Apatite-Forming Ability and Visible Light-Enhanced Antibacterial Activity of CuO-Supported TiO2 Formed on Titanium by Chemical and Thermal Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Surface Analysis of Samples
2.3. Evaluation of In Vitro Apatite-Forming Ability of Sample
2.4. Measurement of Cu Ion-Release Behavior of Sample
2.5. Evaluation of Antimicrobial Activity of Sample
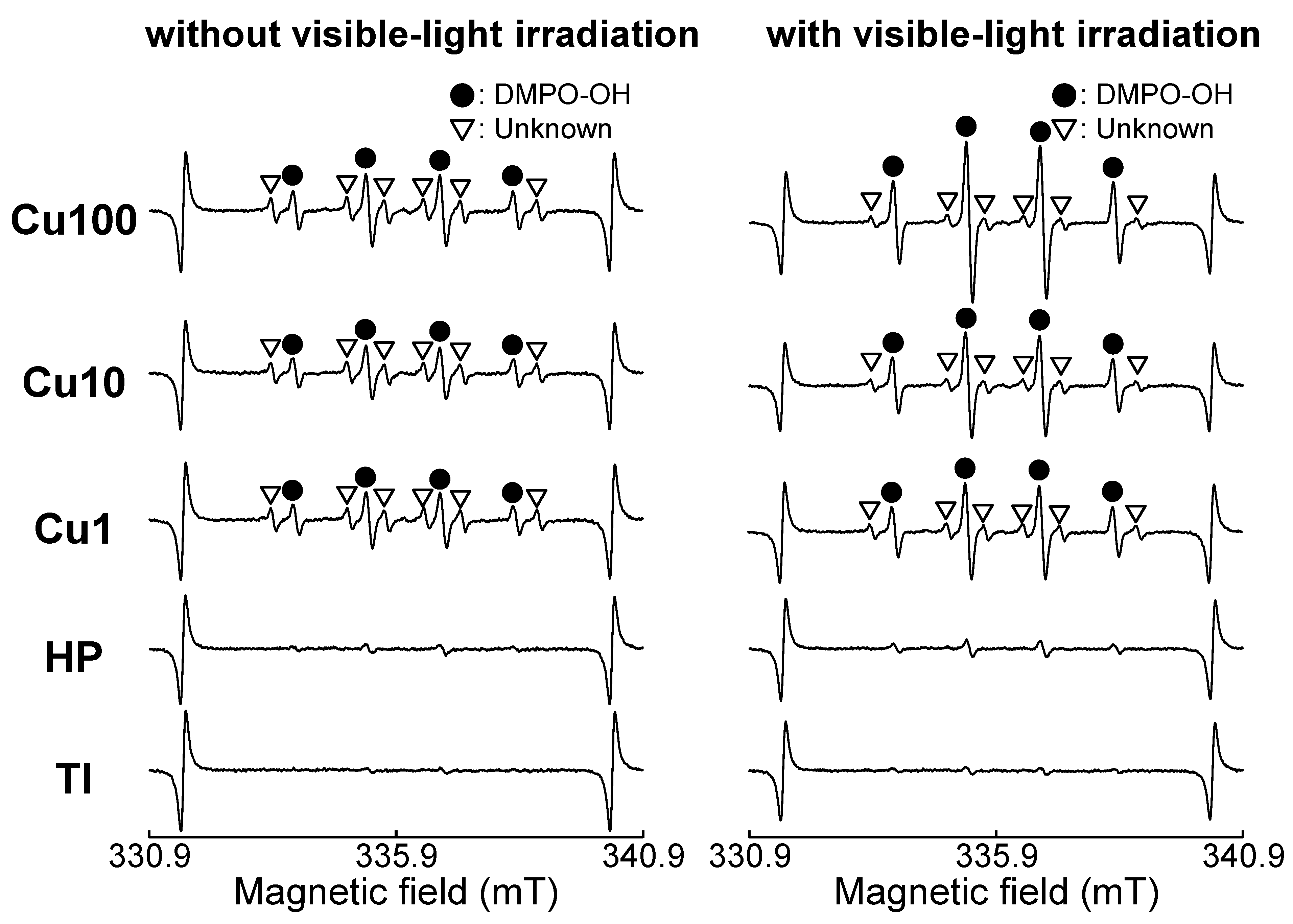
2.6. Measurement of ROS Generated by Samples
2.7. Statistical Analysis
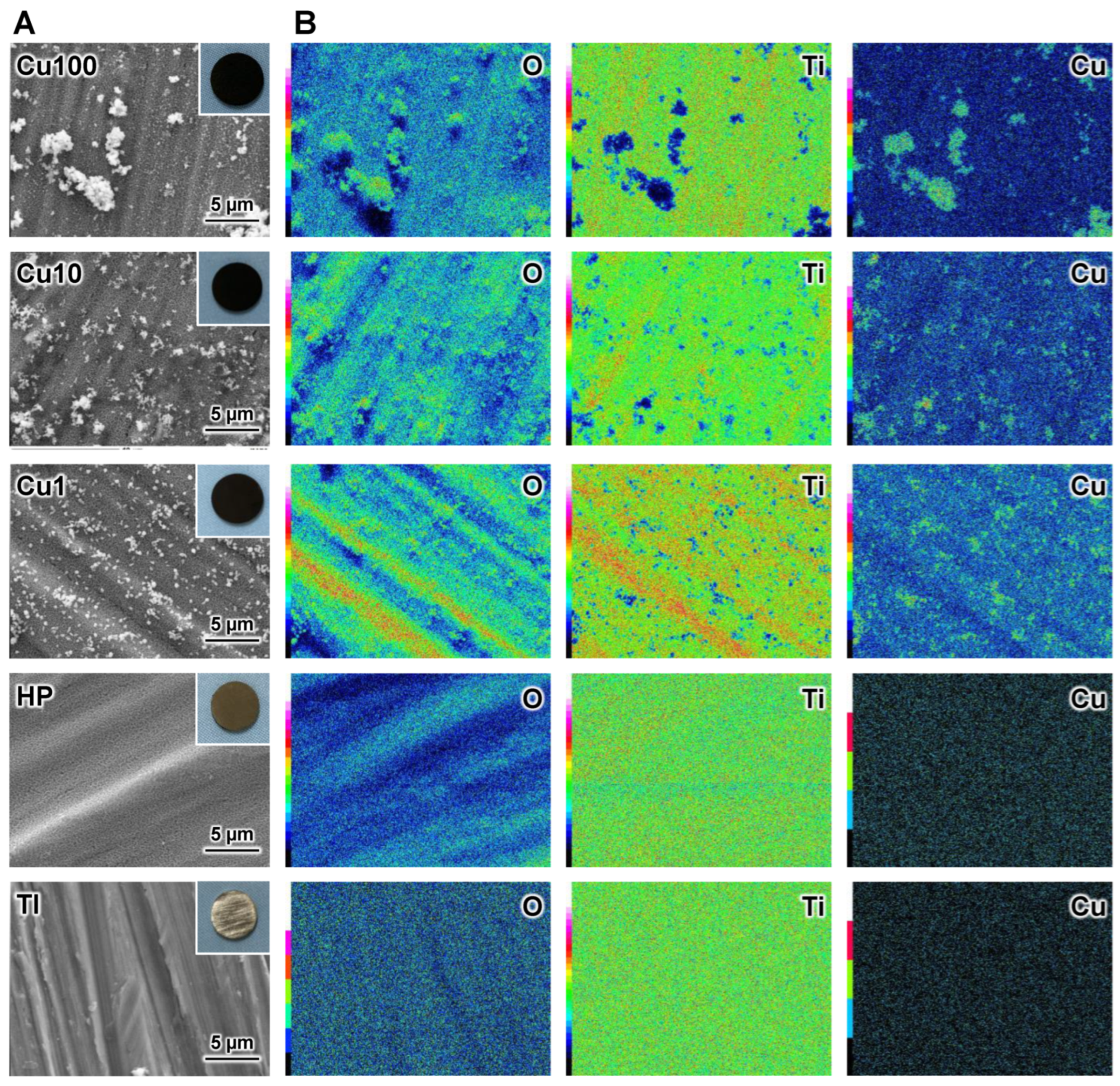
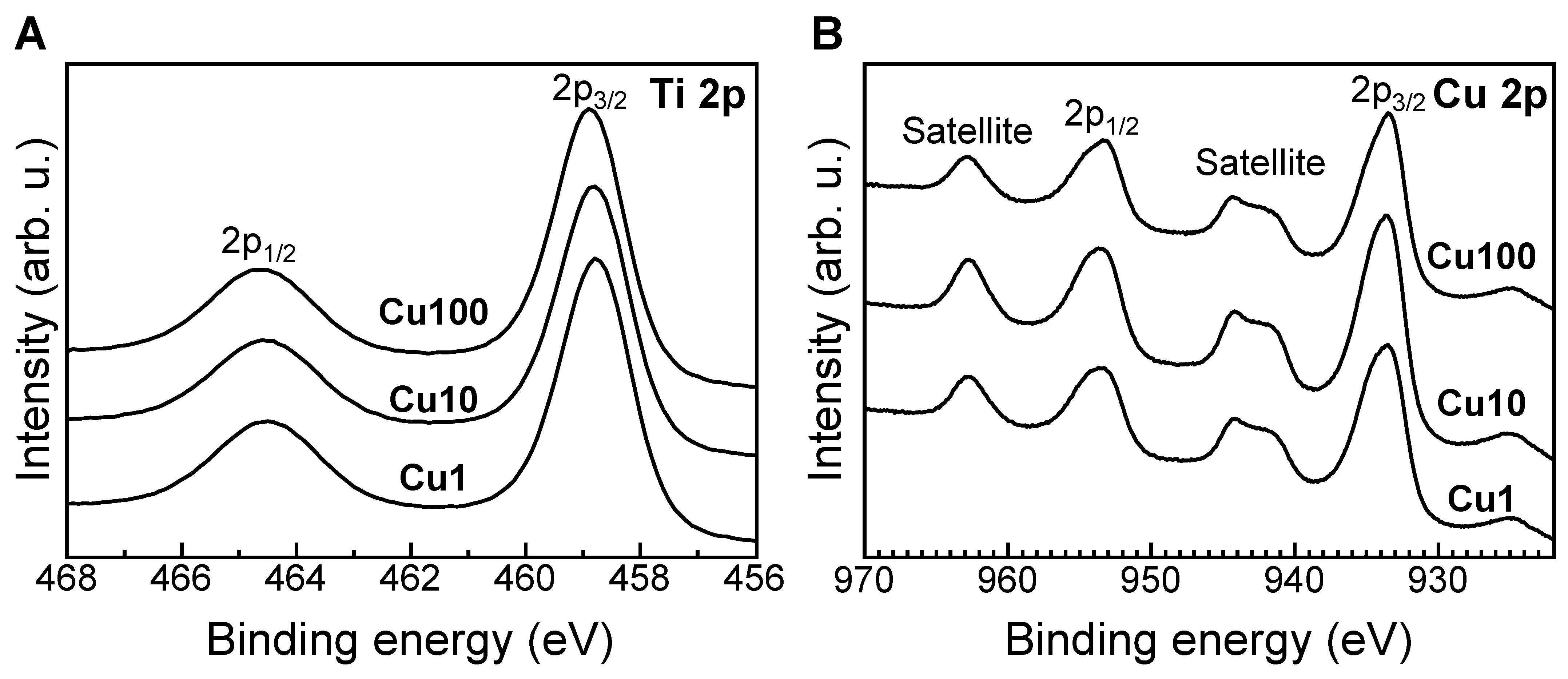
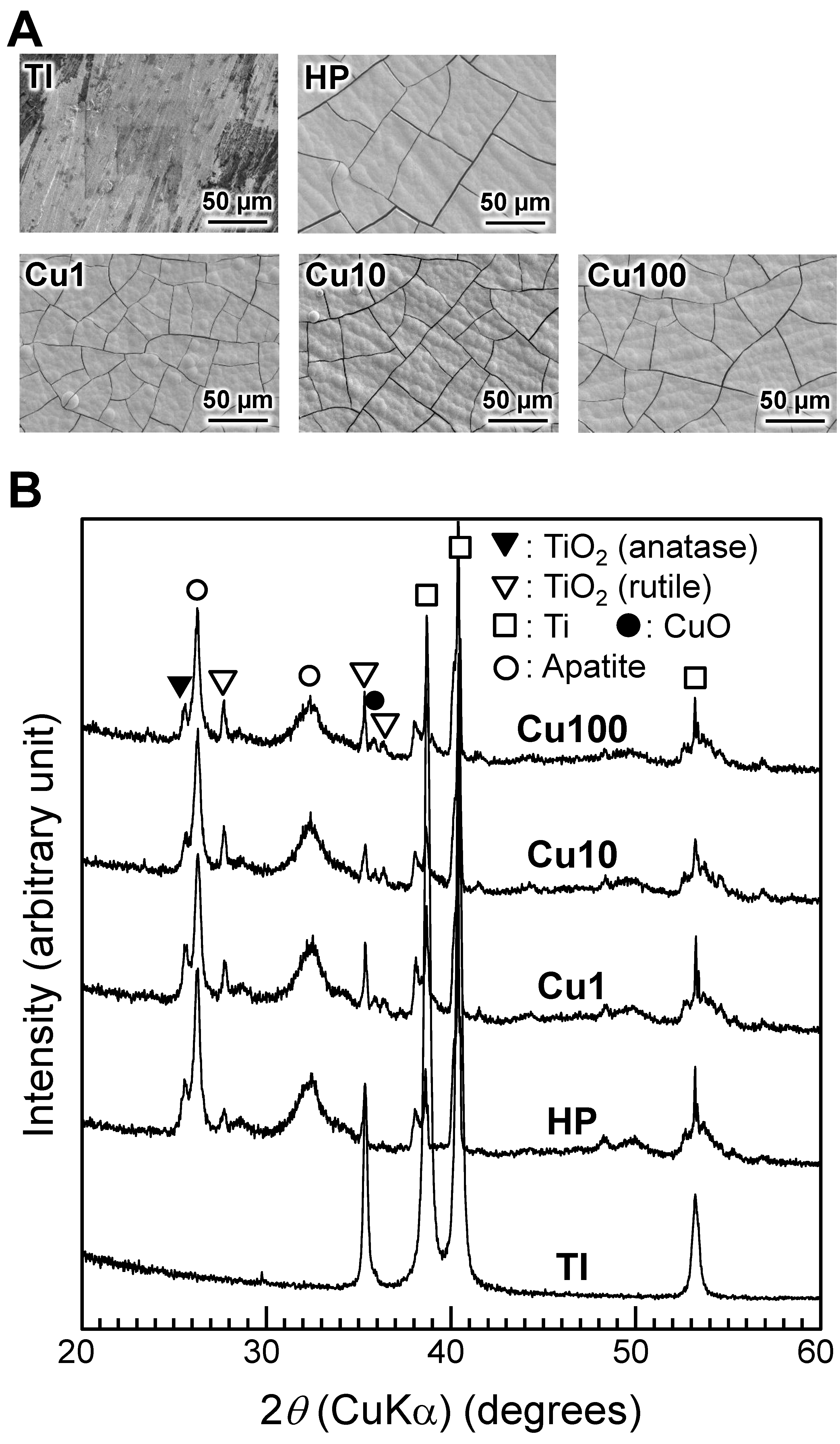
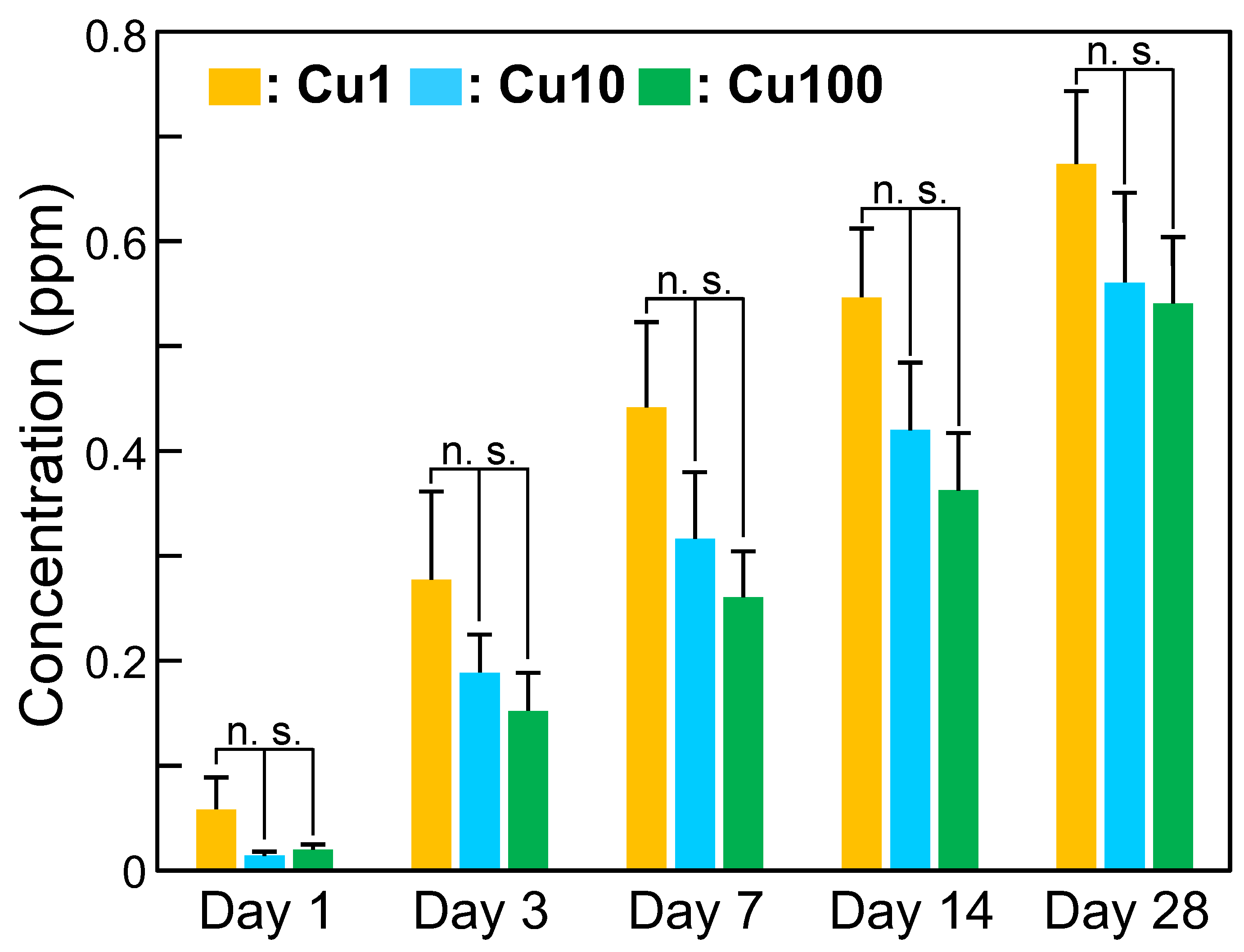
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mombelli, A.; Lang, P. The diagnosis and treatment of peri-implantitis. Periodontology 2000 1998, 17, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Brägger, U.; Aeschlimann, S.; Bürgin, W.; Hämmerle, C.H.; Lang, N.P. Biological and technical complications and failures with fixed partial dentures (FPD) on implants and teeth after four to five years of function. Clin. Oral Implant. Res. 2001, 12, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Gruica, B.; Wang, H.Y.; Lang, N.P.; Buser, D. Impact of IL-1 genotype and smoking status on the prognosis of osseointegrated implants. Clin. Oral Implants Res. 2004, 15, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Fransson, C.; Lekholm, U.; Jemt, T.; Berglundh, T. Prevalence of subjects with progressive bone loss at implants. Clin. Oral Implant. Res. 2005, 16, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Roos-Jansåker, A.M.; Lindahl, C.; Renvert, H.; Renvert, S. Nine-to fourteen-year follow-up of implant treatment, Part I: Implant loss and associations to various factors. J. Clin. Periodontol. 2006, 33, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, G.; Arnhart, C.; Heuberer, S.; Christian, D.; Huber, C.D.; Watzek, G.; Gruber, R. Peri-implantitis and late implant failures in postmenopausal women: A cross-sectional study. J. Clin. Periodontol. 2011, 38, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.Y.; Mattheos, N.; Nixon, K.C.; Ivanovski, S. Residual periodontal pockets are a risk indicator for peri-implantitis in patients treated for periodontitis. Clin. Oral Implant. Res. 2012, 23, 325–333. [Google Scholar]
- Renvert, S.; Lessem, J.; Dahlén, G.; Renvert, H.; Lindah, C. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri-implantitis: A randomized clinical trial. J. Periodontol. 2008, 79, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Schär, D.; Wicki, B.; Eick, S.; Ramseier, C.A.; Arweiler, N.B.; Sculean, A.; Salvi, G.E. Anti-infective therapy of peri-implantitis with adjunctive local drug delivery or photodynamic therapy: 12-month outcomes of a randomized controlled clinical trial. Clin. Oral Implant. Res. 2014, 25, 279–287. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial resistance: Implications and costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Chen, J.Y.; Cheng, F.; Luo, D.W.; Huang, J.F.; Ouyang, J.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.Q.; Peng, Y.Q. Recent advances in Ti-based MOFs in biomedical applications. Dalton Trans. 2022, 51, 14817–14832. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Cai, J.; Peng, G.; Zhou, H.; Zhang, W.; Deyuan Zhang, D.; Gong, D. Metal organic framework-loaded biohybrid magnetic microrobots for enhanced antibacterial treatment. Colloids Surf. A 2024, 685, 133295. [Google Scholar] [CrossRef]
- Veerapandian, M.; Yun, K. Functionalization of biomolecules on nanoparticles: Specialized for antibacterial applications. Appl. Microbiol. Biotechnol. 2011, 90, 1655–1667. [Google Scholar] [CrossRef]
- Geng, Z.; Cao, Z.; Liu, J. Recent advances in targeted antibacterial therapy basing on nanomaterials. Exploration 2023, 3, 20210117. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Gervinskas, G.; Juodkazis, S.; Truong, V.K.; Wu, A.H.F.; Lamb, R.N.; Baulin, V.A.; Watson, G.S.; et al. Bactericidal activity of black silicon. Nat. Commun. 2013, 4, 2838. [Google Scholar] [CrossRef] [PubMed]
- Tarannum, T.; Ahmed, S. Recent development in antiviral surfaces: Impact of topography and environmental conditions. Helyon 2023, 9, e16698. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Bose, S.; Bandyopadhyay, A.; Karandikar, B.; Gibbins, B.L. Surface coatings for improvement of bone cell materials and antimicrobial activities of Ti implants. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 87, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, T.; Miyamoto, H.; Ando, Y.; Noda, I.; Yonekura, Y.; Kawano, S.; Miyazaki, M.; Mawatari, M.; Hotokebuchi, T. In vivo antibacterial and silver-releasing properties of novel thermal sprayed silver-containing hydroxyapatite coating. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 92, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Shirai, T.; Shimizu, T.; Ohtani, K.; Zen, Y.; Takaya, M.; Tsuchiya, H. Antibacterial iodine-supported titanium implants. Acta Biomater. 2011, 7, 1928–1933. [Google Scholar] [CrossRef]
- Kizuki, T.; Matsushita, T.; Kokubo, T. Antibacterial and bioactive calcium titanate layers formed on Ti metal and its alloys. J. Mater. Sci. Mater. Med. 2014, 25, 1737–1746. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, Y.; Yamada, R.; Ashida, M.; Chen, P.; Doi, H.; Nozaki, K.; Nagai, A.; Hanawa, T. Investigation of realizing both antibacterial property and osteogenic cell compatibility on titanium surface by simple electrochemical treatment. ACS Biomater. Sci. Eng. 2019, 5, 5623–5630. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Yamada, R.; Ashida, M.; Doi, H.; Nagai, A.; Hanawa, T. Investigation of antibacterial effect of copper introduced titanium surface by electrochemical treatment against facultative anaerobic bacteria. Dent. Mater. J. 2020, 39, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M.; Manaka, T.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Ashida, M.; Nagai, A.; Hanawa, T. Corrosion behavior and bacterial viability on different surface states of copper. Mater. Trans. 2020, 61, 1143–1148. [Google Scholar] [CrossRef]
- Shimabukuro, M. Antibacterial property and biocompatibility of silver, copper, and zinc in titanium dioxide layers incorporated by one-step micro-arc oxidation: A review. Antibiotics 2020, 9, 716. [Google Scholar] [CrossRef]
- Aoki, S.; Shimabukuro, M.; Kishida, R.; Kyuno, K.; Noda, K.; Yokoi, T.; Kawashita, M. Electrochemical deposition of copper on bioactive porous titanium dioxide layer: Antibacterial and pro-osteogenic activities. ACS Appl. Bio Mater. 2023, 6, 5759–5767. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, T.; Hirata, H.; Eto, S.; Hashimoto, A.; Kii, S.; Kobayashi, T.; Tsukamoto, M.; Yoshihara, T.; Toda, Y.; Mawatari, M. Development of silver-containing hydroxyapatite-coated antimicrobial implants for orthopaedic and spinal surgery. Medicina 2022, 58, 519. [Google Scholar] [CrossRef]
- Kawashita, M.; Iwabuchi, Y.; Suzuki, K.; Furuya, M.; Yokota, K.K.; Kanetaka, H. Surface structure and in vitro apatite-forming ability of titanium doped with various metals. Colloids Surf. A 2018, 555, 558–564. [Google Scholar] [CrossRef]
- Suzuki, K.; Yokoi, T.; Iwatsu, M.; Mokudai, T.; Kanetaka, H.; Kawashita, M. Antibacterial properties of Cu-doped TiO2 prepared by chemical and heat treatment of Ti metal. J. Asian Ceram. Soc. 2021, 9, 1448–1456. [Google Scholar] [CrossRef]
- Fujibayashi, S.; Nakamura, T.; Nishiguchi, S.; Tamura, J.; Uchida, M.; Kim, H.M.; Kokubo, T. Bioactive titanium: Effect of sodium removal on the bone-bonding ability of bioactive titanium prepared by alkali and heat treatment. J. Biomed. Mater. Res. 2001, 56, 562–570. [Google Scholar] [CrossRef]
- Yadav, H.M.; Otari, S.V.; Koli, V.B.; Mali, S.S.; Hong, C.K.; Pawar, S.H.; Delekar, S.D. Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J. Photochem. Photobiol. A 2014, 280, 32–38. [Google Scholar] [CrossRef]
- Liu, M.; Sunada, K.; Hashimoto, K.; Miyauchi, M. Visible-light sensitive Cu(II)-TiO2 with sustained anti-viral activity for efficient indoor environmental remediation. J. Mater. Chem. A 2015, 3, 17312–17319. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-doped TiO2: Visible light assisted photocatalytic antimicrobial activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J. Biomed. Mater. Res. Part A 2003, 64, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuki, C.; Iida, H.; Hayakawa, S.; Osaka, A. Bioactivity of titanium treated with hydrogen peroxide solutions containing metal chlorides. J. Biomed. Mater. Res. 1997, 35, 39–47. [Google Scholar] [CrossRef]
- Kaneko, S.; Tsuru, K.; Hayakawa, S.; Takemoto, S.; Ohtsuki, C.; Ozaki, T.; Inoue, H.; Osaka, A. In vivo evaluation of bone-bonding of titanium metal chemically treated with a hydrogen peroxide solution containing tantalum chloride. Biomaterials 2001, 22, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.M.; Hayakawa, S.; Tsuru, K.; Osaka, A. Crystallization of anatase from amorphous titania in hot water and in vitro biomineralization. J. Ceram. Soc. Jpn. 2002, 110, 78–80. [Google Scholar] [CrossRef]
- Wang, X.X.; Hayakawa, S.; Tsuru, K.; Osaka, A. Bioactive titania gel layers formed by chemical treatment of Ti substrate with a H2O2/HCl solution. Biomaterials 2002, 23, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Osaka, A.; Tsuru, K.; Hayakawa, S. Titania derived from combined chemical and thermal treatments of titanium: In vitro apatite forming ability. Phosphorus Res. Bull. 2004, 17, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Iwatsu, M.; Mokudai, T.; Furuya, M.; Yokota, K.; Kanetaka, H.; Shimabukuro, M.; Yokoi, T.; Kawashita, M. Visible-light-enhanced antibacterial activity of silver and copper co-doped titania formed on titanium via chemical and thermal treatments. Molecules 2023, 28, 650. [Google Scholar] [CrossRef]
- Shirley, D.A. High-resolution X-ray photoemission spectrum of the valence bands of gold. Phys. Rev. B 1972, 5, 4709–4714. [Google Scholar] [CrossRef]
- Biesinger, M.C. Advanced analysis of copper X-ray photoelectron spectra. Surf. Interf. Anal. 2017, 49, 1325–1334. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef]
- ISO 23317:2014; Implants for Surgery—In Vitro Evaluation for Apatite-Forming Ability of Implant Materials. International Organization for Standardization: Geneva, Switzerland, 2014.
- JIS R 1752:2020; Fine Ceramics (Advanced Ceramics, Advanced Technical Ceramics)—Test Method for Antibacterial Activity of Photocatalytic Materials and Efficacy under Indoor Lighting Environment. Japanese Standards Association: Tokyo, Japan, 2020.
- Georgiadou, I.; Spanos, N.; Papadopoulou, C.; Matralis, H.; Kordulis, C.; Lycourghiotis, A. Preparation and characterization of various titanias (anatase) used as supports for vanadia-supported catalysts. Colloids Surf. A 1995, 98, 155–165. [Google Scholar] [CrossRef]
- Shimabukuro, M.; Kobayashi, M.; Kawashita, M. Metallic substrate influences on the osteogenic cell compatibility and antibacterial activity of silver-incorporated porous oxide layers formed by micro-arc oxidation. ACS Appl. Eng. Mater. 2023, 1, 2288–2294. [Google Scholar] [CrossRef]
- Iida, Y.; Ozaki, S. Grain growth and phase transformation of titanium oxide during calcination. J. Am. Ceram. Soc. 1961, 44, 120–127. [Google Scholar] [CrossRef]
- Shannon, R.D.; Pask, J.A. Kinetics of the anatase-rutile transformation. J. Am. Ceram. Soc. 1965, 48, 391–398. [Google Scholar] [CrossRef]
- MacKenzie, K.J.D. Calcination of titania V. Kinetics and mechanism of the anatase-rutile transformation in the presence of additives. Trans. J. Br. Ceram. Soc. 1975, 74, 77–84. [Google Scholar]
- Kim, D.W.; Kim, T.G.; Hong, K.S. Low-firing of CuO-doped anatase. Mater. Res. Bull. 1999, 34, 771–781. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- Sajjad, H.; Sajjad, A.; Haya, R.T.; Khan, M.M.; Zia, M. Copper oxide nanoparticles: In vitro and in vivo toxicity, mechanisms of action and factors influencing their toxicology. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2023, 271, 109682. [Google Scholar] [CrossRef]
- Kasuga, T.; Kondo, H.; Nogami, M. Apatite formation on TiO2 in simulated body fluid. J. Crystal Growth 2002, 235, 235–240. [Google Scholar] [CrossRef]
- Kokubo, T.; Yamaguchi, S. Novel bioactive materials developed by simulated body fluid evaluation: Surface-modified Ti metal and its alloys. Acta Biomater. 2016, 44, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, S.; Yamaguchi, S.; Barbani, N.; Cazzola, M.; Cristallini, C.; Miola, M.; Vernè, E.; Spriano, S. Bioactive materials: In vitro investigation of different mechanisms of hydroxyapatite precipitation. Acta Biomater. 2020, 102, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Miyazaki, T.; Kawashita, M. Preparation and in vitro apatite-forming ability of porous and non-porous titania microspheres. J. Ceram. Soc. Jpn. 2013, 121, 782–787. [Google Scholar] [CrossRef]
- Hans, M.; Erbe, A.; Mathews, S.; Chen, Y.; Solioz, M.; Mücklich, F. Role of copper oxides in contact killing of bacteria. Langmuir 2013, 29, 16160–16166. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Trüper, H.G.; Galinski, E.A. Survival of Escherichia coli during drying and storage in the presence of compatible solutes. Appl. Microbiol. Biotechnol. 1994, 41, 684–688. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Hricovíni, M.; Mazúr, M.; Sîrbu, A.; Palamarciuc, O.; Arion, V.B.; Brezová, V. Copper (II) thiosemicarbazone complexes and their proligands upon UVA irradiation: An EPR and spectrophotometric steady-state study. Molecules 2018, 23, 721. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Miao, D.; Zhu, K.; Tao, K.; Wang, C.; Sharma, V.K.; Jia, H. Interaction of benzo [a] pyrene with Cu (II)-montmorillonite: Generation and toxicity of environmentally persistent free radicals and reactive oxygen species. Environ. Int. 2019, 129, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Kuppusamy, P.; Zweier, J.L. Characterization of free radical generation by xanthine oxidase: Evidence for hydroxyl radical generation. J. Biol. Chem. 1989, 264, 9880–9884. [Google Scholar] [CrossRef]
- Colón, G.; Maicu, M.; Hidalgo, M.C.; Navío, J.A. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Ni, Y.; Zhu, Y.; Ma, X. A simple solution combustion route for the preparation of metal-doped TiO2 nanoparticles and their photocatalytic degradation properties. Dalton Trans. 2011, 14, 3689–3694. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, T.N.; Vaz, M.d.O.; Teixeira, S.R. The effects of surfactant in the sol-gel synthesis of CuO/TiO2 nanocomposites on its photocatalytic activities under UV-visible and visible light illuminations. New J. Chem. 2020, 44, 1888–1904. [Google Scholar] [CrossRef]
- Hernández-Gordillo, A.; Arriaga, S. Mesoporous TiO2 monoliths impregnated with CdS and CuO nanoparticles for airborne bacteria inactivation under visible light. Catal. Lett. 2022, 152, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Irie, H.; Kamiya, K.; Shibanuma, T.; Miura, S.; Tryk, D.A.; Yokoyama, T.; Hashimoto, K. Visible light-sensitive Cu(II)-grafted TiO2 photocatalysts: Activities and X-ray absorption fine structure analyses. J. Phys. Chem. C 2009, 113, 10761–10766. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Tang, J. Charge transfer and photocatalytic activity in CuO/TiO2 nanoparticle heterojunctions synthesised through a rapid, one-pot, microwave solvothermal route. ChemCatChem 2015, 7, 1659–1667. [Google Scholar] [CrossRef]
- Kangwansupamonkon, W.; Lauruengtana, V.; Surassmo, S.; Ruktanonchai, U. Antibacterial effect of apatite-coated titanium dioxide for textiles applications. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 240–249. [Google Scholar] [CrossRef]
- Ueda, T.; Koizumi, R.; Ueda, K.; Ito, K.; Ogasawara, K.; Kanetaka, H.; Narushima, T. Antibacterial properties of TiO2 layers formed by Au-sputtering and thermal oxidation of titanium under visible light. Mater. Trans. 2023, 64, 155–164. [Google Scholar] [CrossRef]



| Surface Treatment | Sample Name |
|---|---|
| None | TI |
| H2O2 + heat | HP |
| H2O2 + 1 mM Cu(OAc)2 + heat | Cu1 |
| H2O2 + 10 mM Cu(OAc)2 + heat | Cu10 |
| H2O2 + 100 mM Cu(OAc)2 + heat | Cu100 |
| Sample | Concentration of Cu (at.%) | Proportion of Cu Species (%) | |
|---|---|---|---|
| Cu0 + Cu+ | Cu2+ | ||
| Cu1 | 6.8 | 20.1 | 79.9 |
| Cu10 | 15.8 | 19.2 | 80.8 |
| Cu100 | 12.9 | 26.5 | 73.5 |
| Sample | ·OH Concentration (µM) | |
|---|---|---|
| Without Visible-Light Irradiation | With Visible-Light Irradiation | |
| TI | 0.09 | 0.19 |
| HP | 0.21 | 0.51 |
| Cu1 | 1.08 | 3.03 |
| Cu10 | 1.44 | 3.23 |
| Cu100 | 1.50 | 4.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, P.-C.; Yokoi, T.; Shimabukuro, M.; Mokudai, T.; Kawashita, M. Apatite-Forming Ability and Visible Light-Enhanced Antibacterial Activity of CuO-Supported TiO2 Formed on Titanium by Chemical and Thermal Treatments. J. Funct. Biomater. 2024, 15, 114. https://doi.org/10.3390/jfb15050114
Sung P-C, Yokoi T, Shimabukuro M, Mokudai T, Kawashita M. Apatite-Forming Ability and Visible Light-Enhanced Antibacterial Activity of CuO-Supported TiO2 Formed on Titanium by Chemical and Thermal Treatments. Journal of Functional Biomaterials. 2024; 15(5):114. https://doi.org/10.3390/jfb15050114
Chicago/Turabian StyleSung, Po-Cheng, Taishi Yokoi, Masaya Shimabukuro, Takayuki Mokudai, and Masakazu Kawashita. 2024. "Apatite-Forming Ability and Visible Light-Enhanced Antibacterial Activity of CuO-Supported TiO2 Formed on Titanium by Chemical and Thermal Treatments" Journal of Functional Biomaterials 15, no. 5: 114. https://doi.org/10.3390/jfb15050114





