Characterization and In Vitro Evaluation of Porous Polymer-Blended Scaffolds Functionalized with Tricalcium Phosphate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Scaffolds
2.3. Physicochemical Characterization of Composite Scaffolds
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. Differential Scanning Calorimetry
2.3.3. Optical and Scanning Electron Microscopy
2.3.4. Contact Angle Measurement
2.3.5. Degradation Studies
2.4. Biological Evaluation
2.4.1. Studies on Extracts
2.4.2. In Vitro Cytocompatibility
2.5. Statistical Analysis
3. Results
3.1. ATR-FTIR Spectroscopy
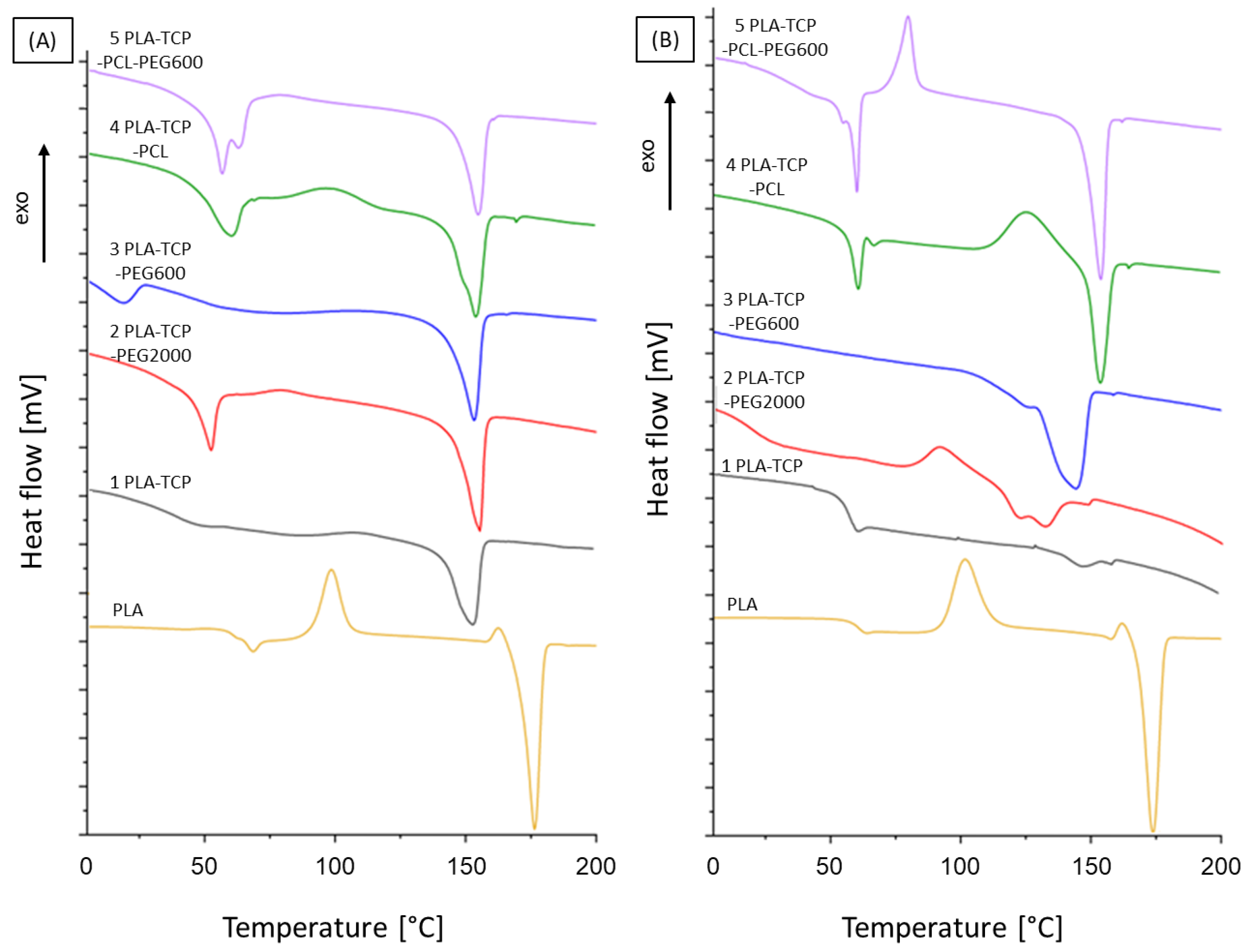
3.2. Differential Scanning Calorimetry
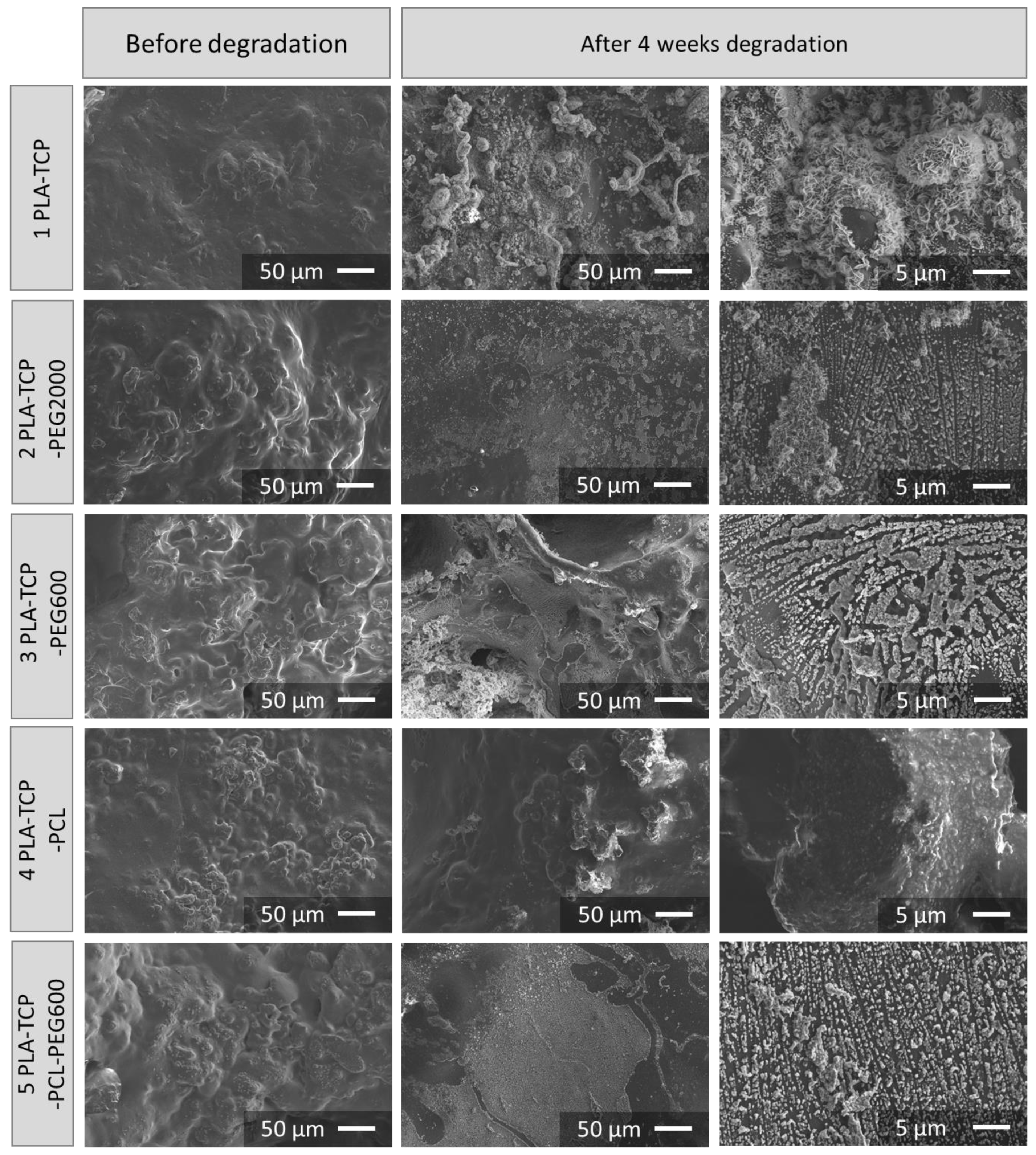
3.3. Morphology and Microstructure of Fabricated Scaffolds
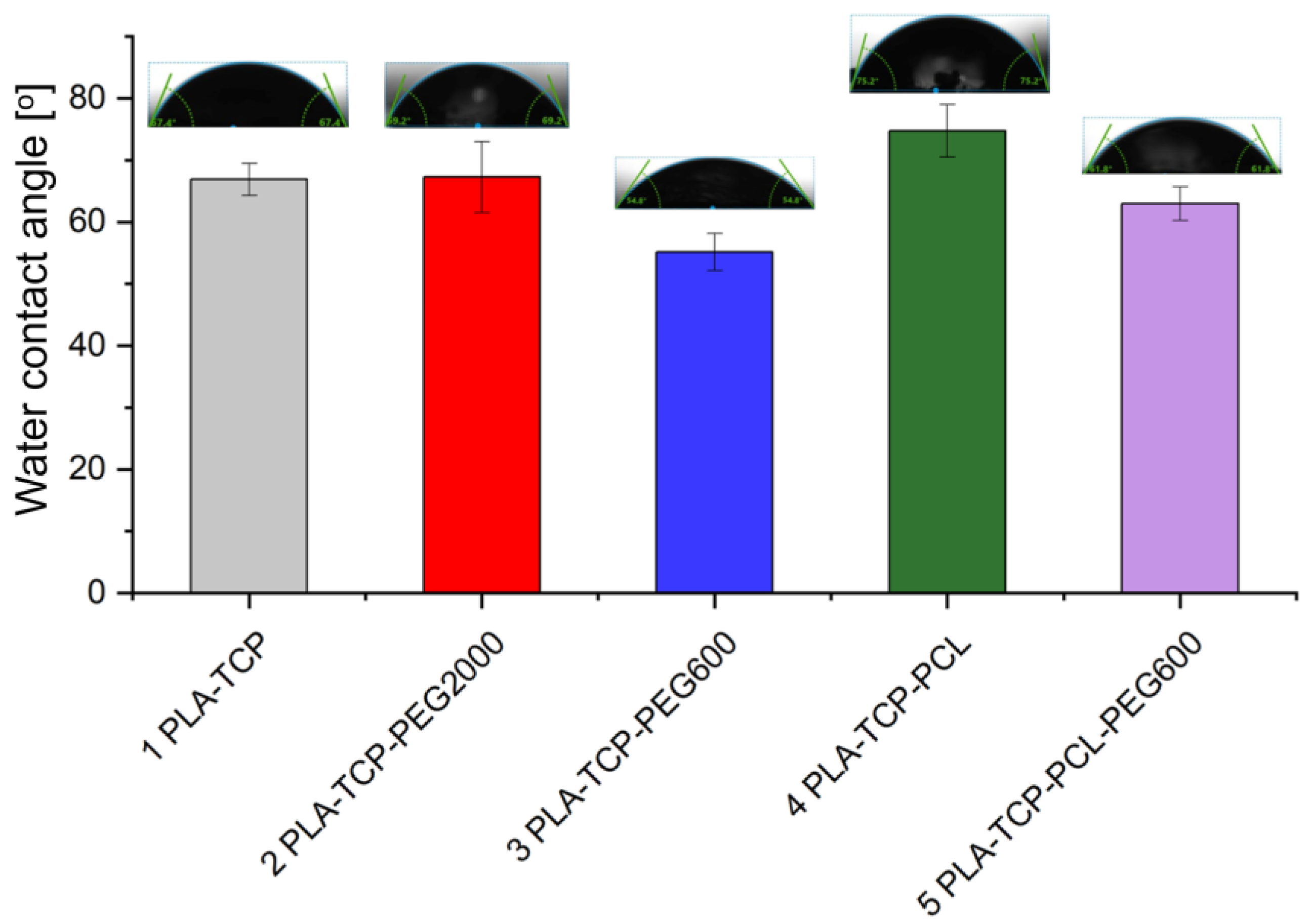
3.4. Water Contact Angle Measurement
3.5. Degradation Studies
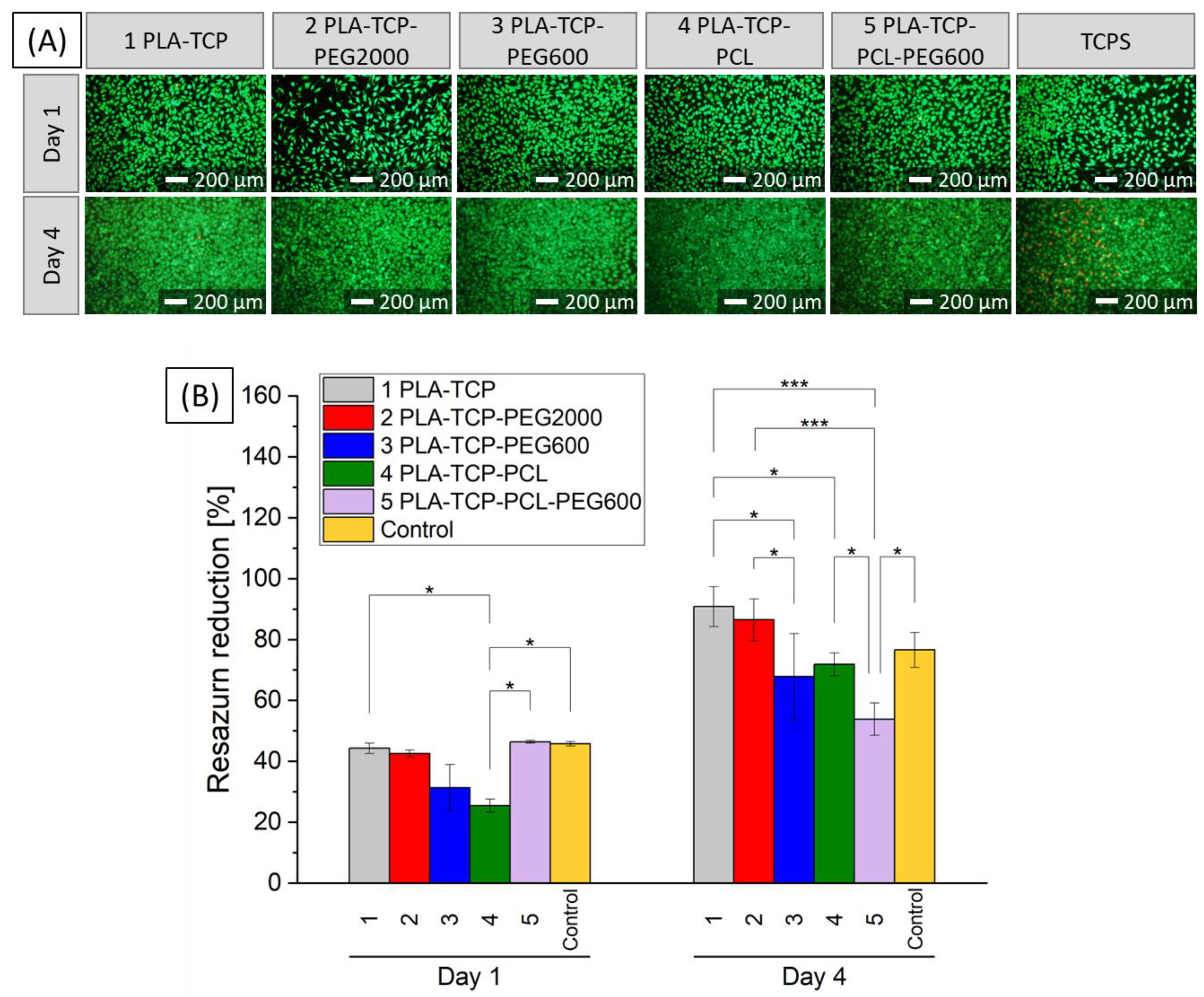
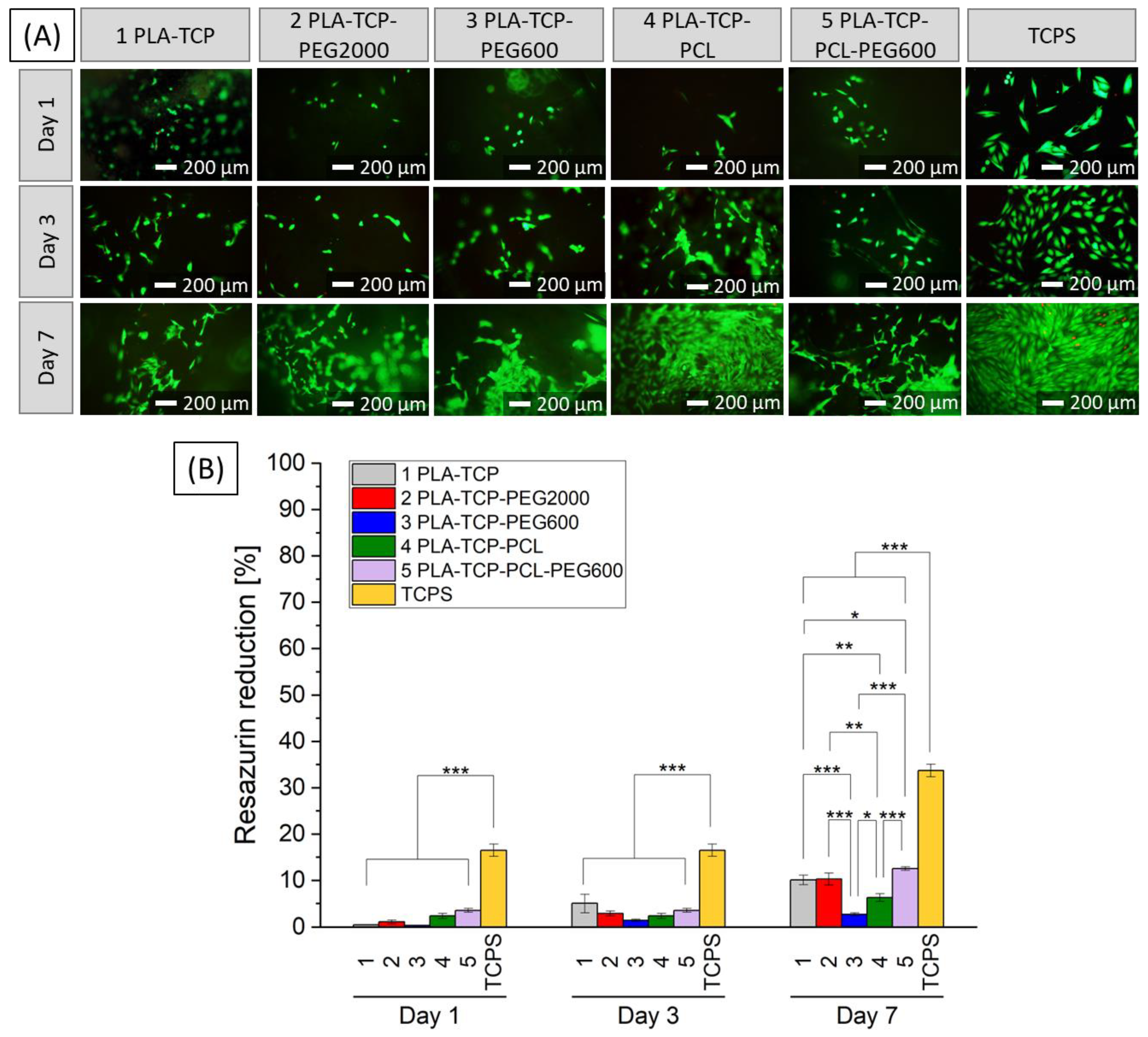
3.6. Biological Performance
3.6.1. Cytotoxicity Tests with Extracts
3.6.2. In Vitro Studies with MG-63 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Roseti, L.; Parisi, V.; Petretta, M.; Cavallo, C.; Desando, G.; Bartolotti, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the Art and New Perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Yeung, K.W.K.; Liu, C.; Yang, X. Biomimetic Porous Scaffolds for Bone Tissue Engineering. Mater. Sci. Eng. R Rep. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for Bone Tissue Engineering Scaffolds: A Review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef]
- Ghassemi, T.; Shahroodi, A.; Ebrahimzadeh, M.H.; Mousavian, A.; Movaffagh, J.; Moradi, A. Current Concepts in Scaffolding for Bone Tissue Engineering. Arch. Bone Jt. Surg. 2018, 6, 90–99. [Google Scholar]
- Abdelaziz, A.G.; Nageh, H.; Abdo, S.M.; Abdalla, M.S.; Amer, A.A.; Abdal-hay, A.; Barhoum, A. A Review of 3D Polymeric Scaffolds for Bone Tissue Engineering: Principles, Fabrication Techniques, Immunomodulatory Roles, and Challenges. Bioengineering 2023, 10, 204. [Google Scholar] [CrossRef]
- Lee, S.S.; Du, X.; Kim, I.; Ferguson, S.J. Scaffolds for Bone-Tissue Engineering. Matter 2022, 5, 2722–2759. [Google Scholar] [CrossRef]
- Abbasi, N.; Hamlet, S.; Love, R.M.; Nguyen, N.-T. Porous Scaffolds for Bone Regeneration. J. Sci. Adv. Mater. Devices 2020, 5, 1–9. [Google Scholar] [CrossRef]
- Wang, C.; Liu, J.; Min, S.; Liu, Y.; Liu, B.; Hu, Y.; Wang, Z.; Mao, F.; Wang, C.; Ma, X.; et al. The Effect of Pore Size on the Mechanical Properties, Biodegradation and Osteogenic Effects of Additively Manufactured Magnesium Scaffolds after High Temperature Oxidation: An in Vitro and in Vivo Study. Bioact. Mater. 2023, 28, 537–548. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in Modern Medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Bhandari, P.; Singh, T.; Sharma, S.; Singh, J.; Singh, S. Promising Role of Polylactic Acid as an Ingenious Biomaterial in Scaffolds, Drug Delivery, Tissue Engineering, and Medical Implants: Research Developments, and Prospective Applications. Molecules 2023, 28, 485. [Google Scholar] [CrossRef]
- Shahverdi, M.; Seifi, S.; Akbari, A.; Mohammadi, K.; Shamloo, A.; Movahhedy, M.R. Melt Electrowriting of PLA, PCL, and Composite PLA/PCL Scaffolds for Tissue Engineering Application. Sci. Rep. 2022, 12, 19935. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.P.; Da Silva, B.C.R.; Hamouda, A.E.I.; De Toledo, M.A.S.; Schalla, C.; Rütten, S.; Goetzke, R.; Mattoso, L.H.C.; Zenke, M.; Sechi, A. PLA/Hydroxyapatite Scaffolds Exhibit in Vitro Immunological Inertness and Promote Robust Osteogenic Differentiation of Human Mesenchymal Stem Cells without Osteogenic Stimuli. Sci. Rep. 2022, 12, 2333. [Google Scholar] [CrossRef]
- Patrício, T.; Domingos, M.; Gloria, A.; Bártolo, P. Characterisation of PCL and PCL/PLA Scaffolds for Tissue Engineering. Procedia CIRP 2013, 5, 110–114. [Google Scholar] [CrossRef]
- Czwartos, J.; Zaszczyńska, A.; Nowak-Stępniowska, A.; Fok, T.; Budner, B.; Bartnik, A.; Wachulak, P.; Kołbuk, D.; Sajkiewicz, P.; Fiedorowicz, H. The Novel Approach to Physico-Chemical Modification and Cytocompatibility Enhancement of Fibrous Polycaprolactone (PCL) Scaffolds Using Soft X-Ray/Extreme Ultraviolet (SXR/EUV) Radiation and Low-Temperature, SXR/EUV Induced, Nitrogen and Oxygen Plasmas. Appl. Surf. Sci. 2022, 606, 154779. [Google Scholar] [CrossRef]
- Salehi, S.; Ghomi, H.; Hassanzadeh-Tabrizi, S.A.; Koupaei, N.; Khodaei, M. The Effect of Polyethylene Glycol on Printability, Physical and Mechanical Properties and Osteogenic Potential of 3D-Printed Poly (l-Lactic Acid)/Polyethylene Glycol Scaffold for Bone Tissue Engineering. Int. J. Biol. Macromol. 2022, 221, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Nazari, T.; Bayandori Moghaddam, A.; Davoodi, Z. Optimized Polylactic Acid/Polyethylene Glycol (PLA/PEG) Electrospun Fibrous Scaffold for Drug Delivery: Effect of Graphene Oxide on the Cefixime Release Mechanism. Mater. Res. Express 2019, 6, 115351. [Google Scholar] [CrossRef]
- Szymczyk-Ziółkowska, P.; Łabowska, M.B.; Detyna, J.; Michalak, I.; Gruber, P. A Review of Fabrication Polymer Scaffolds for Biomedical Applications Using Additive Manufacturing Techniques. Biocybern. Biomed. Eng. 2020, 40, 624–638. [Google Scholar] [CrossRef]
- Kirillova, A.; Yeazel, T.R.; Asheghali, D.; Petersen, S.R.; Dort, S.; Gall, K.; Becker, M.L. Fabrication of Biomedical Scaffolds Using Biodegradable Polymers. Chem. Rev. 2021, 121, 11238–11304. [Google Scholar] [CrossRef]
- Eltom, A.; Zhong, G.; Muhammad, A. Scaffold Techniques and Designs in Tissue Engineering Functions and Purposes: A Review. Adv. Mater. Sci. Eng. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Toh, H.W.; Toong, D.W.Y.; Ng, J.C.K.; Ow, V.; Lu, S.; Tan, L.P.; Wong, P.E.H.; Venkatraman, S.; Huang, Y.; Ang, H.Y. Polymer Blends and Polymer Composites for Cardiovascular Implants. Eur. Polym. J. 2021, 146, 110249. [Google Scholar] [CrossRef]
- Du, X.; Fu, S.; Zhu, Y. 3D Printing of Ceramic-Based Scaffolds for Bone Tissue Engineering: An Overview. J. Mater. Chem. B 2018, 6, 4397–4412. [Google Scholar] [CrossRef] [PubMed]
- Aydogdu, M.O.; Oner, E.T.; Ekren, N.; Erdemir, G.; Kuruca, S.E.; Yuca, E.; Bostan, M.S.; Eroglu, M.S.; Ikram, F.; Uzun, M.; et al. Comparative Characterization of the Hydrogel Added PLA/β-TCP Scaffolds Produced by 3D Bioprinting. Bioprinting 2019, 13, e00046. [Google Scholar] [CrossRef]
- Wang, W.; Liu, P.; Zhang, B.; Gui, X.; Pei, X.; Song, P.; Yu, X.; Zhang, Z.; Zhou, C. Fused Deposition Modeling Printed PLA/Nano β-TCP Composite Bone Tissue Engineering Scaffolds for Promoting Osteogenic Induction Function. Int. J. Nanomed. 2023, 18, 5815–5830. [Google Scholar] [CrossRef]
- Wei, S.; Ma, J.-X.; Xu, L.; Gu, X.-S.; Ma, X.-L. Biodegradable Materials for Bone Defect Repair. Mil. Med. Res. 2020, 7, 54. [Google Scholar] [CrossRef]
- Ruiz-Aguilar, C. Porous Phosphate-Based Bioactive Glass/β-TCP Scaffold for Tooth Remineralization. PLoS ONE 2023, 18, e0284885. [Google Scholar] [CrossRef] [PubMed]
- Elhattab, K.; Bhaduri, S.B.; Sikder, P. Influence of Fused Deposition Modelling Nozzle Temperature on the Rheology and Mechanical Properties of 3D Printed β-Tricalcium Phosphate (TCP)/Polylactic Acid (PLA) Composite. Polymers 2022, 14, 1222. [Google Scholar] [CrossRef] [PubMed]
- Onak Pulat, G.; Sunal, G.; Karaman, O. Enhanced osteogenic differentiation of human mesenchymal stem cells BY flexible β-TCP/PLA bone grafts with silicate additive. Konya J. Eng. Sci. 2023, 11, 770–782. [Google Scholar] [CrossRef]
- Donate, R.; Monzón, M.; Alemán-Domínguez, M.E.; Ortega, Z. Enzymatic Degradation Study of PLA-Based Composite Scaffolds. Rev. Adv. Mater. Sci. 2020, 59, 170–175. [Google Scholar] [CrossRef]
- Wang, Q.; Ye, W.; Ma, Z.; Xie, W.; Zhong, L.; Wang, Y.; Rong, Q. 3D Printed PCL/β-TCP Cross-Scale Scaffold with High-Precision Fiber for Providing Cell Growth and Forming Bones in the Pores. Mater. Sci. Eng. C 2021, 127, 112197. [Google Scholar] [CrossRef]
- Helaehil, J.V.; Lourenço, C.B.; Huang, B.; Helaehil, L.V.; De Camargo, I.X.; Chiarotto, G.B.; Santamaria-Jr, M.; Bártolo, P.; Caetano, G.F. In Vivo Investigation of Polymer-Ceramic PCL/HA and PCL/β-TCP 3D Composite Scaffolds and Electrical Stimulation for Bone Regeneration. Polymers 2021, 14, 65. [Google Scholar] [CrossRef]
- Tabatabaei, F.; Gelin, A.; Rasoulianboroujeni, M.; Tayebi, L. Coating of 3D Printed PCL/TCP Scaffolds Using Homogenized-Fibrillated Collagen. Colloids Surf. B Biointerfaces 2022, 217, 112670. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, L.D.; Passador, F.R.; Lobo, A.O.; Trichês, E.D.S. Morphological, Thermal and Bioactivity Evaluation of Electrospun PCL/β-TCP Fibers for Tissue Regeneration. Polímeros 2019, 29, e2019005. [Google Scholar] [CrossRef]
- Pae, H.; Kang, J.; Cha, J.; Lee, J.; Paik, J.; Jung, U.; Kim, B.; Choi, S. 3D-printed Polycaprolactone Scaffold Mixed with Β-tricalcium Phosphate as a Bone Regenerative Material in Rabbit Calvarial Defects. J. Biomed. Mater. Res. 2019, 107, 1254–1263. [Google Scholar] [CrossRef] [PubMed]
- Senatov, F.; Zimina, A.; Chubrik, A.; Kolesnikov, E.; Permyakova, E.; Voronin, A.; Poponova, M.; Orlova, P.; Grunina, T.; Nikitin, K.; et al. Effect of Recombinant BMP-2 and Erythropoietin on Osteogenic Properties of Biomimetic PLA/PCL/HA and PHB/HA Scaffolds in Critical-Size Cranial Defects Model. Biomater. Adv. 2022, 135, 112680. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Sun, J.; Zhu, Q.; Jin, X.; Zhang, Z.; Zhao, Z.; Chen, G.; Wang, C.; Jiang, H.; Zhang, P. Microstructures and Properties of Polycaprolactone/Tricalcium Phosphate Scaffolds Containing Polyethylene Glycol Fabricated by 3D Printing. Ceram. Int. 2022, 48, 24032–24043. [Google Scholar] [CrossRef]
- Sankaralingam, P.; Sakthivel, P.; Chinnaswamy Thangavel, V. Novel Composites for Bone Tissue Engineering. In Biomedical Engineering; Haidar, Z.S., Ed.; IntechOpen: London, UK, 2023; Volume 17, ISBN 978-1-80356-896-6. [Google Scholar]
- Revati, R.; Majid, M.S.A.; Ridzuan, M.J.M.; Basaruddin, K.S.; Rahman, Y.M.N.; Cheng, E.M.; Gibson, A.G. In Vitro Degradation of a 3D Porous Pennisetum Purpureum/PLA Biocomposite Scaffold. J. Mech. Behav. Biomed. Mater. 2017, 74, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Qader, I.N.; Pekdemir, R.M.E.; Coşkun, M.; Kanca, M.S.; Kök, M.; Dağdelen, F. Biocompatible PLA/PCL Blends Nanocomposites Doped with Nanographite: Physico-Chemical, and Thermal Behaviour. J. Polym. Res. 2022, 29, 264. [Google Scholar] [CrossRef]
- Fernández-Tena, A.; Otaegi, I.; Irusta, L.; Sebastián, V.; Guerrica-Echevarria, G.; Müller, A.J.; Aranburu, N. High-Impact PLA in Compatibilized PLA/PCL Blends: Optimization of Blend Composition and Type and Content of Compatibilizer. Macro. Mater. Eng. 2023, 308, 2300213. [Google Scholar] [CrossRef]
- Shojaee Kang Sofla, M.; Mortazavi, S.; Seyfi, J. Preparation and Characterization of Polyvinyl Alcohol/Chitosan Blends Plasticized and Compatibilized by Glycerol/Polyethylene Glycol. Carbohydr. Polym. 2020, 232, 115784. [Google Scholar] [CrossRef]
- Chavalitpanya, K.; Phattanarudee, S. Poly(Lactic Acid)/Polycaprolactone Blends Compatibilized with Block Copolymer. Energy Procedia 2013, 34, 542–548. [Google Scholar] [CrossRef]
- Knap, K.; Reczyńska-Kolman, K.; Kwiecień, K.; Niewolik, D.; Płonka, J.; Ochońska, D.; Jeleń, P.; Mielczarek, P.; Kazek-Kęsik, A.; Jaszcz, K.; et al. Poly(Sebacic Acid) Microparticles Loaded with Azithromycin as Potential Pulmonary Drug Delivery System: Physicochemical Properties, Antibacterial Behavior, and Cytocompatibility Studies. Biomater. Adv. 2023, 153, 213540. [Google Scholar] [CrossRef] [PubMed]
- Desante, G.; Pudełko, I.; Krok-Borkowicz, M.; Pamuła, E.; Jacobs, P.; Kazek-Kęsik, A.; Nießen, J.; Telle, R.; Gonzalez-Julian, J.; Schickle, K. Surface Multifunctionalization of Inert Ceramic Implants by Calcium Phosphate Biomimetic Coating Doped with Nanoparticles Encapsulating Antibiotics. ACS Appl. Mater. Interfaces 2023, 15, 21699–21718. [Google Scholar] [CrossRef] [PubMed]
- Savaris, M.; Braga, G.L.; Dos Santos, V.; Carvalho, G.A.; Falavigna, A.; Machado, D.C.; Viezzer, C.; Brandalise, R.N. Biocompatibility Assessment of Poly(Lactic Acid) Films after Sterilization with Ethylene Oxide in Histological Study In Vivo with Wistar Rats and Cellular Adhesion of Fibroblasts In Vitro. Int. J. Polym. Sci. 2017, 2017, 7158650. [Google Scholar] [CrossRef]
- Chieng, B.; Ibrahim, N.; Yunus, W.; Hussein, M. Poly(Lactic Acid)/Poly(Ethylene Glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2013, 6, 93–104. [Google Scholar] [CrossRef]
- Kandi, R.; Pandey, P.M.; Majood, M.; Mohanty, S. Fabrication and Characterization of Customized Tubular Scaffolds for Tracheal Tissue Engineering by Using Solvent Based 3D Printing on Predefined Template. Rapid Prototyp. J. 2021, 27, 421–428. [Google Scholar] [CrossRef]
- Afriani, F.; Dahlan, K.; Nikmatin, S.; Zuas, O. Alginate affecting the characteristics of porous β-TCP/alginate composite scaffolds. J. Optoelectron. Biomed. Mater. 2015, 7, 67–76. [Google Scholar]
- De Aza, P.N.; Guitián, F.; Santos, C.; De Aza, S.; Cuscó, R.; Artús, L. Vibrational Properties of Calcium Phosphate Compounds. 2. Comparison between Hydroxyapatite and β-Tricalcium Phosphate. Chem. Mater. 1997, 9, 916–922. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the Structure of Solution Grown Crystals of Lactide Copolymers by Means of Chemical Reactions. Kolloid Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Al-Azzam, N.; Alazzam, A. Micropatterning of Cells via Adjusting Surface Wettability Using Plasma Treatment and Graphene Oxide Deposition. PLoS ONE 2022, 17, e0269914. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Yang, W.; Liang, W.; Yu, H.; Liu, L. Recent Advance in Surface Modification for Regulating Cell Adhesion and Behaviors. Nanotechnol. Rev. 2020, 9, 971–989. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization of Standardization: Geneva, Switzerland, 2009.
- Pant, S.; Thomas, S.; Loganathan, S.; Valapa, R.B. 3D Bioprinted Poly(Lactic Acid)/Mesoporous Bioactive Glass Based Biomimetic Scaffold with Rapid Apatite Crystallization and in-Vitro Cytocompatability for Bone Tissue Engineering. Int. J. Biol. Macromol. 2022, 217, 979–997. [Google Scholar] [CrossRef] [PubMed]
- Sabee, M.M.S.M.; Awang, M.S.; Bustami, Y.; Hamid, Z.A.A. Antimicrobial Activity Evaluation for Gentamicin Loaded PLA Microspheres. Mater. Today Proc. 2019, 16, 2060–2066. [Google Scholar] [CrossRef]
- Wang, K.; Wang, R.; Hu, K.; Ma, Z.; Zhang, C.; Sun, X. Crystallization-Driven Formation Poly (l-Lactic Acid)/Poly (d-Lactic Acid)-Polyethylene Glycol-Poly (l-Lactic Acid) Small-Sized Microsphere Structures by Solvent-Induced Self-Assembly. Int. J. Biol. Macromol. 2024, 254, 127924. [Google Scholar] [CrossRef] [PubMed]
- Gautam, A.; Pal, K. Gefitinib Conjugated PEG Passivated Graphene Quantum Dots Incorporated PLA Microspheres for Targeted Anticancer Drug Delivery. Heliyon 2022, 8, e12512. [Google Scholar] [CrossRef]
- Kaniuk, E.; Lechowska-Liszka, A.; Gajek, M.; Nikodem, A.; Ścisłowska-Czarnecka, A.; Rapacz-Kmita, A.; Stodolak-Zych, E. Correlation between Porosity and Physicochemical and Biological Properties of Electrospinning PLA/PVA Membranes for Skin Regeneration. Biomater. Adv. 2023, 152, 213506. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ming, J.; Cai, S.; Wang, X.; Ning, X. One-Step Fabrication of Polylactic Acid (PLA) Nanofibrous Membranes with Spider-Web-like Structure for High-Efficiency PM0.3 Capture. J. Hazard. Mater. 2024, 465, 133232. [Google Scholar] [CrossRef] [PubMed]
- Mohammed-Sadhakathullah, A.H.M.; Paulo-Mirasol, S.; Molina, B.G.; Torras, J.; Armelin, E. PLA-PEG-Cholesterol Biomimetic Membrane for Electrochemical Sensing of Antioxidants. Electrochim. Acta 2024, 476, 143716. [Google Scholar] [CrossRef]
- Gangolphe, L.; Leon-Valdivieso, C.Y.; Nottelet, B.; Déjean, S.; Bethry, A.; Pinese, C.; Bossard, F.; Garric, X. Electrospun Microstructured PLA-Based Scaffolds Featuring Relevant Anisotropic, Mechanical and Degradation Characteristics for Soft Tissue Engineering. Mater. Sci. Eng. C 2021, 129, 112339. [Google Scholar] [CrossRef]
- Ren, Q.; Zhu, X.; Li, W.; Wu, M.; Cui, S.; Ling, Y.; Ma, X.; Wang, G.; Wang, L.; Zheng, W. Fabrication of Super-Hydrophilic and Highly Open-Porous Poly (Lactic Acid) Scaffolds Using Supercritical Carbon Dioxide Foaming. Int. J. Biol. Macromol. 2022, 205, 740–748. [Google Scholar] [CrossRef]
- Molina, M.I.E.; Komvopoulos, K. In Vitro Degradation of Fibrous Bilayer Poly-L-Lactic Acid Scaffolds. J. Biomech. 2023, 161, 111823. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Q.; He, F.; Zuo, F.; Shi, X. Fabrication of Cancellous-Bone-Mimicking β-Tricalcium Phosphate Bioceramic Scaffolds with Tunable Architecture and Mechanical Strength by Stereolithography 3D Printing. J. Eur. Ceram. Soc. 2022, 42, 6713–6720. [Google Scholar] [CrossRef]
- Nadi, A.; Khodaei, M.; Javdani, M.; Mirzaei, S.A.; Soleimannejad, M.; Tayebi, L.; Asadpour, S. Fabrication of Functional and Nano-Biocomposite Scaffolds Using Strontium-Doped Bredigite Nanoparticles/Polycaprolactone/Poly Lactic Acid via 3D Printing for Bone Regeneration. Int. J. Biol. Macromol. 2022, 219, 1319–1336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Bai, T.; Wang, P.; Wang, Y.; Liu, C.; Shen, C. Selective Dispersion of Carbon Nanotubes and Nanoclay in Biodegradable Poly(ε-Caprolactone)/Poly(Lactic Acid) Blends with Improved Toughness, Strength and Thermal Stability. Int. J. Biol. Macromol. 2020, 153, 1272–1280. [Google Scholar] [CrossRef] [PubMed]
- Dodda, J.M.; Azar, M.G.; Bělský, P.; Šlouf, M.; Gajdošová, V.; Kasi, P.B.; Anerillas, L.O.; Kovářík, T. Bioresorbable Films of Polycaprolactone Blended with Poly(Lactic Acid) or Poly(Lactic-Co-Glycolic Acid). Int. J. Biol. Macromol. 2023, 248, 126654. [Google Scholar] [CrossRef] [PubMed]
- Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical Processing of PCL and PCL-PEG Blends to Produce Improved PCL-Based Porous Scaffolds. J. Supercrit. Fluids 2022, 186, 105611. [Google Scholar] [CrossRef]
- Haimi, S.; Suuriniemi, N.; Haaparanta, A.-M.; Ellä, V.; Lindroos, B.; Huhtala, H.; Räty, S.; Kuokkanen, H.; Sándor, G.K.; Kellomäki, M.; et al. Growth and Osteogenic Differentiation of Adipose Stem Cells on PLA/Bioactive Glass and PLA/β-TCP Scaffolds. Tissue Eng. Part A 2009, 15, 1473–1480. [Google Scholar] [CrossRef]
- Ferri, J.M.; Motoc, D.L.; Bou, S.F.; Balart, R. Thermal Expansivity and Degradation Properties of PLA/HA and PLA/βTCP in Vitro Conditioned Composites. J. Therm. Anal. Calorim. 2019, 138, 2691–2702. [Google Scholar] [CrossRef]
- Luyt, A.S.; Gasmi, S. Influence of Blending and Blend Morphology on the Thermal Properties and Crystallization Behaviour of PLA and PCL in PLA/PCL Blends. J. Mater. Sci. 2016, 51, 4670–4681. [Google Scholar] [CrossRef]
- Jia, S.; Yu, D.; Wang, Z.; Zhang, X.; Chen, L.; Fu, L. Morphologies, Crystallization, and Mechanical Properties of PLA-based Nanocomposites: Synergistic Effects of PEG/HNTs. J. Appl. Polym. Sci. 2019, 136, 47385. [Google Scholar] [CrossRef]
- Bijarimi, M.; Ahmad, S.; Rasid, R.; Khushairi, M.A.; Zakir, M. Poly(Lactic Acid)/Poly(Ethylene Glycol) Blends: Mechanical, Thermal and Morphological Properties. AIP Conf. Proc. 2016, 1727, 020002. [Google Scholar]
- Zhang, S.; Yan, D.; Zhao, L.; Lin, J. Composite Fibrous Membrane Comprising PLA and PCL Fibers for Biomedical Application. Compos. Commun. 2022, 34, 101268. [Google Scholar] [CrossRef]
- Zain, S.K.M.; Sazali, E.S.; Ghoshal, S.K.; Hisam, R. In Vitro Bioactivity and Biocompatibility Assessment of PCL/PLA–Scaffolded Mesoporous Silicate Bioactive Glass: Role of Boron Activation. J. Non Cryst. Solids 2024, 625, 122763. [Google Scholar] [CrossRef]
- Soh, E.; Kolos, E.; Ruys, A.J. Foamed High Porosity Alumina for Use as a Bone Tissue Scaffold. Ceram. Int. 2015, 41, 1031–1047. [Google Scholar] [CrossRef]
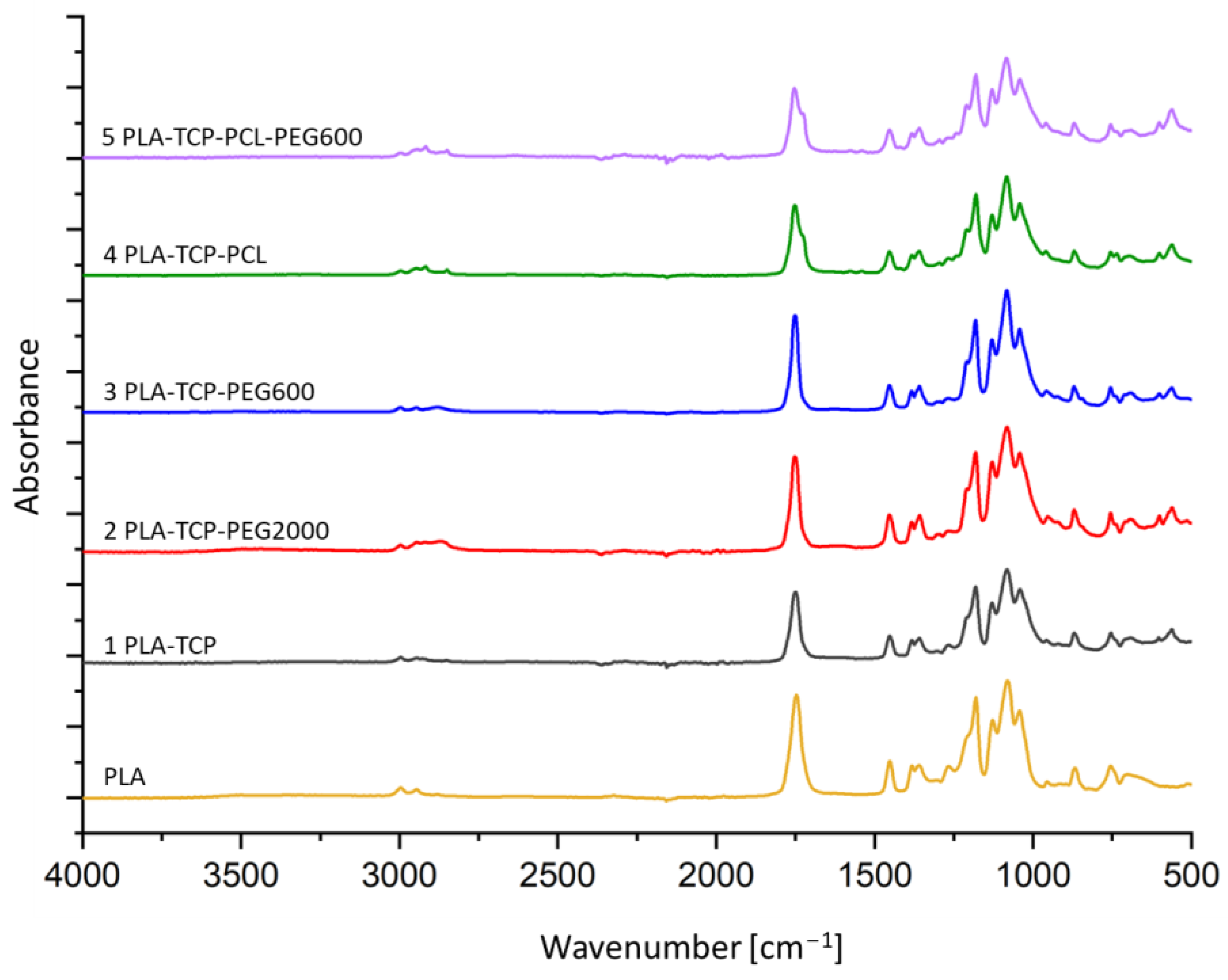


| Sample | Component [wt.%] | ||||
|---|---|---|---|---|---|
| PLA | TCP | PEG600 | PEG2000 | PCL | |
| 1 PLA-TCP | 72 | 28 | - | - | - |
| 2 PLA-TCP-PEG2000 | 60 | 28 | - | 12 | - |
| 3 PLA-TCP-PEG600 | 60 | 28 | 12 | - | - |
| 4 PLA-TCP-PCL | 60 | 28 | - | - | 12 |
| 5 PLA-TCP-PCL-PEG600 | 50 | 28 | 10 | - | 12 |
| Sample | Tg [°C] | Tcc [°C] | Tm [°C] | Xc [%] | ||||
|---|---|---|---|---|---|---|---|---|
| 1st Heating | 2nd Heating | 1st Heating | 2nd Heating | 1st Heating | 2nd Heating | 1st Heating | 2nd Heating | |
| PLA | 66 | 61 | 99 | 102 | 170 | 168 | 58 | 55 |
| 1 PLA-TCP | 41 | 54 | 109 | nd | 154 | 146 | 27 | 3 |
| 2 PLA-TCP -PEG2000 | 51 | 14 | 80 | 89 | 155 | 130 | 39 | 35 |
| 3 PLA-TCP -PEG600 | 46 | 42 | nd | nd | 152 | 143 | 39 | 17 |
| 4 PLA-TCP -PCL | nd | nd | 97 | 124 | 56 (PCL) 153 (PLA) | 57 (PCL) 153 (PLA) | 26 | 22 |
| 5 PLA-TCP-PCL-PEG600 | nd | 35 | nd | 77 | 154 (PLA) | 56 (PCL) 152 (PLA) | 37 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pudełko-Prażuch, I.; Balasubramanian, M.; Ganesan, S.M.; Marecik, S.; Walczak, K.; Pielichowska, K.; Chatterjee, S.; Kandaswamy, R.; Pamuła, E. Characterization and In Vitro Evaluation of Porous Polymer-Blended Scaffolds Functionalized with Tricalcium Phosphate. J. Funct. Biomater. 2024, 15, 57. https://doi.org/10.3390/jfb15030057
Pudełko-Prażuch I, Balasubramanian M, Ganesan SM, Marecik S, Walczak K, Pielichowska K, Chatterjee S, Kandaswamy R, Pamuła E. Characterization and In Vitro Evaluation of Porous Polymer-Blended Scaffolds Functionalized with Tricalcium Phosphate. Journal of Functional Biomaterials. 2024; 15(3):57. https://doi.org/10.3390/jfb15030057
Chicago/Turabian StylePudełko-Prażuch, Iwona, Mareeswari Balasubramanian, Sundara Moorthi Ganesan, Stanisław Marecik, Kamila Walczak, Kinga Pielichowska, Suvro Chatterjee, Ravichandran Kandaswamy, and Elżbieta Pamuła. 2024. "Characterization and In Vitro Evaluation of Porous Polymer-Blended Scaffolds Functionalized with Tricalcium Phosphate" Journal of Functional Biomaterials 15, no. 3: 57. https://doi.org/10.3390/jfb15030057





