey, K.A. A comparison of coronal tooth discoloration elicited by various endodontic reparative materials. J. Endod. 2016, 42, 470–473. [Google Scholar] [CrossRef]
- Arandi, N.Z.; Thabet, M. Minimal intervention in dentistry: A literature review on Biodentine as a bioactive pulp capping material. BioMed Res. Int. 2021, 2021, 5569313. [Google Scholar] [CrossRef]
- German, M.J. Developments in resin-based composites. Dent. Mater. 2022, 232, 638–643. [Google Scholar] [CrossRef]
- Hashimoto, M.; Hirose, M.; Kitagawa, H.; Yamaguchi, S.; Imazato, S. Improving the durability of resin-dentin bonds with an antibacterial monomer MDPB. Dent. Mater. J. 2018, 37, 620–627. [Google Scholar] [CrossRef]
- Thongthai, P.; Kitagawa, H.; Noree, S.; Iwasaki, Y.; Liu, Y.; Laranjeira Abe, G.; Yamaguchi, S.; Imazato, S. Evaluation of the long-term antibiofilm effect of a surface coating with dual functionality of antibacterial and protein-repellent effects. Dent. Mater. J. 2022, 41, 189–196. [Google Scholar] [CrossRef]
- Moecke, S.E.; Silva, A.G.C.S.; Andrade, A.C.M.; Borges, A.B.; Torres, C.R.G. Efficacy of S-PRG filler varnishes on enamel caries remineralization. J. Dent. 2022, 119, 104074. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Mogi, M.; Miura, F.; Nakabayashi, N. Effect of 4-MET on bond strength and penetration of monomers into enamel. Dent. Mater. 1992, 8, 173–175. [Google Scholar] [CrossRef]
- Ito, S.; Iijima, M.; Motai, F.; Mizoguchi, I.; Saito, T. Effects of calcium salts of acidic monomers on mineral induction of phosphoprotein immobilized to agarose beads. J. Biomed. Mater. Res. A 2012, 100, 2760–2765. [Google Scholar] [CrossRef] [PubMed]
- Thaweboon, S.; Saito, T.; Nagano, K.; Thaweboon, B. Evaluation of an adhesive containing calcium salt of acidic monomers on inhibition of biofilm formation ofbacteria related to root caries. Key Engin. Mater. 2020, 853, 41–45. [Google Scholar] [CrossRef]
- Qiu, Y.J.; Tang, J.; Saito, T. A novel bio-active adhesive monomer induces odontoblast differentiation: A comparative study. Int. Endod. J. 2020, 53, 1413–1429. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Saito, T. Novel bioactive adhesive monomer CMET promotes odontogenic differentiation and dentin regeneration. Int. J. Mol. Sci. 2021, 22, 12728. [Google Scholar] [CrossRef]
- Mizuno, M.; Yasukawa, K.; Inoue, K. Insight into the mechanism of the stabilization of moloney murine leukaemia virus reverse transcriptase by eliminating RNase H activity. Biosci. Biotechnol. Biochem. 2010, 74, 440–442. [Google Scholar] [CrossRef]
- Villa, O.; Wohlfahrt, J.C.; Koldsland, O.C.; Brookers, S.J.; Lyngstadaas, S.P.; Aass, A.M.; Reseland, J.E. EMD in periodontal regenerative surgery modulates cytokine profiles: A randomised controlled clinical trial. Sci. Rep. 2016, 15, 23060. [Google Scholar] [CrossRef]
- Takayama, S.I.; Murakami, S. Efficacy of FGF-2 in periodontal regeneration in a case of severe intrabony defect and furcation involvement with 15-month follow-up. Clin. Adv. Periodontics 2021, 11, 74–79. [Google Scholar] [CrossRef] [PubMed]
- BaniHani, A.; Santamaría, R.M.; Hu, S.; Maden, M.; Albadri, S. Minimal intervention dentistry for managing carious lesions into dentine in primary teeth: An umbrella review. Eur. Arch. Paediatr. Dent. 2022, 23, 667–693. [Google Scholar] [CrossRef] [PubMed]
- Ritter, A.V. Posterior resin-based composite restorations: Clinical recommendations for optimal success. J. Esthet. Dent. 2001, 13, 88–99. [Google Scholar] [CrossRef]
- Chandrasekhar, V.; Rudrapati, L.; Badami, V.; Tummala, M. Incremental techniques in direct composite restoration. J. Conserv. Dent. 2017, 20, 386–391. [Google Scholar]
- Nakabayashi, N.; Nakamura, M.; Yasuda, N. Hybrid layer as a dentin-bonding mechanism. J. Esthet. Dent. 1991, 3, 133–138. [Google Scholar] [CrossRef]
- Sano, H.; Takatsu, T.; Ciucchi, B.; Horner, J.A.; Matthews, W.G.; Pashley, D.H. Nanoleakage: Leakage within the hybrid layer. Oper. Dent. 1995, 20, 18–25. [Google Scholar]
- Putzeys, E.; Duca, R.C.; Coppens, L.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. In-vitro transdentinal diffusion of monomers from adhesives. J. Dent. 2018, 75, 91–97. [Google Scholar] [CrossRef]
- Nishida, M.; Imazato, S.; Takahashi, Y.; Ebisu, S.; Ishimoto, T.; Nakano, T.; Yasuda, Y.; Saito, T. The influence of the antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide on the proliferation, differentiation and mineralization of odontoblast-like cells. Biomaterials 2010, 31, 1518–1532. [Google Scholar] [CrossRef]
- Bianchi, L.; Ribeiro, A.P.; Carrilho, M.R.; Pashley, D.H.; de Souza Costa, C.A.; Hebling, J. Cytotoxicity of adhesive systems of different hydrophilicities on cultured odontoblast-like cells. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 1498–1507. [Google Scholar] [CrossRef] [PubMed]
- Lagadic-Gossmann, D.; Huc, L.; Lecureur, V. Alterations of intracellular pH homeostasis in apoptosis: Origins and roles. Cell Death Differ. 2004, 11, 953–961. [Google Scholar] [CrossRef]
- Sreenath, T.; Thyagarajan, T.; Hall, B.; Longenecker, G.; D’Souza, R.; Hong, S.; Wright, J.T.; MacDougall, M.; Sauk, J.; Kulkarni, A.B. Dentin sialophosphoprotein knockout mouse teeth display widened predentin zone and develop defective dentin mineralization similar to human dentinogenesis imperfecta type III. J. Biol. Chem. 2003, 278, 24874–24880. [Google Scholar] [CrossRef] [PubMed]
- Napierala, D.; Sun, Y.; Maciejewska, I.; Bertin, T.K.; Dawson, B.; D’Souza, R.; Qin, C.; Lee, B. Transcriptional repression of the Dspp gene leads to dentinogenesis imperfecta phenotype in Col1a1-Trps1 transgenic mice. J. Bone Miner. Res. 2012, 27, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Y.; Ramachandran, A.; George, A. DSPP is essential for normal development of the dental-craniofacial complex. J. Dent. Res. 2016, 95, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Prasad, M.; Butler, W.T.; Qin, C. Dentin sialophosphoprotein in biomineralization. Connect. Tissue Res. 2010, 51, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Cook, R.G.; Orkiszewski, R.S.; Butler, W.T. Identification and characterization of the carboxyl-terminal region of rat dentin sialoprotein. J. Biol. Chem. 2001, 276, 904–909. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Dummer, P.M. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int. Endod. J. 2011, 44, 697–730. [Google Scholar] [CrossRef]
- Ju, Y.; Ge, J.; Ren, X.; Zhu, X.; Xue, Z.; Feng, Y.; Zhao, S. Cav1.2 of L-type calcium channel is a key factor for the differentiation of dental pulp stem cells. J. Endod. 2015, 41, 1048–1055. [Google Scholar] [CrossRef]
- Staquet, M.J.; Couble, M.L.; Roméas, A.; Connolly, M.; Magloire, H.; Hynes, R.O.; Clezardin, P.; Bleicher, F.; Farges, J.C. Expression and localisation of alphav integrins in human odontoblasts. Cell Tissue Res. 2006, 323, 457–463. [Google Scholar] [CrossRef]

| Bonding Agent | Components | Chemical Formulation |
|---|---|---|
| Bioactive universal bond (Code: DP-023) (BA bond) + functional brush | Bonding agent | MDP, HEMA, ethanol, silane compound, others |
| Functional brush | CMET, others | |
| Scotchbond Universal Plus adhesive (SC, 3M ESPE, USA) | Bonding agent | MDP, HEMA, dimethacrylates, vitrebond copolymer, filler, photoinitiator, ethanol, water, silane |
| Gene Name | Forward (3′ to 5′) | Backward (5′ to 3′) | Fragment Size (bp) | Tm |
|---|---|---|---|---|
| DSPP | TCAATGGCGGGTGCTTTAGA | TGCTCACTGCACAACATGAAGA | 111 | 62 |
| DMP-1 | CGTTCCTCTGGGGGCTGTCC | CCGGGATCATCGCTCTGCATC | 577 | 60 |
| β-Actin | AACCCTAAGGCCAACAGTGAAAAG | TCATGAAGTAGTCTGTGAGGT | 241 | 53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, Y.; Qiu, Y.; Altankhishig, B.; Matsuda, Y.; Hasan, M.R.; Saito, T. Novel Universal Bond Containing Bioactive Monomer Promotes Odontoblast Differentiation In Vitro. J. Funct. Biomater. 2023, 14, 506. https://doi.org/10.3390/jfb14100506
Rao Y, Qiu Y, Altankhishig B, Matsuda Y, Hasan MR, Saito T. Novel Universal Bond Containing Bioactive Monomer Promotes Odontoblast Differentiation In Vitro. Journal of Functional Biomaterials. 2023; 14(10):506. https://doi.org/10.3390/jfb14100506
Chicago/Turabian StyleRao, Yaxin, Youjing Qiu, Bayarchimeg Altankhishig, Yasuhiro Matsuda, Md Riasat Hasan, and Takashi Saito. 2023. "Novel Universal Bond Containing Bioactive Monomer Promotes Odontoblast Differentiation In Vitro" Journal of Functional Biomaterials 14, no. 10: 506. https://doi.org/10.3390/jfb14100506









