Active and Passive Mineralization of Bio-Gide® Membranes in Rat Calvaria Defects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Surgery
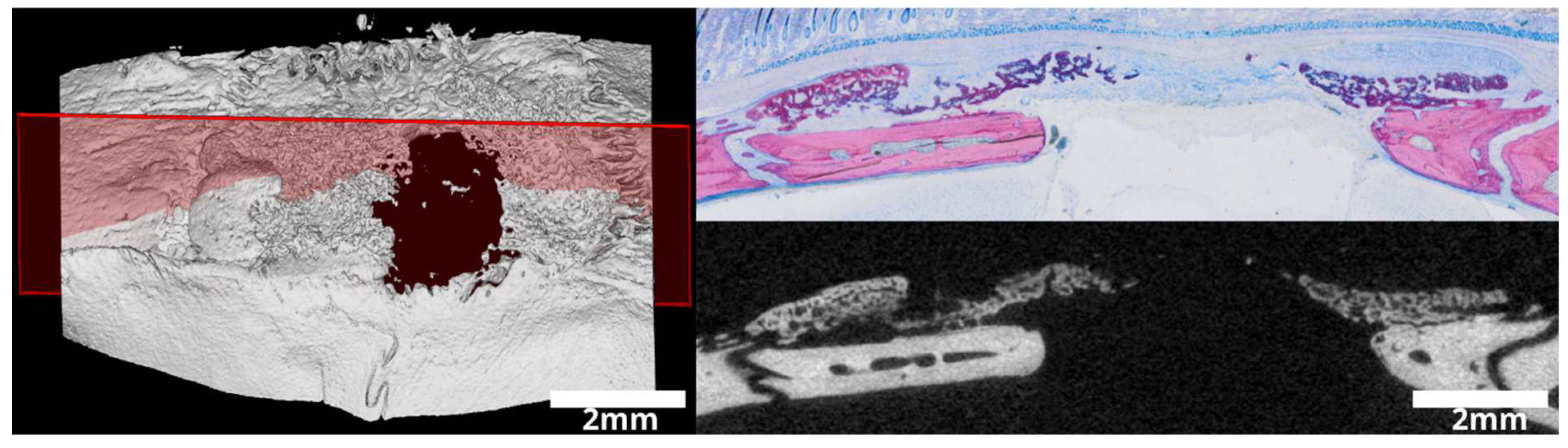
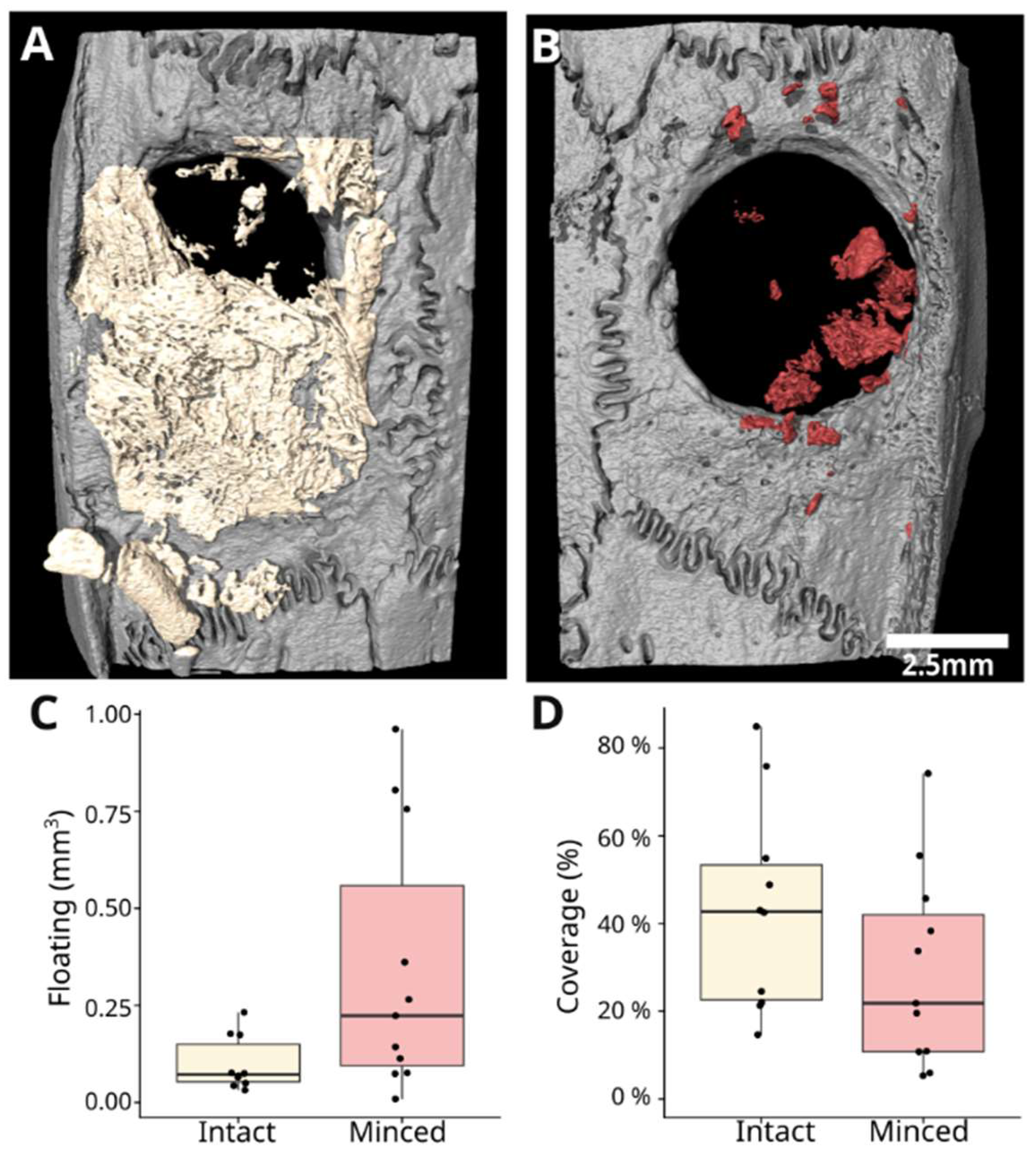
2.3. Micro-CT Analysis
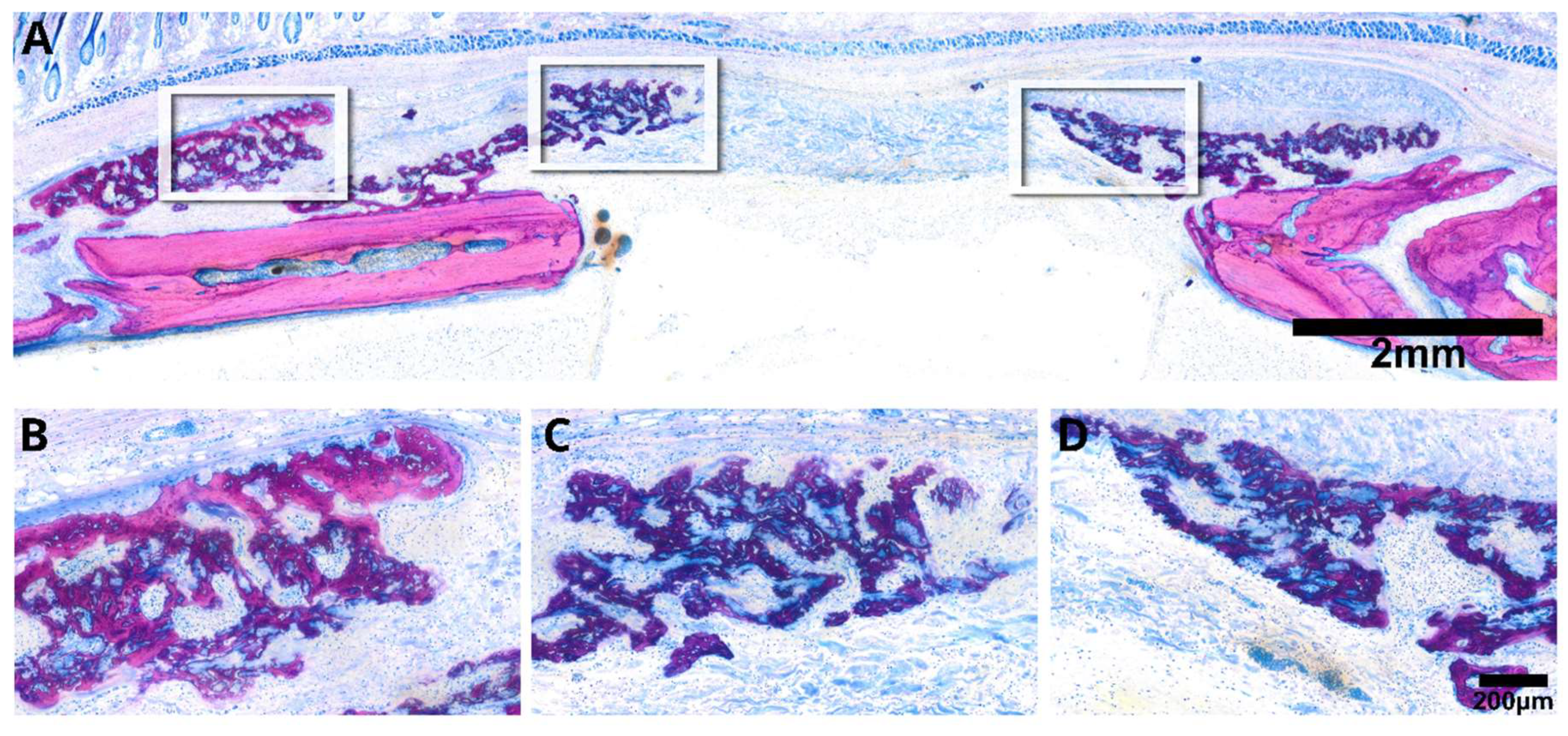
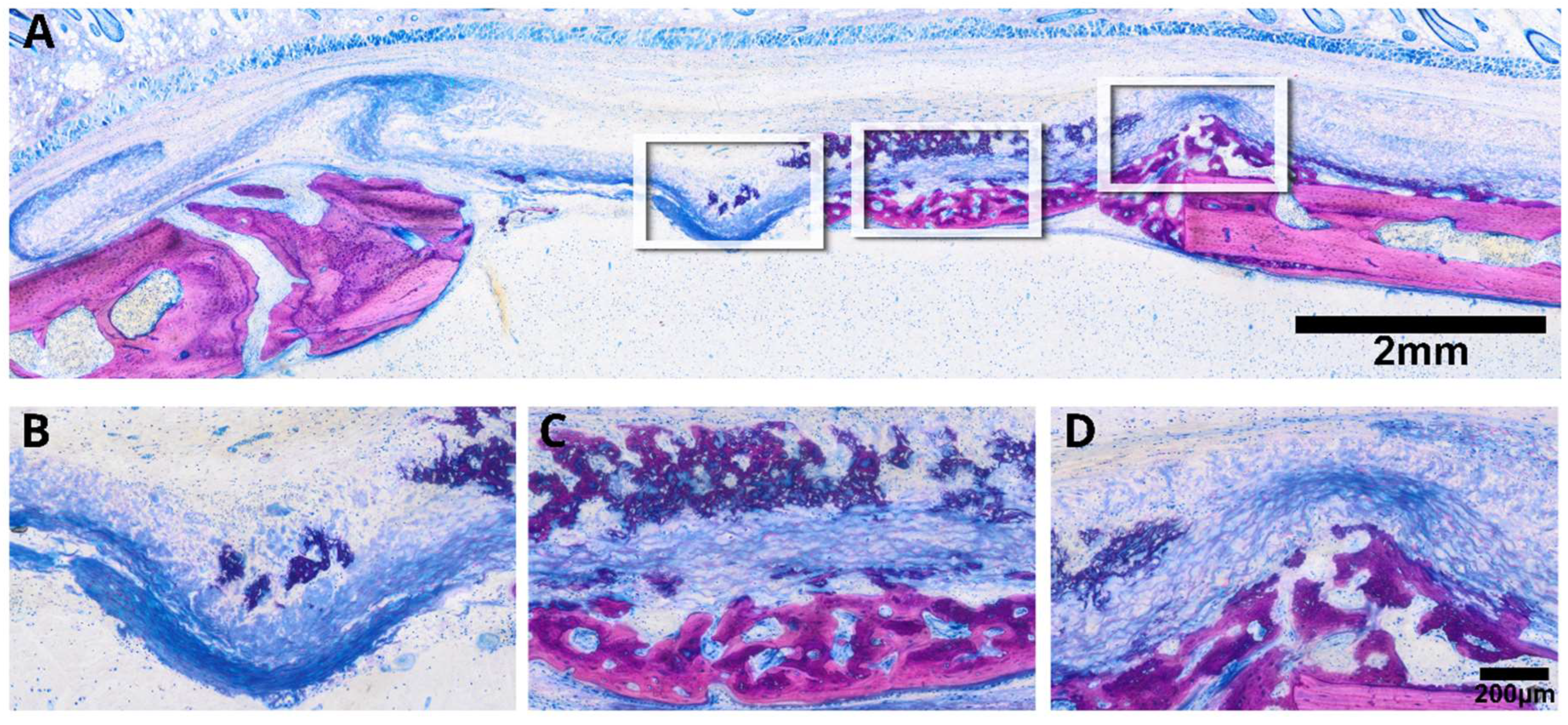
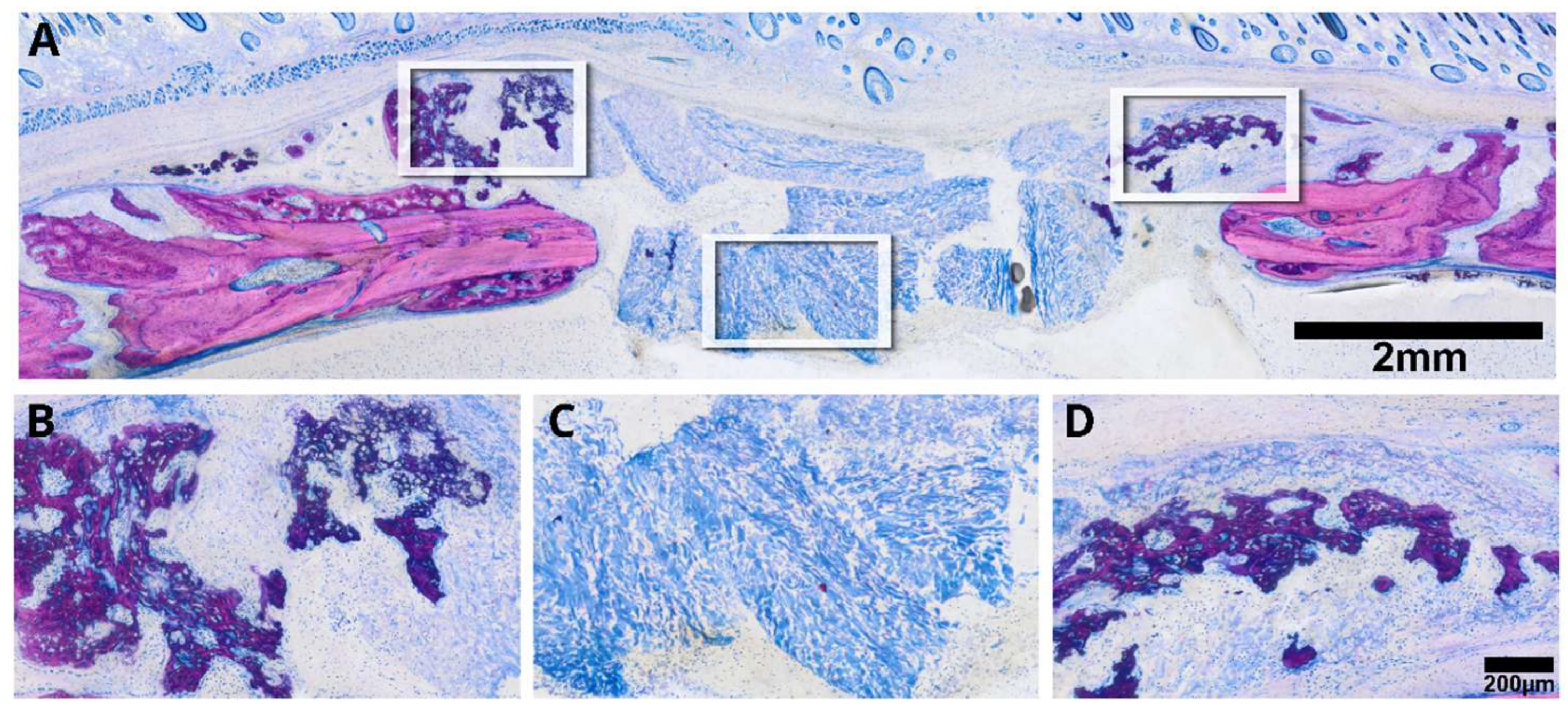
2.4. Histological Analysis
2.5. Statistics
3. Results
3.1. Intact and Minced Collagen Membranes: µCT Analysis
3.2. Intact Collagen Membranes: Histological Analysis
3.3. Minced Collagen Membranes: Histological Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ren, Y.; Fan, L.; Alkildani, S.; Liu, L.; Emmert, S.; Najman, S.; Rimashevskiy, D.; Schnettler, R.; Jung, O.; Xiong, X.; et al. Barrier Membranes for Guid ed Bone Regeneration (GBR): A Focus on Recent Advances in Collagen Membranes. Int. J. Mol. Sci. 2022, 23, 14987. [Google Scholar] [CrossRef]
- Mizraji, G.; Davidzohn, A.; Gursoy, M.; Gursoy, U.K.; Shapira, L.; Wilensky, A. Membrane barriers for guided bone regeneration: An overview of available biomaterials. Periodontol. 2000 2023, 93, 56–76. [Google Scholar] [CrossRef]
- Kim, K.; Su, Y.; Kucine, A.J.; Cheng, K.; Zhu, D. Guided Bone Regeneration Using Barrier Membrane in Dental Applications. ACS Biomater. Sci. Eng. 2023, 9, 5457–5478. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, C.; Shi, H.; Luo, X.; Sun, H.; Wang, Q.; Zhang, D. Advances in Barrier Membranes for Guided Bone Regeneration Techniques. Front. Bioeng. Biotechnol. 2022, 10, 921576. [Google Scholar] [CrossRef]
- Omar, O.; Elgali, I.; Dahlin, C.; Thomsen, P. Barrier membranes: More than the barrier effect? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 103–123. [Google Scholar] [CrossRef]
- Carter, D.R.; Beaupre, G.S.; Giori, N.J.; Helms, J.A. Mechanobiology of skeletal regeneration. Clin. Orthop. Relat. Res. 1998, S41–S55. [Google Scholar] [CrossRef] [PubMed]
- Josephson, T.O.; Morgan, E.F. Harnessing mechanical cues in the cellular microenvironment for bone regeneration. Front. Physiol. 2023, 14, 1232698. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Duan, B.; Hou, K.; Mao, L.; Wang, X. A comparative in vitro and in vivo study of porcine- and bovine-derived non-cross-linked collagen membranes. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 568–578. [Google Scholar] [CrossRef]
- Shi, X.; Li, X.; Tian, Y.; Qu, X.; Zhai, S.; Liu, Y.; Jia, W.; Cui, Y.; Chu, S. Physical, mechanical, and biological properties of collagen membranes for guided bone regeneration: A comparative in vitro study. BMC Oral. Health 2023, 23, 510. [Google Scholar] [CrossRef] [PubMed]
- Panahipour, L.; Kargarpour, Z.; Luza, B.; Lee, J.S.; Gruber, R. TGF-beta Activity Related to the Use of Collagen Membranes: In Vitro Bioassays. Int. J. Mol. Sci. 2020, 21, 6636. [Google Scholar] [CrossRef] [PubMed]
- Stahli, A.; Miron, R.J.; Bosshardt, D.D.; Sculean, A.; Gruber, R. Collagen Membranes Adsorb the Transforming Growth Factor-beta Receptor I Kinase-Dependent Activity of Enamel Matrix Derivative. J. Periodontol. 2016, 87, 583–590. [Google Scholar] [CrossRef]
- Di Summa, F.; Kargarpour, Z.; Nasirzade, J.; Stahli, A.; Mitulovic, G.; Panic-Jankovic, T.; Koller, V.; Kaltenbach, C.; Muller, H.; Panahipour, L.; et al. TGFbeta activity released from platelet-rich fibrin adsorbs to titanium surface and collagen membranes. Sci. Rep. 2020, 10, 10203. [Google Scholar] [CrossRef]
- Hillmann, G.; Steinkamp-Zucht, A.; Geurtsen, W.; Gross, G.; Hoffmann, A. Culture of primary human gingival fibroblasts on biodegradable membranes. Biomaterials 2002, 23, 1461–1469. [Google Scholar] [CrossRef]
- Willershausen, I.; Barbeck, M.; Boehm, N.; Sader, R.; Willershausen, B.; Kirkpatrick, C.J.; Ghanaati, S. Non-cross-linked collagen type I/III materials enhance cell proliferation: In vitro and in vivo evidence. J. Appl. Oral. Sci. 2014, 22, 29–37. [Google Scholar] [CrossRef]
- Silva, E.C.; Omonte, S.V.; Martins, A.G.; de Castro, H.H.; Gomes, H.E.; Zenobio, E.G.; de Oliveira, P.A.; Horta, M.C.; Souza, P.E. Hyaluronic acid on collagen membranes: An experimental study in rats. Arch. Oral. Biol. 2017, 73, 214–222. [Google Scholar] [CrossRef]
- Song, J.M.; Shin, S.H.; Kim, Y.D.; Lee, J.Y.; Baek, Y.J.; Yoon, S.Y.; Kim, H.S. Comparative study of chitosan/fibroin-hydroxyapatite and collagen membranes for guided bone regeneration in rat calvarial defects: Micro-computed tomography analysis. Int. J. Oral. Sci. 2014, 6, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, Y.; Amizuka, N.; Nakadate, M.; Ohnishi, H.; Fujii, N.; Oda, K.; Nomura, S.; Maeda, T. A histological evaluation for guided bone regeneration induced by a collagenous membrane. Biomaterials 2005, 26, 6158–6166. [Google Scholar] [CrossRef] [PubMed]
- Feher, B.; Apaza Alccayhuaman, K.A.; Strauss, F.J.; Lee, J.S.; Tangl, S.; Kuchler, U.; Gruber, R. Osteoconductive properties of upside-down bilayer collagen membranes in rat calvarial defects. Int. J. Implant. Dent. 2021, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Alccayhuaman, K.A.A.; Tangl, S.; Blouin, S.; Hartmann, M.A.; Heimel, P.; Kuchler, U.; Lee, J.S.; Gruber, R. Osteoconductive Properties of a Volume-Stable Collagen Matrix in Rat Calvaria Defects: A Pilot Study. Biomedicines 2021, 9, 732. [Google Scholar] [CrossRef] [PubMed]
- Kuchler, U.; Rybaczek, T.; Dobask, T.; Heimel, P.; Tangl, S.; Klehm, J.; Menzel, M.; Gruber, R. Bone-conditioned medium modulates the osteoconductive properties of collagen membranes in a rat calvaria defect model. Clin. Oral. Implants Res. 2018, 29, 381–388. [Google Scholar] [CrossRef]
- Strauss, F.J.; Kuchler, U.; Kobatake, R.; Heimel, P.; Tangl, S.; Gruber, R. Acid bone lysates reduce bone regeneration in rat calvaria defects. J. Biomed. Mater. Res. A 2021, 109, 659–665. [Google Scholar] [CrossRef]
- Nasirzade, J.; Alccayhuaman, K.A.A.; Kargarpour, Z.; Kuchler, U.; Strauss, F.J.; Panahipour, L.; Kampleitner, C.; Heimel, P.; Schwarz, F.; Gruber, R. Acid Dentin Lysate Failed to Modulate Bone Formation in Rat Calvaria Defects. Biology 2021, 10, 196. [Google Scholar] [CrossRef]
- Kusumbe, A.P.; Ramasamy, S.K.; Adams, R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 2014, 507, 323–328. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Imber, J.C.; Bosshardt, D.D.; Stahli, A.; Saulacic, N.; Deschner, J.; Sculean, A. Pre-clinical evaluation of the effect of a volume-stable collagen matrix on periodontal regeneration in two-wall intrabony defects. J. Clin. Periodontol. 2021, 48, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Katagiri, H.; El Tawil, Y.; Lang, N.P.; Imber, J.C.; Sculean, A.; Fujioka-Kobayashi, M.; Saulacic, N. Collagen-Based Matrices for Osteoconduction: A Preclinical In Vivo Study. Biomedicines 2021, 9, 143. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Wei, M. Biomineralization of Collagen-Based Materials for Hard Tissue Repair. Int. J. Mol. Sci. 2021, 22, 944. [Google Scholar] [CrossRef]
- Tang, S.; Dong, Z.; Ke, X.; Luo, J.; Li, J. Advances in biomineralization-inspired materials for hard tissue repair. Int. J. Oral. Sci. 2021, 13, 42. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Acri, T.; Geary, S.; Salem, A.K. Biomimetic Mineralization of Biomaterials Using Simulated Body Fluids for Bone Tissue Engineering and Regenerative Medicine. Tissue Eng. Part. A 2017, 23, 1169–1180. [Google Scholar] [CrossRef]
- Agis, H.; Magdalenko, M.; Stogerer, K.; Watzek, G.; Gruber, R. Collagen barrier membranes decrease osteoclastogenesis in murine bone marrow cultures. Clin. Oral. Implants Res. 2010, 21, 656–661. [Google Scholar] [CrossRef]
- Behring, J.; Junker, R.; Walboomers, X.F.; Chessnut, B.; Jansen, J.A. Toward guided tissue and bone regeneration: Morphology, attachment, proliferation, and migration of cells cultured on collagen barrier membranes. A systematic review. Odontology 2008, 96, 1–11. [Google Scholar] [CrossRef]
- Bianco, P.; Kuznetsov, S.A.; Riminucci, M.; Gehron Robey, P. Postnatal skeletal stem cells. Methods Enzymol. 2006, 419, 117–148. [Google Scholar] [CrossRef] [PubMed]
- Gerstenfeld, L.C.; Cullinane, D.M.; Barnes, G.L.; Graves, D.T.; Einhorn, T.A. Fracture healing as a post-natal developmental process: Molecular, spatial, and temporal aspects of its regulation. J. Cell Biochem. 2003, 88, 873–884. [Google Scholar] [CrossRef] [PubMed]
- Permuy, M.; Guede, D.; Lopez-Pena, M.; Munoz, F.; Caeiro, J.R.; Gonzalez-Cantalapiedra, A. Effects of diacerein on cartilage and subchondral bone in early stages of osteoarthritis in a rabbit model. BMC Vet. Res. 2015, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Wildemann, B.; Ignatius, A.; Leung, F.; Taitsman, L.A.; Smith, R.M.; Pesantez, R.; Stoddart, M.J.; Richards, R.G.; Jupiter, J.B. Non-union bone fractures. Nat. Rev. Dis. Primers 2021, 7, 57. [Google Scholar] [CrossRef]
- Mwale, F.; Stachura, D.; Roughley, P.; Antoniou, J. Limitations of using aggrecan and type X collagen as markers of chondrogenesis in mesenchymal stem cell differentiation. J. Orthop. Res. 2006, 24, 1791–1798. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apaza Alccayhuaman, K.A.; Heimel, P.; Tangl, S.; Lettner, S.; Kampleitner, C.; Panahipour, L.; Kuchler, U.; Gruber, R. Active and Passive Mineralization of Bio-Gide® Membranes in Rat Calvaria Defects. J. Funct. Biomater. 2024, 15, 54. https://doi.org/10.3390/jfb15030054
Apaza Alccayhuaman KA, Heimel P, Tangl S, Lettner S, Kampleitner C, Panahipour L, Kuchler U, Gruber R. Active and Passive Mineralization of Bio-Gide® Membranes in Rat Calvaria Defects. Journal of Functional Biomaterials. 2024; 15(3):54. https://doi.org/10.3390/jfb15030054
Chicago/Turabian StyleApaza Alccayhuaman, Karol Ali, Patrick Heimel, Stefan Tangl, Stefan Lettner, Carina Kampleitner, Layla Panahipour, Ulrike Kuchler, and Reinhard Gruber. 2024. "Active and Passive Mineralization of Bio-Gide® Membranes in Rat Calvaria Defects" Journal of Functional Biomaterials 15, no. 3: 54. https://doi.org/10.3390/jfb15030054








