Hydroxyapatite-Based Coatings on Silicon Wafers and Printed Zirconia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Zirconia Substrate
2.2. HAp Solution
2.3. PEI-HAp Solution
2.4. PEI-HAp Coating on Zirconia
2.5. Characterization of the PEI-HAp Coating
3. Results and Discussion
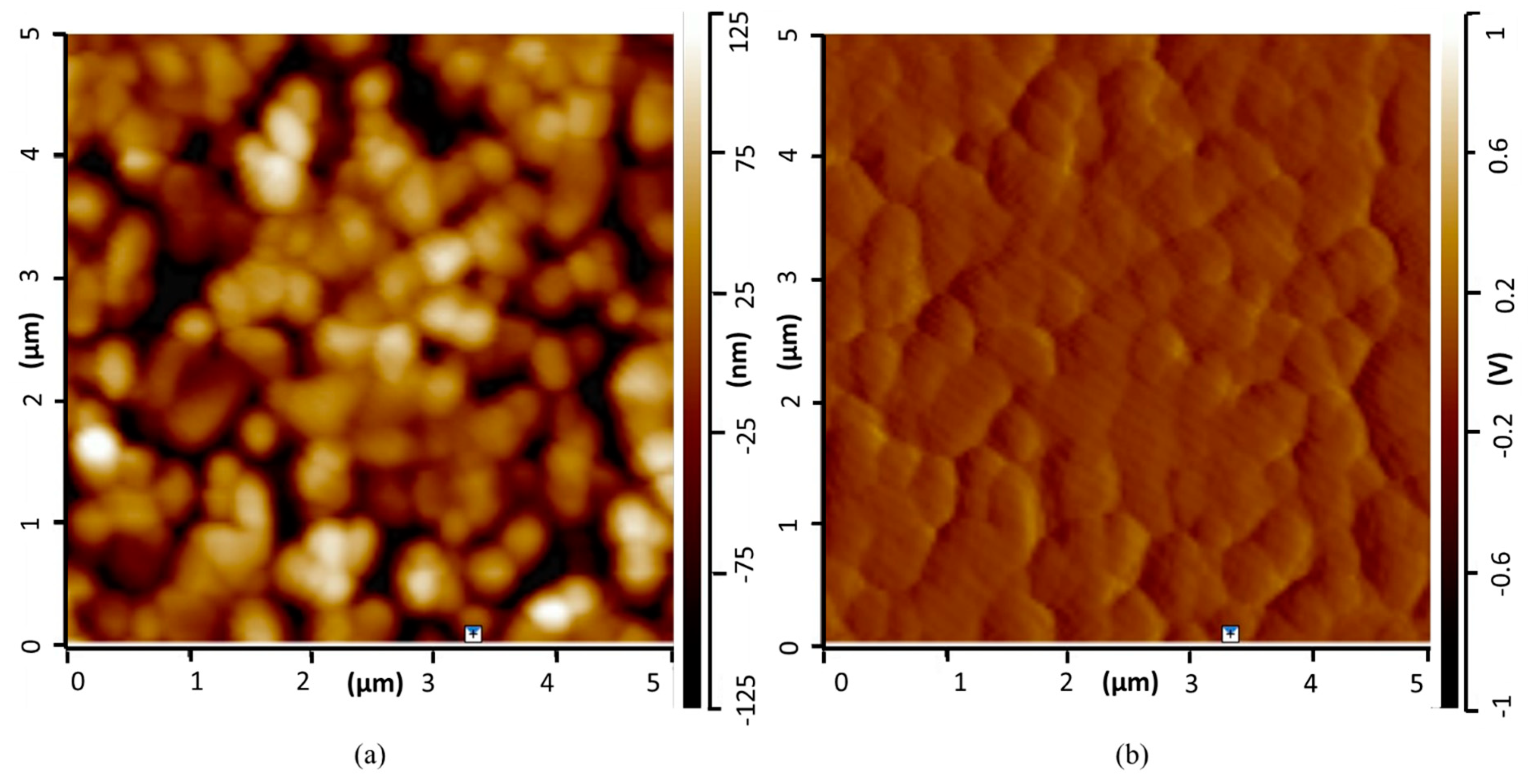
3.1. Coating Topography
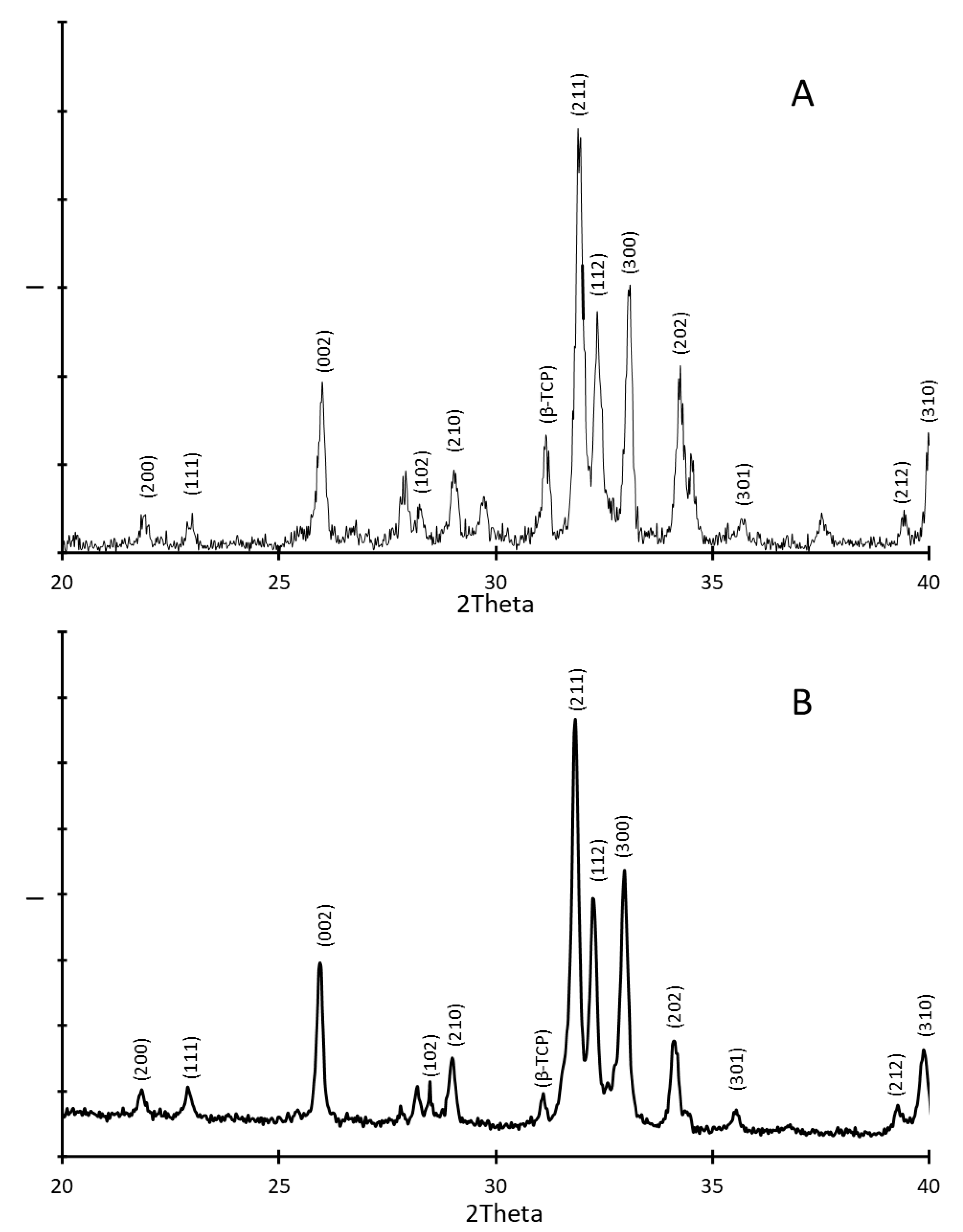
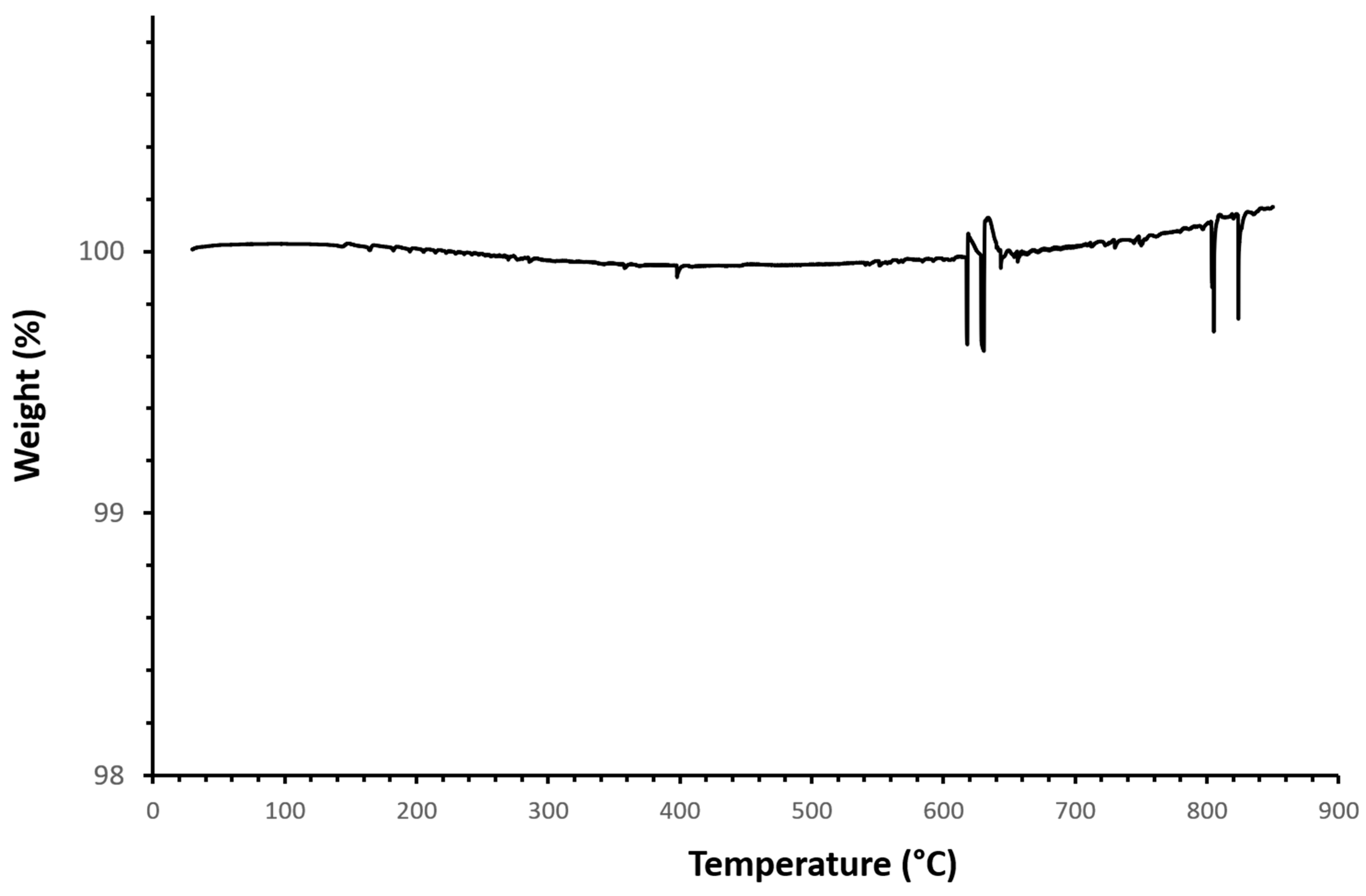
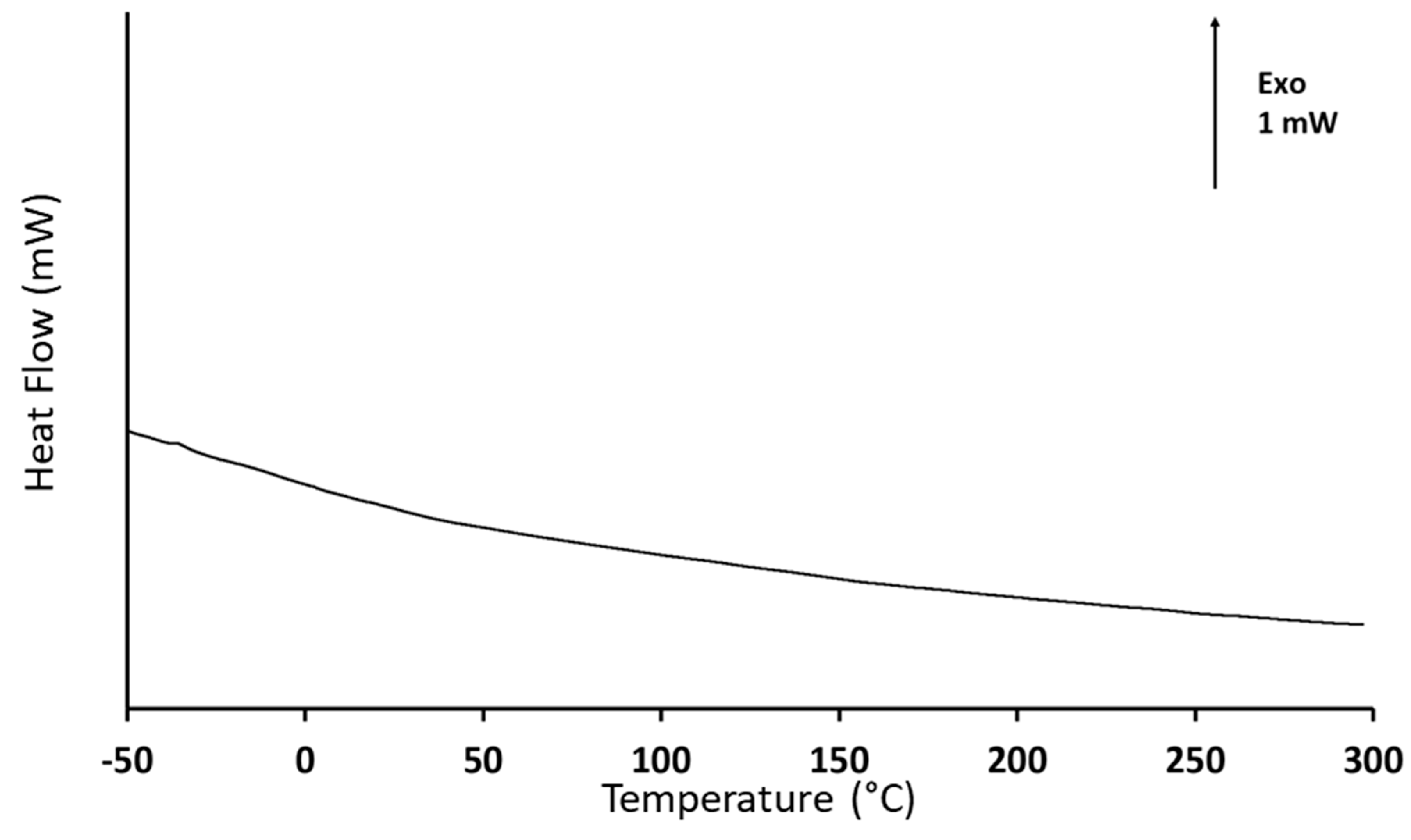
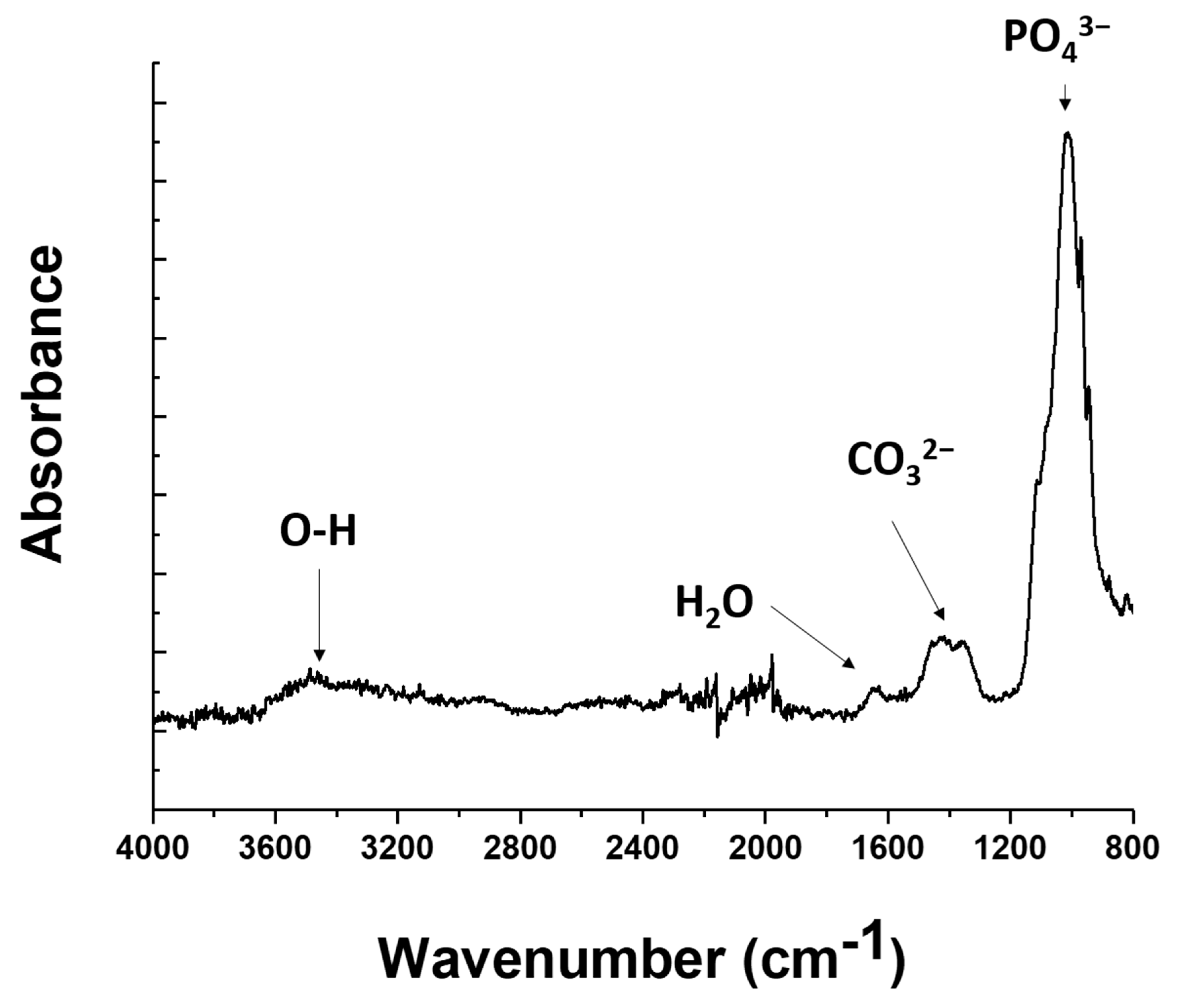
3.2. Coating Characterization
3.3. Coating Morphology on Printed Zirconia
3.4. Benefits and Drawbacks of DLP and Organic–Mineral Interactions for HAp Coating
4. Conclusions
- Zirconia substrate obtained via digital light processing (DLP) can be used as a scaffold for coating deposition.
- The coating of HAp on porous substrates is not uniform and its adhesion is poor. The results are significantly better when Hap is mixed with PEI.
- The adhesion of HAp mixed with PEI is possible on several surfaces including silicon and zirconia in the present case.
- A subsequent annealing procedure successfully removes PEI. It does not damage the HAp coating.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Londoño, J.J.; Ramos, A.M.; Correa, S.A.; Mesnard, M. Review of expandable dental implants. Brit. J. Oral Max. Surg. 2021, 59, 546–554. [Google Scholar] [CrossRef]
- De Stefano, M.; Lanza, A.; Faia, E.; Ruggiero, A. A novel ultrashort dental implant design for the reduction of the bone stress/strain: A comparative numerical investigation. Biomed. Eng. Adv. 2023, 5, 100077. [Google Scholar] [CrossRef]
- Panchal, M.; Khare, S.; Khamkar, P.; Bhole, K.S. Dental implants: A review of types, design analysis, materials, additive manufacturing methods, and future scope. Mater. Today Proc. 2022, 68, 1860–1867. [Google Scholar] [CrossRef]
- Matsko, A.; França, R. Design, manufacturing, and clinical outcomes for additively manufactured titanium dental implants: A systematic review. Dent. Rev. 2022, 2, 100041. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, W.; Tang, Y.; Yang, G.; Wu, X.; Lin, S.; Liu, X.; Cao, H.; Jiang, X. Preservation of alveolar ridge height through mechanical memory: A novel dental implant design. Bioact. Mater. 2021, 6, 75–83. [Google Scholar] [CrossRef]
- Moreira, A.; Madeira, S.; Buciumeanu, M.; Fialho, J.; Carvalho, A.; Silva, F.; Monteiro, F.J.; Caramês, J. Design and surface characterization of micropatterned silica coatings for zirconia dental implants. J. Mech. Behav. Biomed. 2022, 126, 105060. [Google Scholar] [CrossRef]
- Dantas, T.A.; Pinto, P.; Vaz, P.C.S.; Silva, F.S. Design and optimization of zirconia functional surfaces for dental implants applications. Ceram. Int. 2020, 46, 16328–16336. [Google Scholar] [CrossRef]
- Zanetti, E.M.; Pascoletti, G.; Cali, M.; Bignardi, G.; Franceschini, G. Clinical Assessment of Dental Implant Stability During Follow-Up: What Is Actually Measured, and Perspectives. Biosensors 2018, 8, 68. [Google Scholar] [CrossRef]
- Kittur, N.; Oak, R.; Dekate, D.; Jadhav, S.; Dhatrak, P. Dental implant stability and its measurements to improve osseointegration at the bone-implant interface: A review. Mater. Today Proc. 2021, 43, 1064–1070. [Google Scholar] [CrossRef]
- Qin, J.; Yang, D.; Maher, S.; Lima-Marques, L.; Zhou, Y.; Chen, Y.; Atkins, G.J.; Losic, D. Micro- and nano-structured 3D printed titanium implants with a hydroxyapatite coating for improved osseointegration. J. Mater. Chem. B 2018, 6, 3136–3144. [Google Scholar] [CrossRef]
- Olander, J.; Ruud, A.; Wennerberg, A.; Stenport, V.F. Wear particle release at the interface of dental implant components: Effects of different material combinations. An in vitro study. Dent. Mater. 2022, 38, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Das, I.; Chattopadhyay, S.; Mahato, A.; Kundu, B.; De, G. Fabrication of a cubic zirconia nanocoating on a titanium dental implant with excellent adhesion, hardness and biocompatibility. RSC Adv. 2016, 6, 59030–59038. [Google Scholar] [CrossRef]
- Bose, S.; Koski, C.; Vu, A.A. Additive manufacturing of natural biopolymers and composites for bone tissue engineering. Mater. Horiz. 2020, 7, 2011–2027. [Google Scholar] [CrossRef]
- Zou, R.; Bi, L.; Huang, Y.; Wang, Y.; Wang, Y.; Li, L.; Liu, J.; Feng, L.; Jiang, X.; Deng, B. A biocompatible silicon nitride dental implant material prepared by digital light processing technology. J. Mech. Behav. Biomed. 2023, 141, 105756. [Google Scholar] [CrossRef]
- Hasan, J.; Bright, R.; Hayles, A.; Palms, D.; Zilm, P.; Barker, D.; Vasilev, K. Preventing peri-implantitis: The quest for a next generation of titanium dental implants. ACS Biomater. Sci. Eng. 2022, 8, 4697–4737. [Google Scholar] [CrossRef]
- Gallegos, S.I.; Parsaei, S.; Siddiqui, D.A.; Biguetti, C.C.; Palmer, K.L.; Rodrigues, D.C. Can dental cement composition affect dental implant success? ACS Biomater. Sci. Eng. 2019, 5, 5116–5127. [Google Scholar] [CrossRef]
- Milone, D.; Fiorillo, L.; Alberti, F.; Cervino, G.; Filardi, V.; Pistone, A.; Cicciù, M.; Risitano, G. Stress distribution and failure analysis comparison between Zirconia and Titanium dental implants. Procedia Struct. Integr. 2022, 41, 680–691. [Google Scholar] [CrossRef]
- Kitagawa, I.L.; Miyazaki, C.M.; Pitol-Palin, L.; Okamoto, R.; de Vasconcellos, L.M.R.; Constantiono, C.J.L.; Lisboa-Filho, P.N. Titanium-based alloy surface modification with TiO2 and poly(sodium 4-styrenesulfonate) multilayers for dental implants. ACS Appl. Bio Mater. 2021, 4, 3055–3066. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.-H.; Shin, Y.C.; Lee, S.-H.; Lee, S.-H.; Kang, M.S.; Lee, M.-S.; Lee, J.H.; Lee, J.-H.; Han, D.-W.; Kim, B. Dental implants with electrochemical nanopattern formation to increase osseointegration. J. Ind. Eng. Chem. 2022, 116, 543–555. [Google Scholar] [CrossRef]
- Wu, Y.; Feng, F.; Xin, H.; Li, K.; Tang, Z.; Guo, Y.; Qin, D.; An, B.; Diao, X.; Dou, C. Fracture strength and osseointegration of an ultrafine-grained titanium mini dental implant after macromorphology optimization. ACS Biomater. Sci. Eng. 2019, 5, 4122–4130. [Google Scholar] [CrossRef]
- Gautam, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia based dental ceramics: Structure, mechanical properties, biocompatibility and applications. Dalton Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhao, M.; Deng, H.; Liu, Z.; Wang, L.; Ge, L. Facile and versatile strategy for fabrication of highly bacteriostatic and biocompatible SLA-Ti surfaces with the regulation of Mg/Cu coimplantation ratio for dental implant applications. Colloids Surf. B 2023, 223, 113180. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, E.; Çalişkan, F. A new functional graded dental implant design with biocompatible and antibacterial properties. Mater. Chem. Phys. 2022, 277, 125481. [Google Scholar] [CrossRef]
- Jayasree, A.; Gómez-Cerezo, M.N.; Verron, E.; Ivanovski, S.; Gulati, K. Gallium-doped dual micro-nano titanium dental implants towards soft-tissue integration and bactericidal functions. Mater. Today Adv. 2022, 16, 100297. [Google Scholar] [CrossRef]
- Ren, X.; van der Mei, H.C.; Ren, Y.; Busscher, H.J. Keratinocytes protect soft-tissue integration of dental implant materials against bacterial challenges in a 3D-tissue infection model. Acta Biomater. 2019, 96, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Mühl, A.; Szabó, P.; Krafcsik, O.; Aigner, Z.; Kopniczky, J.; Nagy, A.; Marada, G.; Turzó, K. Comparison of surface aspects of turned and anodized titanium dental implant, or abutment material for an optimal soft tissue integration. Heliyon 2022, 8, e10263. [Google Scholar] [CrossRef]
- Kim, J.C.; Lee, M.; Yeo, I.-S.L. Three interfaces of the dental implant system and their clinical effects on hard and soft tissues. Mater. Horiz. 2022, 9, 1387–1411. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, M.; Yang, Y.; Zheng, M.; Yang, Y.; Liu, X.; Tan, J. Biofunctionalization of zirconia with cell-adhesion peptides via polydopamine crosslinking for soft tissue engineering: Effects on the biological behaviors of human gingival fibroblasts and oral bacteria. RSC Adv. 2020, 10, 6200–6212. [Google Scholar] [CrossRef]
- Matter, M.T.; Maliqi, L.; Keevend, K.; Guimond, S.; Ng, J.; Armagan, E.; Rottmar, M.; Herrmann, I.K. One-step synthesis of versatile antimicrobial nano-architected implant coatings for hard and soft tissue healing. ACS Appl. Mater. Interfaces 2021, 13, 33300–33310. [Google Scholar] [CrossRef]
- Fischer, N.G.; Moussa, D.G.; Skoe, E.P.; De Jong, D.A.; Aparicio, C. Keratinocyte-specific peptide-based surfaces for hemidesmosome upregulation and prevention of bacterial colonization. ACS Biomater. Sci. Eng. 2020, 6, 4929–4939. [Google Scholar] [CrossRef]
- Fischer, N.G.; Munchow, E.A.; Tamerler, C.; Bottino, M.C.; Aparicio, C. Harnessing biomolecules for bioinspired dental biomaterials. J. Mater. Chem. B 2020, 8, 8713–8747. [Google Scholar] [CrossRef]
- De Avila, E.D.; Castro, A.G.B.; Tagit, O.; Krom, B.P.; Lőwik, D.; van Well, A.A.; Bannenberg, L.J.; Vergani, C.E.; van den Beucken, J.J.J.P. Anti-bacterial efficacy via drug-delivery system from layer-by-layer coating for percutaneous dental implant components. Appl. Surf. Sci. 2019, 488, 194–204. [Google Scholar] [CrossRef]
- Scarano, A.; Piattelli, M.; Caputi, S.; Favero, G.A.; Piattelli, A. Bacterial adhesion on commercially pure titanium and zirconium oxide disks: An in vivo human study. J. Periodontol. 2004, 75, 292–296. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, C.; Pita, M.S.; Fernandes, F.H.N.C.; Pedrazzi, V.; de Albuquerque Junior, R.F.; Ribeiro, R.F. Bacterial adhesion on the titanium and zirconia abutment surfaces. Clin. Oral Implant. Res. 2014, 25, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Caravaca, C.; Shi, L.; Balvay, S.; Rivory, P.; Laurenceau, E.; Chevolot, Y.; Hartmann, D.; Gremillard, L.; Chevalier, J. Direct silanization of zirconia for increased biointegration. Acta Biomater. 2016, 46, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Seesala, V.S.; Rajasekaran, R.; Ojha, A.K.; Mahato, A.; Korrayi, R.R.; Das, B.; Venugopal, S.P.; Roy, S.; Dhara, S. A novel functional gradient hydroxyapatite coating for zirconia-based implants. Surf. Coat. Technol. 2023, 469, 129817. [Google Scholar] [CrossRef]
- Gledhill, H.C.; Turner, I.G.; Doyle, C. In vitro dissolution behaviour of two morphologically different thermally sprayed hydroxyapatite coatings. Biomaterials 2001, 22, 695–700. [Google Scholar] [CrossRef]
- Heimann, R.B. Plasma-sprayed hydroxylapatite-based coatings: Chemical, mechanical, microstructural, and biomedical properties. J. Therm. Spray Technol. 2016, 25, 827–850. [Google Scholar] [CrossRef]
- Bøe, B.G.; Röhrl, S.M.; Heier, T.; Snorrason, F.; Nordsletten, L. A prospective randomized study comparing electrochemically deposited hydroxyapatite and plasma-sprayed hydroxyapatite on titanium stems. Acta Orthop. 2011, 82, 13–19. [Google Scholar] [CrossRef]
- Ganvir, A.; Nagar, S.; Markocsan, N.; Balani, K. Deposition of hydroxyapatite coatings by axial plasma spraying: Influence of feedstock characteristics on coating microstructure, phase content and mechanical properties. J. Eur. Ceram. 2021, 41, 4637–4649. [Google Scholar] [CrossRef]
- Ansari, Z.; Kalantar, M.; Kharaziha, M.; Ambrosio, L.; Raucci, M.G. Polycaprolactone/fluoride substituted-hydroxyapatite (PCL/FHA) nanocomposite coatings prepared by in-situ sol-gel process for dental implant applications. Prog. Org. Coat. 2020, 147, 105873. [Google Scholar] [CrossRef]
- González-Estrada, O.A.; Pertuz Comas, A.D.; Ospina, R. Characterization of hydroxyapatite coatings produced by pulsed-laser deposition on additive manufacturing Ti6Al4V ELI. Thin Solid Films 2022, 763, 139592. [Google Scholar] [CrossRef]
- Lissandrello, F.; Magagnin, L. Pulsed electrochemical deposition of calcium phosphate coatings for biomedical applications. Trans. Inst. Mater. Finish. 2023, 101, 173–178. [Google Scholar] [CrossRef]
- Bianchi, M.; Lopomo, N.; Boi, M.; Gambardella, A.; Marchiori, G.; Berni, M.; Pavan, P.; Marcacci, M.; Russo, A. Ceramic thin films realized by means of pulsed plasma deposition technique: Applications for orthopedics. J. Mech. Med. Biol. 2015, 15, 1540002. [Google Scholar] [CrossRef]
- Garrido, B.; Martin-Morata, A.; Dosta, S.; Cano, I.G. Improving the bond strength of bioactive glass coatings obtained by atmospheric plasma spraying. Surf. Coat. Technol. 2023, 470, 129837. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, B.; Gong, Y.; Zhou, P.; Li, H. Mechanical properties of nanodiamond-reinforced hydroxyapatite composite coatings deposited by suspension plasma spraying. Appl. Surf. Sci. 2018, 439, 60–65. [Google Scholar] [CrossRef]
- Oyane, A.; Kakehata, M.; Sakamaki, I.; Pyatenko, A.; Yashiro, H.; Ito, A.; Torizuka, K. Biomimetic apatite coating on yttria-stabilized tetragonal zirconia utilizing femtosecond laser surface processing. Surf. Coat. Technol. 2016, 296, 88–95. [Google Scholar] [CrossRef]
- Singh, P.P.; Dixit, K.; Sinha, N. A sol-gel based bioactive glass coating on laser textured 316L stainless steel substrate for enhanced biocompatibility and anti-corrosion properties. Ceram. Int. 2022, 48, 18704–18715. [Google Scholar] [CrossRef]
- García-Arnáez, I.; Cerqueira, A.; Romero-Gavilán, F.; Elortza, F.; Azkargorta, M.; Iloro, I.; Suay, J.; Goñi, I.; Gurruchaga, M. Development and characterisation of strontium-doped sol-gel coatings to optimise the initial bone regeneration processes. Mater. Today Commun. 2022, 33, 104674. [Google Scholar] [CrossRef]
- Catauro, M.; Bollino, F.; Giovanardi, R.; Veronesi, P. Modification of Ti6Al4V implant surfaces by biocompatible TiO2/PCL hybrid layers prepared via sol-gel dip coating: Structural characterization, mechanical and corrosion behavior. Mater. Sci. Eng. C 2017, 74, 501–507. [Google Scholar] [CrossRef]
- Alavi, S.E.; Panah, N.; Page, F.; Gholami, M.; Dastfal, A.; Sharma, L.A.; Shahmabadi, H.E. Hydrogel-based therapeutic coatings for dental implants. Eur. Polym. J. 2022, 181, 111652. [Google Scholar] [CrossRef]
- Vieira, M.; Tavares, C.R.; Bergamasco, R.; Petrus, J.C.C. Application of ultrafiltration-complexation process for metal removal from pulp and paper industry wastewater. J. Membr. Sci. 2001, 194, 273–276. [Google Scholar] [CrossRef]
- Lin, X.; Tran, D.T.; Song, M.-H.; Yun, Y.-S. Development of polyethyleneimine-starch fibers stable over the broad pH range for selective adsorption of gold from actual leachate solutions of waste electrical and electronic equipment. J. Clean. Prod. 2021, 328, 129545. [Google Scholar] [CrossRef]
- Li, S.; Tu, L.; Lu, Y.; Lin, M.; Ma, J.; Bai, C.; Gao, C.; Xue, L. Polyethyleneimine (PEI) based thin film nanocomposite (TFN) total heat exchange membranes (THEMs) composed of shaped ZIF-8 crystalline micro-leaves (ZIF-L). Sep. Purif. Technol. 2023, 324, 124435. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, F.-G.; Hu, P.; Chen, Z. Interaction of polyethylenimine with model cell membranes studied by linear and nonlinear spectroscopic techniques. J. Phys. Chem. C 2014, 118, 12195–12205. [Google Scholar] [CrossRef]
- Kong, L.; Mu, Z.; Yu, Y.; Zhang, L.; Hu, J. Polyethyleneimine-stabilized hydroxyapatite nanoparticles modified with hyaluronic acid for targeted drug delivery. RSC Adv. 2016, 6, 101790–101799. [Google Scholar] [CrossRef]
- Li, X.; Zhong, H.; Zhang, J.; Duan, Y.; Bai, H.; Li, J.; Jiang, D. Dispersion and properties of zirconia suspensions for stereolithography. Int. J. Appl. Ceram. Technol. 2020, 17, 239–247. [Google Scholar] [CrossRef]
- Borlaf, M.; Serra-Capdevila, A.; Colominas, C.; Graule, T. Development of UV-curable ZrO2 slurries for additive manufacturing (LCM-DLP) technology. J. Eur. Ceram. Soc. 2019, 39, 3797–3803. [Google Scholar] [CrossRef]
- Jang, K.J.; Kang, J.H.; Fisher, J.G.; Park, S.W. Effect of the volume fraction of zirconia suspensions on the microstructure and physical properties of products produced by additive manufacturing. Dent. Mater. 2019, 35, e97–e106. [Google Scholar] [CrossRef]
- Coppola, B.; Schmitt, J.; Lacondemine, T.; Tardivat, C.; Montanaro, L.; Palmero, P. Digital light processing stereolithography of zirconia ceramics: Slurry elaboration and orientation-reliant mechanical properties. J. Eur. Ceram. Soc. 2022, 42, 2974–2982. [Google Scholar] [CrossRef]
- Ji, S.H.; Da, S.K.; Park, M.S.; Ji, S.Y. Sintering process optimization for 3YSZ ceramic 3D-printed objects manufactured by stereolithography. Nanomaterials 2021, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Snyder, N. Digital Light Processing (DLP) of Yttria-Stabilized-Zirconia (YSZ). Master’s Thesis, The University of Nebraska Lincoln, Lincoln, NE, USA, May 2022. [Google Scholar]
- Manafi, S.A.; Yazdani, B.; Rahimiopour, M.R.; Sadrnezhaad, S.K.; Amin, M.H.; Razavi, M. Synthesis of nano-hydroxyapatite under a sonochemical/hydrothermal condition. Biomed. Mater. 2008, 3, 025002. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.H.; Pai, Y.; Chang, S. Effect of thermal treatment of the hydroxyapatite powders on the micropore and microstructure of porous biphasic calcium phosphate composite granules. J. Biomater. Nanobiotechnol. 2013, 4, 114–118. [Google Scholar] [CrossRef]
- Bohner, M.; Le Gars Santoni, B.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef] [PubMed]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Lin, H.K.; Pan, Y.H.; Salamanca, E.; Lin, Y.T.; Chang, W.J. Prevention of bone resorption by ha/β-TCP + collagen composite after tooth extraction: A case series. Int. J. Environ. Res. Public Health 2019, 16, 4616. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.T.; Nam, N.H.; Hong, C.T.; Dac, D.V.Q.; Thu, L.P.; Hai, D.T.; Osial, M.; Giersig, M.; Thanh, D.T.M. Apatite Ore-based Nanostructures: Novel and Eco-friendly Sorbent for Efficient Removal of Wastewater Containing Pb2+ and Fe3+. Water Air Soil Pollut. 2023, 234, 550. [Google Scholar] [CrossRef]
- Lazić, S.; Zec, S.; Miljević, N.; Milonjić, S. The effect of temperature on the properties of hydroxyapatite precipitated from calcium hydroxide and phosphoric acid. Thermochim. Acta 2001, 11, 13–22. [Google Scholar] [CrossRef]
- Jagadale, P.N.; Jagtap, P.P.; Joshi, M.G.; Bamane, S.R. A prototype synthesis and characterization of hydroxyapatite bioceramics nanocrystallites. Adv. Mater. Lett. 2016, 7, 325–329. [Google Scholar] [CrossRef]
- Safarzadeh, M.; Ramesh, S.; Tan, C.Y.; Chandran, H.; Ching, Y.C.; Noor, A.F.M.; Krishnasamy, S.; Teng, W.D. Sintering behaviour of carbonated hydroxyapatite prepared at different carbonate and phosphate ratios. Bol. Soc. Esp. Ceram. Vidr. 2020, 59, 73–80. [Google Scholar] [CrossRef]
- Román, F.; Colomer, P.; Calventus, Y.; Hutchinson, J.M. Study of hyperbranched poly(ethyleneimine) polymers of different molecular weight and their interaction with epoxy resin. Materials 2018, 11, 410. [Google Scholar] [CrossRef] [PubMed]
- Grenda, K.; Idström, A.; Evenäs, L.; Persson, M.; Holmberg, K.; Bordes, R. An analytical approach to elucidate the architecture of polyethyleneimines. J. Appl. Polym. Sci. 2022, 139, 51657. [Google Scholar] [CrossRef]
- Feng, C.F.; Khor, K.A.; Gu, Y.W.; Cheang, P. Analysis of phase changes in plasma-sprayed Ti-6Al-4V/hydroxyapatite composite coatings by DSC. Mater. Lett. 2001, 51, 88–93. [Google Scholar] [CrossRef]
- Payne, M.E.; Lou, Y.; Zhang, X.; Sahiner, N.; Sandoval, N.R.; Shantz, D.F.; Grayson, S.M. Comparison of cross-linked branched and linear poly(ethylene imine) microgel microstructures and their impact in antimicrobial behavior, copper chelation, and carbon dioxide capture. ACS Appl. Polym. Mater. 2020, 2, 826–836. [Google Scholar] [CrossRef]
- Chang, M.C.; Tanaka, J. FT-IR study for hydroxyapatite/collagen nanocomposite cross-linked by glutaraldehyde. Biomaterials 2002, 23, 4811–4818. [Google Scholar] [CrossRef] [PubMed]
- Abidi, S.S.A.; Murtaza, Q. Synthesis and characterization of nano-hydroxyapatite powder using wet chemical precipitation reaction. J. Mater. Sci. Technol. 2014, 30, 307–310. [Google Scholar] [CrossRef]
- Prekajski, M.; Mirković, M.; Todorović, B.; Matković, A.; Marinović-Cincović, M.; Luković, J.; Matović, B. Ouzo effect—New simple nanoemulsion method for synthesis of strontium hydroxyapatite nanospheres. J. Eur. Ceram. Soc. 2016, 36, 1293–1298. [Google Scholar] [CrossRef]
- Jiang, B.; Ke, S.; Yang, B.; Chen, J.; Li, W.; Fang, M.; Huang, Z.; Sun, J.; Min, X.; Hu, X. Interfacial characteristics of bioglass diffusion zone between scaffold-like hydroxyapatite/wollastonite coating and dense zirconia substrate. Ceram. Int. 2023, 49, 37388–37395. [Google Scholar] [CrossRef]

| Methodology | Objectives | Results |
|---|---|---|
| Pulsed laser deposition [42] |
|
|
| Pulsed electrochemical deposition [43] |
|
|
| Pulsed plasma deposition [44] |
|
|
| Atmospheric plasma spraying [45] |
|
|
| Suspension plasma spraying [46] |
|
|
| Axial suspension plasma spraying [40] |
|
|
| In-situ sol–gel process [41] |
|
|
| DLP + dip-coating [this study] |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chauvin, A.; Garda, M.-R.; Snyder, N.; Cui, B.; Delpouve, N.; Tan, L. Hydroxyapatite-Based Coatings on Silicon Wafers and Printed Zirconia. J. Funct. Biomater. 2024, 15, 11. https://doi.org/10.3390/jfb15010011
Chauvin A, Garda M-R, Snyder N, Cui B, Delpouve N, Tan L. Hydroxyapatite-Based Coatings on Silicon Wafers and Printed Zirconia. Journal of Functional Biomaterials. 2024; 15(1):11. https://doi.org/10.3390/jfb15010011
Chicago/Turabian StyleChauvin, Antoine, Marie-Rose Garda, Nathan Snyder, Bai Cui, Nicolas Delpouve, and Li Tan. 2024. "Hydroxyapatite-Based Coatings on Silicon Wafers and Printed Zirconia" Journal of Functional Biomaterials 15, no. 1: 11. https://doi.org/10.3390/jfb15010011




