Multifunctional ZnO@DOX/ICG-LMHP Nanoparticles for Synergistic Multimodal Antitumor Activity
Abstract
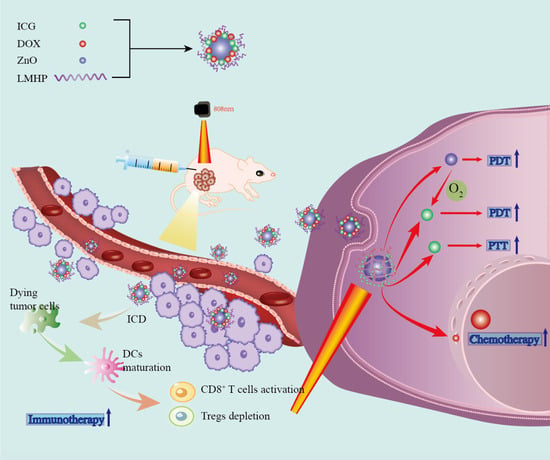
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZNIDL NPs
2.3. Characterization of ZNIDL NPs
2.4. Drug Release of ZNIDL NPs
2.5. Oxygen Generation of ZNIDL NPs
2.6. Photodynamic and Photothermal Effect of ZNIDL NPs
2.7. Intracellular Behavior of ZNIDL NPs
2.8. In Vitro Antitumor Activity of ZNIDL NPs
2.9. ICD Signaling Molecules of ZNIDL NPs
2.10. In Vivo Antitumor Activity of ZNIDL NPs
2.11. In Vivo Antitumor Immune Response of ZNIDL NPs
2.12. In Vivo Antitumor Abscopal Effect of ZNIDL NPs
2.13. Postoperative Tumor Recurrence Model of ZNIDL NPs
2.14. Statistical Analysis
3. Results and Discussion
3.1. Characterization of ZNIDL NPs
3.2. Intracellular Behavior of ZNIDL NPs
3.3. In Vitro Evaluation of the Immunogenic Cell Death Effect
3.4. In Vivo Antitumor Activity of ZNIDL NPs
3.5. In Vivo Antitumor Immune Response of ZNIDL NPs
3.6. Abscopal Effects and Antitumor Recurrence of ZNIDL NPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leon, R.; Goetz, M. Advances in systemic therapies for triple negative breast cancer. BMJ 2023, 381, e071674. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, Y.T.; Xue, J.; Li, J.; Yi, J.; Bu, J.; Zhang, Z.; Qiu, P.; Gu, X. Advances in immunotherapy for triple-negative breast cancer. Mol Cancer 2023, 22, 145. [Google Scholar] [CrossRef] [PubMed]
- Nolan, E.; Lindeman, G.; Visvader, J. Deciphering breast cancer: From biology to the clinic. Cell 2023, 186, 1708–1728. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.; Scalzo, R.; Wellberg, E. Diabetes mellitus in breast cancer survivors: Metabolic effects of endocrine therapy. Nat. Rev. Endocrinol. 2023, 20, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Meng, Q.; Zhang, Y.; Su, R.; Xing, F.; Yang, H.; Hou, Y.; Ma, P.; Huang, K.; Feng, S. π Bridge Engineering-Boosted Dual Enhancement of Type-I Photodynamic and Photothermal Performance for Mitochondria-Targeting Multimodal Phototheranostics of Tumor. ACS Nano 2023, 17, 21553–21566. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, M.; Lv, B.; Xue, G.; Jiang, H.; Chen, G.; Ma, Y.; Sun, Y.; Cao, J. “Closed-Loop” O2-Economizer Induced In Situ Therapeutic Vaccine against Hypoxic Tumors. ACS Nano 2023, 17, 21170–21181. [Google Scholar] [CrossRef] [PubMed]
- Juengpanich, S.; Li, S.; Yang, T.; Xie, T.; Chen, J.; Shan, Y.; Lee, J.; Lu, Z.; Chen, T.; Zhang, B.; et al. Pre-activated nanoparticles with persistent luminescence for deep tumor photodynamic therapy in gallbladder cancer. Nat. Commun. 2023, 14, 5699. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xu, K.; Liu, S.; He, Y.; Tan, M.; Mao, Y.; Yang, Y.; Wu, J.; Feng, Q.; Luo, Z.; et al. All-in-One Engineering Multifunctional Nanoplatforms for Sensitizing Tumor Low-Temperature Photothermal Therapy In Vivo. ACS Nano 2023, 17, 20218–20236. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Xia, J.; Ma, Q.; Zhu, W.; Gao, Z.; Han, S.; Liang, Y.; Cao, J.; Sun, Y. Tumor Microenvironment-triggered Nanosystems as dual-relief Tumor Hypoxia Immunomodulators for enhanced Phototherapy. Theranostics 2020, 10, 9132–9152. [Google Scholar] [CrossRef]
- Zheng, X.; Shi, Y.; Tang, D.; Xiao, H.; Shang, K.; Zhou, X.; Tan, G. Near-Infrared-II Nanoparticles for Vascular Normalization Combined with Immune Checkpoint Blockade via Photodynamic Immunotherapy Inhibit Uveal Melanoma Growth and Metastasis. Adv. Sci. 2023, 10, e2206932. [Google Scholar] [CrossRef]
- Liu, J.; Bu, W.; Shi, J. Chemical Design and Synthesis of Functionalized Probes for Imaging and Treating Tumor Hypoxia. Chem. Rev. 2017, 117, 6160–6224. [Google Scholar] [CrossRef]
- Li, Z.; Li, X.; Lu, Y.; Zhu, X.; Zheng, W.; Chen, K.; Liu, S.; Wu, J.; Guan, W. Improved Photodynamic Therapy Based on Glutaminase Blockage via Tumor Membrane Coated CB-839/IR-780 Nanoparticles. Small 2023, e2305174. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Wang, Y.; Qu, S.; Zhang, N.; Nie, K.; Wang, J.; Huang, Y.; Sui, D.; Yu, B.; Qin, M.; et al. Controllable Star Cationic poly (Disulfide)s Achieve Genetically Cascade Catalytic Therapy by Delivering Bifunctional Fusion Plasmids. Adv. Mater. 2023, 35, e2307190. [Google Scholar] [CrossRef]
- Wan, Y.; Fu, L.; Li, C.; Lin, J.; Huang, P. Conquering the Hypoxia Limitation for Photodynamic Therapy. Adv. Mater. 2021, 33, e2103978. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mao, Y.; Sun, C.; Zhao, Q.; Gao, Y.; Wang, S. A versatile gas-generator promoting drug release and oxygen replenishment for amplifying photodynamic-chemotherapy synergetic anti-tumor effects. Biomaterials 2021, 276, 120985. [Google Scholar] [CrossRef] [PubMed]
- Gulzar, A.; Xu, J.; Wang, C.; He, F.; Yang, D.; Gai, S.; Yang, P.; Lin, J.; Jin, D.; Xing, B. Tumour microenvironment responsive nanoconstructs for cancer theranostic. Nano Today 2019, 26, 16–56. [Google Scholar] [CrossRef]
- Sahu, A.; Kwon, I.; Tae, G. Improving cancer therapy through the nanomaterials-assisted alleviation of hypoxia. Biomaterials 2020, 228, 119578. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Huang, Y.; Xue, Z.; Zeng, S. Tumor microenvironment responsive hollow mesoporous Co9S8@MnO2-ICG/DOX intelligent nanoplatform for synergistically enhanced tumor multimodal therapy. Biomaterials 2020, 262, 120346. [Google Scholar] [CrossRef]
- Zou, P.; Lin, R.; Fang, Z.; Chen, J.; Guan, H.; Yin, J.; Chang, Z.; Xing, L.; Lang, J.; Xue, X.; et al. Implanted, Wireless, Self-Powered Photodynamic Therapeutic Tablet Synergizes with Ferroptosis Inducer for Effective Cancer Treatment. Adv. Sci. 2023, 10, e2302731. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Li, Z.; Wang, G.; Liao, A.; Wang, J.; Li, H.; Guo, Z.; Cheng, B.; Zhang, X. Intelligent Nanodelivery System-Generated 1O2 Mediates Tumor Vessel Normalization by Activating Endothelial TRPV4-eNOS Signaling. Small 2022, 18, e2200038. [Google Scholar] [CrossRef]
- Joel, E.; Silvia, G.; Kevin, K.; Enrico, D. Colloidal Approaches to Zinc Oxide Nanocrystals. Chem. Rev. 2023, 123, 271–326. [Google Scholar]
- Cui, T.; Yan, Z.; Qin, H.; Sun, Y.; Ren, J.; Qu, X. A Sequential Target-Responsive Nanocarrier with Enhanced Tumor Penetration and Neighboring Effect In Vivo. Small 2019, 15, e1903323. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Zhen, W.; Wang, Y.; Zhang, S.; Zhao, Y.; Song, S.; Wu, Z.; Zhang, H. Defect modified zinc oxide with augmenting sonodynamic reactive oxygen species generation. Biomaterials 2020, 251, 120075. [Google Scholar] [CrossRef]
- Ran, M.; Sun, R.; Yan, J.; Pulliainen, A.; Zhang, Y.; Zhang, H. DNA Nanoflower Eye Drops with Antibiotic-Resistant Gene Regulation Ability for MRSA Keratitis Target Treatment. Small 2023, 19, e2304194. [Google Scholar] [CrossRef]
- Tian, B.; Tian, R.; Liu, S.; Wang, Y.; Gai, S.; Xie, Y.; Yang, D.; He, F.; Yang, P.; Lin, J. Doping Engineering to Modulate Lattice and Electronic Structure for Enhanced Piezocatalytic Therapy and Ferroptosis. Adv. Mater. 2023, 35, e2304262. [Google Scholar] [CrossRef] [PubMed]
- Zhi, S.; Zhang, X.; Zhang, J.; Wang, X.; Bi, S. Functional Nucleic Acids-Engineered Bio-Barcode Nanoplatforms for Targeted Synergistic Therapy of Multidrug-Resistant Cancer. ACS Nano 2023, 17, 13533–13544. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Li, X.; Huang, J.; Guo, X.; Zhang, J.; Luo, Z.; Shi, Y.; Jiang, M.; Qin, B.; et al. ER-Targeting PDT Converts Tumors into In Situ Therapeutic Tumor Vaccines. ACS Nano 2022, 16, 9240–9253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xu, J.; Feng, C.; Ren, J.; Bao, L.; Zhao, Y.; Tao, W.; Zhao, Y.; Yang, X. Tailoring Aggregation Extent of Photosensitizers to Boost Phototherapy Potency for Eliciting Systemic Antitumor Immunity. Adv. Mater. 2022, 34, e2106390. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Wang, Y.; Sun, C.; Bao, L.; Zhao, Y.; Yang, X.; Zhao, Y. A Photosensitizer Discretely Loaded Nanoaggregate with Robust Photodynamic Effect for Local Treatment Triggers Systemic Antitumor Responses. ACS Nano 2022, 16, 3070–3080. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Wang, M.; Chen, M.; Chen, Z.; Peng, X.; Zhou, F.; Song, J.; Qu, J. Programming cell pyroptosis with biomimetic nanoparticles for solid tumor immunotherapy. Biomaterials 2020, 254, 120142. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Zhan, M.; Liang, J.; Yang, X.; Zhang, B.; Shi, X.; Hu, Y. Programming Injectable DNA Hydrogels Yields Tumor Microenvironment-Activatable and Immune-Instructive Depots for Augmented Chemo-Immunotherapy. Adv. Sci. 2023, 10, e2302119. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Xie, T.; Liu, Y.; Yan, S.; Ou, F.; Zhang, H.; Lei, L.; He, D.; Wei, H.; Yu, C. A Sodium Alginate-Based Multifunctional Nanoplatform for Synergistic Chemo-Immunotherapy of Hepatocellular Carcinoma. Adv. Mater. 2023, 35, e2301352. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cao, Z.; Chen, C.; Li, H.; He, S.; Hou, X.; Liang, M.; Yang, X.; Wang, J. Nanoassembly of doxorubicin-conjugated polyphosphoester and siRNA simultaneously elicited macrophage- and T cell-mediated anticancer immune response for cancer therapy. Biomaterials 2023, 302, 122339. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Liu, R.; Chen, F.; Zhang, J.; Zheng, S.; Shao, D.; Du, J. Nanoparticle-Mediated CD47-SIRPα Blockade and Calreticulin Exposure for Improved Cancer Chemo-Immunotherapy. ACS Nano 2023, 17, 8966–8979. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Novohradsky, V.; Maji, M.; Babu, T.; Markova, L.; Kostrhunova, H.; Kasparkova, J.; Gandin, V.; Brabec, V.; Gibson, D. Multitargeting Prodrugs that Release Oxaliplatin, Doxorubicin and Gemcitabine are Potent Inhibitors of Tumor Growth and Effective Inducers of Immunogenic Cell Death. Angew. Chem. Int. Ed. 2023, 62, e202310774. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Lyu, R.; Wang, J.; Cheng, Q.; Yu, Y.; Yang, S.; Mao, C.; Yang, M. Metal-Organic Frameworks Nucleated by Silk Fibroin and Modified with Tumor-Targeting Peptides for Targeted Multimodal Cancer Therapy. Adv. Sci. 2023, 10, 2302700. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, R.; He, J.; Yu, L.; Li, X.; Zhang, J.; Li, S.; Zhang, C.; Kagan, J.; Karp, J.; et al. Ultrasound-responsive low-dose doxorubicin liposomes trigger mitochondrial DNA release and activate cGAS-STING-mediated antitumour immunity. Nat. Commun. 2023, 14, 3877. [Google Scholar] [CrossRef]
- Wei, J.; Ji, G.; Zhang, C.; Yan, L.; Luo, Q.; Wang, C.; Chen, Q.; Yang, J.; Chen, L.; Ma, C. Silane-Capped ZnO Nanoparticles for Use as the Electron Transport Layer in Inverted Organic Solar Cells. ACS Nano 2018, 12, 5518–5529. [Google Scholar] [CrossRef]
- Zhao, B.; Zhao, Y.; Huang, Y.; Luo, L.; Song, P.; Wang, X.; Chen, S.; Yu, K.; Zhang, X.; Zhang, Q. The efficiency of tumor-specific pH-responsive peptide-modified polymeric micelles containing paclitaxel. Biomaterials 2012, 33, 2508–2520. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, W.; Zhong, T.; Zhang, S.; Huang, D.; Guo, Y.; Yao, X.; Wang, C.; Zhang, W.; Zhang, X.; et al. Tumor-specific pH-responsive peptide-modified pH-sensitive liposomes containing doxorubicin for enhancing glioma targeting and anti-tumor activity. J. Control Release 2016, 222, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Huang, Y.; Zhao, B.; Zhao, X.; Duan, Y.; Du, R.; Yu, K.; Song, P.; Zhao, Y.; Zhang, X.; et al. Anti-tumor and anti-angiogenic effect of metronomic cyclic NGR-modified liposomes containing paclitaxel. Biomaterials 2013, 34, 1102–1114. [Google Scholar] [CrossRef]
- Tian, B.; Liu, S.; Yu, C.; Liu, S.; Dong, S.; Feng, L.; Hu, N.; Yang, P. A Metal-Free Mesoporous Carbon Dots/Silica Hybrid Type I Photosensitizer with Enzyme-Activity for Synergistic Treatment of Hypoxic Tumor. Adv. Funct. Mater. 2023, 33, 2300818. [Google Scholar] [CrossRef]
- Wu, N.; Tu, Y.; Fan, G.; Ding, J.; Luo, J.; Wang, W.; Zhang, C.; Yuan, C.; Zhang, H.; Chen, P.; et al. Enhanced photodynamic therapy/photothermo therapy for nasopharyngeal carcinoma via a tumour microenvironment-responsive self-oxygenated drug delivery system. Asian J. Pharm. Sci. 2022, 17, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu, W.; Xiao, P.; Kang, M.; Yan, D.; Wen, H.; Song, N.; Wang, D.; Tang, B. Molecular Engineering of High-Performance Aggregation-Induced Emission Photosensitizers to Boost Cancer Theranostics Mediated by Acid-Triggered Nucleus-Targeted Nanovectors. ACS Nano 2021, 15, 10689–10699. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, S.; Liu, M.; Zhong, T.; Li, H.; Wang, J.; Zhao, H.; Tian, Y.; Wang, H.; Wang, J.; et al. Antitumor Activity of the Zinc Oxide Nanoparticles Coated with Low-Molecular-Weight Heparin and Doxorubicin Complex In Vitro and In Vivo. Mol. Pharm. 2022, 19, 4179–4190. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, Z.; Liu, M.; Wang, J.; Xu, M.; Li, Z.; Duan, X.; Hao, Y.; Zheng, X.; Li, H.; et al. Anti-tumour activity of low molecular weight heparin doxorubicin nanoparticles for histone H1 high-expressive prostate cancer PC-3M cells. J. Control Release 2019, 295, 102–117. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Z.; Zhang, S.; Wang, J.; Li, Z.; Xu, M.; Li, H.; Zhang, S.; Wang, G.; Liao, A.; et al. A combination strategy based on an Au nanorod/doxorubicin gel via mild photothermal therapy combined with antigen-capturing liposomes and anti-PD-L1 agent promote a positive shift in the cancer-immunity cycle. Acta Biomater. 2021, 136, 495–507. [Google Scholar] [CrossRef]
- An, S.; Tiruthani, K.; Wang, Y.; Xu, L.; Hu, M.; Li, J.; Song, W.; Jiang, H.; Sun, J.; Liu, R.; et al. Locally Trapping the C-C Chemokine Receptor Type 7 by Gene Delivery Nanoparticle Inhibits Lymphatic Metastasis Prior to Tumor Resection. Small 2019, 15, e1805182. [Google Scholar] [CrossRef]
- Liu, J.; Li, H.; Luo, Y.; Xu, C.; Du, X.; Du, J.; Wang, J. Enhanced Primary Tumor Penetration Facilitates Nanoparticle Draining into Lymph Nodes after Systemic Injection for Tumor Metastasis Inhibition. ACS Nano 2019, 13, 8648–8658. [Google Scholar] [CrossRef]
- Phuengkham, H.; Song, C.; Um, S.; Lim, Y. Implantable Synthetic Immune Niche for Spatiotemporal Modulation of Tumor-Derived Immunosuppression and Systemic Antitumor Immunity: Postoperative Immunotherapy. Adv. Mater. 2018, 30, e1706719. [Google Scholar] [CrossRef]
- Peng, L.; Mei, X.; He, J.; Xu, J.; Zhang, W.; Liang, R.; Wei, M.; Evans, D.; Duan, X. Monolayer Nanosheets with an Extremely High Drug Loading toward Controlled Delivery and Cancer Theranostics. Adv. Mater. 2018, 30, e1707389. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, Z.; Sun, Y.; Zhang, X. Controlled synthesis of a core-shell nanohybrid for effective multimodal image-guided combined photothermal/photodynamic therapy of tumors. Npg. Asia Mater. 2019, 11, 63. [Google Scholar] [CrossRef]
- Liu, X.; Dong, X.; Yang, S.; Lai, X.; Liu, H.; Gao, Y.; Feng, H.; Zhu, M.; Yuan, Y.; Lu, Q.; et al. Biomimetic Liposomal Nanoplatinum for Targeted Cancer Chemophototherapy. Adv. Sci. 2021, 8, 2003679. [Google Scholar] [CrossRef]
- Wang, W.; Chen, C.; Ying, Y.; Lv, S.; Wang, Y.; Zhang, X.; Cai, Z.; Gu, W.; Li, Z.; Jiang, G.; et al. Smart PdH@MnO2 Yolk-Shell Nanostructures for Spatiotemporally Synchronous Targeted Hydrogen Delivery and Oxygen-Elevated Phototherapy of Melanoma. ACS Nano 2022, 16, 5597–5614. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Sun, H.; Guo, D. Type I photodynamic therapy by organic-inorganic hybrid materials: From strategies to applications. Coord. Chem. Rev. 2019, 395, 46–62. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, K.; Bu, W.; Ni, D.; Liu, Y.; Feng, J.; Shi, J. Marriage of Scintillator and Semiconductor for Synchronous Radiotherapy and Deep Photodynamic Therapy with Diminished Oxygen Dependence. Angew. Chem. Int. Ed. 2015, 54, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Huang, P.; Chen, X. Overcoming the Achilles’ heel of photodynamic therapy. Chem. Soc. Rev. 2016, 45, 6488–6519. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Ding, A.; Xu, Y.; Ye, Y.; Li, L.; Xie, R.; Huang, W. Gene and Photothermal Combination Therapy: Principle, Materials, and Amplified Anticancer Intervention. Small 2023, 29, e2307078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yong, Y.; Song, L.; Dong, X.; Zhang, X.; Liu, X.; Gu, Z.; Zhao, Y.; Hu, Z. Multifunctional WS2@Poly(ethylene imine) Nanoplatforms for Imaging Guided Gene-Photothermal Synergistic Therapy of Cancer. Adv. Healthc. Mater. 2016, 5, 2776–2787. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Chan, C.; Lin, W. Nanoparticle-Mediated Immunogenic Cell Death Enables and Potentiates Cancer Immunotherapy. Angew. Chem. Int. Ed. 2019, 58, 670–680. [Google Scholar] [CrossRef]
- Feng, B.; Hou, B.; Xu, Z.; Saeed, M.; Yu, H.; Li, Y. Self-Amplified Drug Delivery with Light-Inducible Nanocargoes to Enhance Cancer Immunotherapy. Adv. Mater. 2019, 31, e1902960. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Patin, E.; Pedersen, M.; Wilkins, A.; Dillon, M.; Melcher, A.; Harrington, K. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer. 2020, 20, 203–217. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Chen, M.; Liu, Z. Local biomaterials-assisted cancer immunotherapy to trigger systemic antitumor responses. Chem. Soc. Rev. 2019, 48, 5506–5526. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.; Dane, E. Enhancing cancer immunotherapy with nanomedicine. Nat. Rev. Immunol. 2020, 20, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Najibi, A.; Sobral, M.; Seo, B.; Lee, J.; Wu, D.; Li, A.; Verbeke, C.; Mooney, D. Biomaterial-based scaffold for in situ chemo-immunotherapy to treat poorly immunogenic tumors. Nat. Commun. 2020, 11, 5696. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Chen, Q.; Liu, Z. Smart Injectable Hydrogels for Cancer Immunotherapy. Adv. Funct. Mater. 2020, 30, 1902785. [Google Scholar] [CrossRef]
- Li, T.; Chen, G.; Xiao, Z.; Li, B.; Zhong, H.; Lin, M.; Cai, Y.; Huang, J.; Xie, X.; Shuai, X. Surgical Tumor-Derived Photothermal Nanovaccine for Personalized Cancer Therapy and Prevention. Nano Lett. 2022, 22, 3095–3103. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, X.; Wang, M.; Liu, W.; Zhang, L.; Zhang, Y.; Hu, Z.; Zhou, X.; Jiang, W.; Zou, Q.; et al. Optogenetic-controlled immunotherapeutic designer cells for post-surgical cancer immunotherapy. Nat. Commun. 2022, 13, 6357. [Google Scholar] [CrossRef]
- Liu, X.; Huang, Y.; Wang, J.; Kong, P.; Tian, W.; Liu, P.; Fang, C.; Li, S.; Nie, Y.; Feng, Z.; et al. Supramolecular Polymer-Nanomedicine Hydrogel Loaded with Tumor Associated Macrophage-Reprogramming polyTLR7/8a Nanoregulator for Enhanced Anti-Angiogenesis Therapy of Orthotopic Hepatocellular Carcinoma. Adv. Sci. 2023, 10, e2300637. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Guo, W.; Cai, C.; Tang, J.; Rao, W.; Wang, Y.; Wang, Y.; Yu, L.; Ding, J. Unified Therapeutic-Prophylactic Vaccine Demonstrated with a Postoperative Filler Gel to Prevent Tumor Recurrence and Metastasis. Adv. Funct. Mater. 2022, 32, 2206084. [Google Scholar] [CrossRef]
- Ke, Y.; Zhu, J.; Chu, Y.; Cen, L.; Fu, Y.; Fan, X.; Shao, J.; Li, R.; Yu, L.; Liu, B.; et al. Bifunctional Fusion Membrane-Based Hydrogel Enhances Antitumor Potency of Autologous Cancer Vaccines by Activating Dendritic Cells. Adv. Funct. Mater. 2022, 32, 2201306. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Wang, J.; Liu, J.; Yu, J.; Wang, J.; Wang, H.; Wei, Q.; Liu, M.; Xu, M.; Feng, Z.; et al. Multifunctional ZnO@DOX/ICG-LMHP Nanoparticles for Synergistic Multimodal Antitumor Activity. J. Funct. Biomater. 2024, 15, 35. https://doi.org/10.3390/jfb15020035
Li Z, Wang J, Liu J, Yu J, Wang J, Wang H, Wei Q, Liu M, Xu M, Feng Z, et al. Multifunctional ZnO@DOX/ICG-LMHP Nanoparticles for Synergistic Multimodal Antitumor Activity. Journal of Functional Biomaterials. 2024; 15(2):35. https://doi.org/10.3390/jfb15020035
Chicago/Turabian StyleLi, Zhuoyue, Jingru Wang, Junwei Liu, Jianming Yu, Jingwen Wang, Hui Wang, Qingchao Wei, Man Liu, Meiqi Xu, Zhenhan Feng, and et al. 2024. "Multifunctional ZnO@DOX/ICG-LMHP Nanoparticles for Synergistic Multimodal Antitumor Activity" Journal of Functional Biomaterials 15, no. 2: 35. https://doi.org/10.3390/jfb15020035