Analysis of Bone Mineral Density and Bone Quality of Cortical Bone in the Human Hyoid Body and Histological Observation of the Entheses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Preparation of Samples
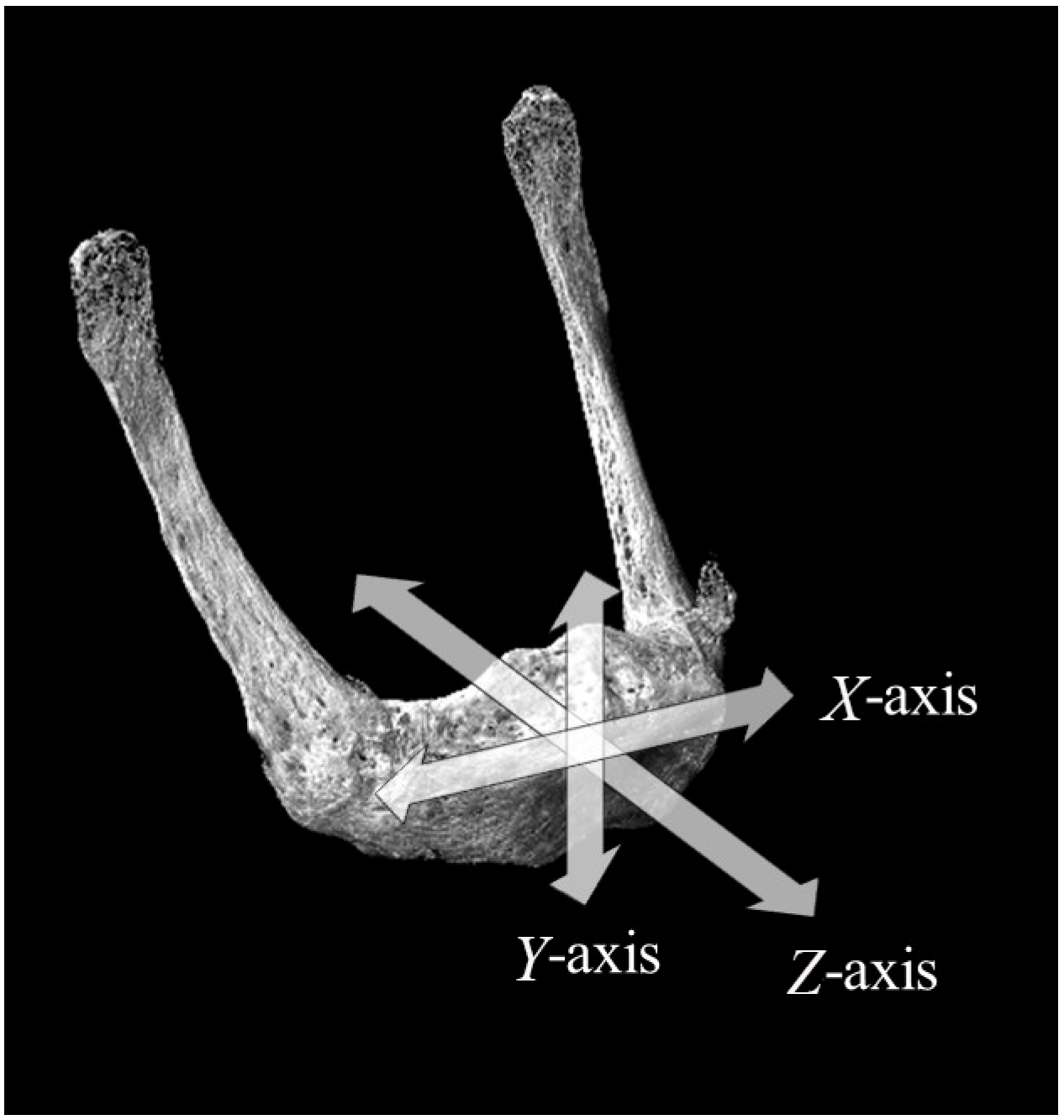
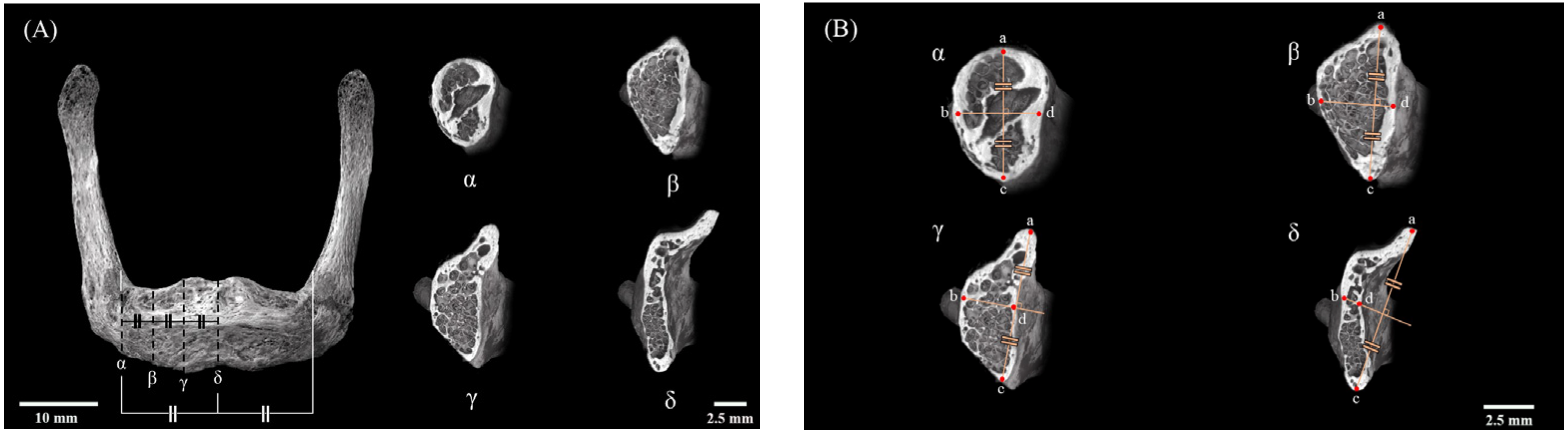
2.3. Setting of Measurement Sites
2.4. Scanning Electron Microscopy
2.5. Measurement of Bone Mineral Density
2.6. Biological Apatite (BAp) Crystallite Orientation
2.7. Statistical Analysis
3. Results
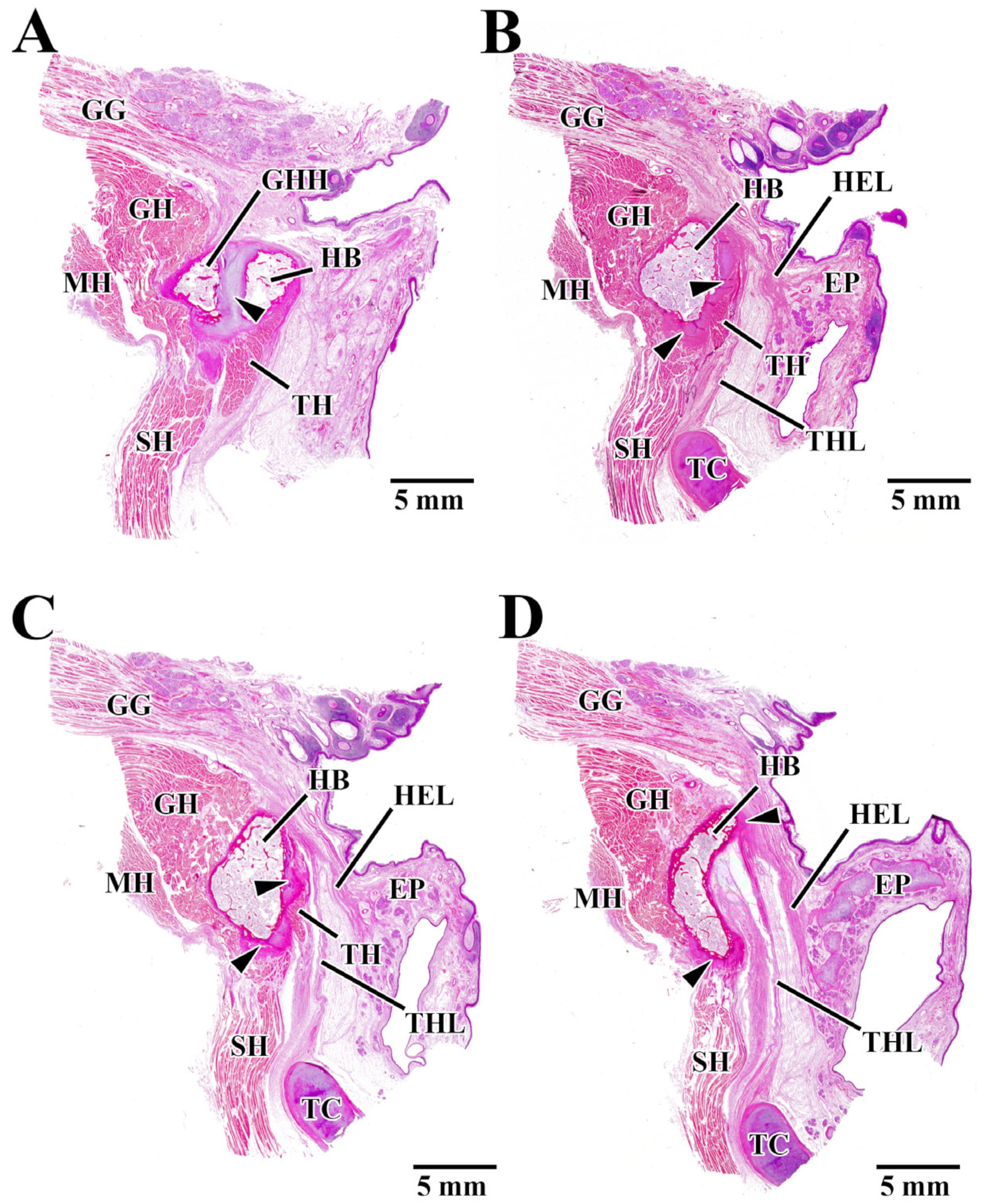
3.1. Observation of Cortical Bone Surface
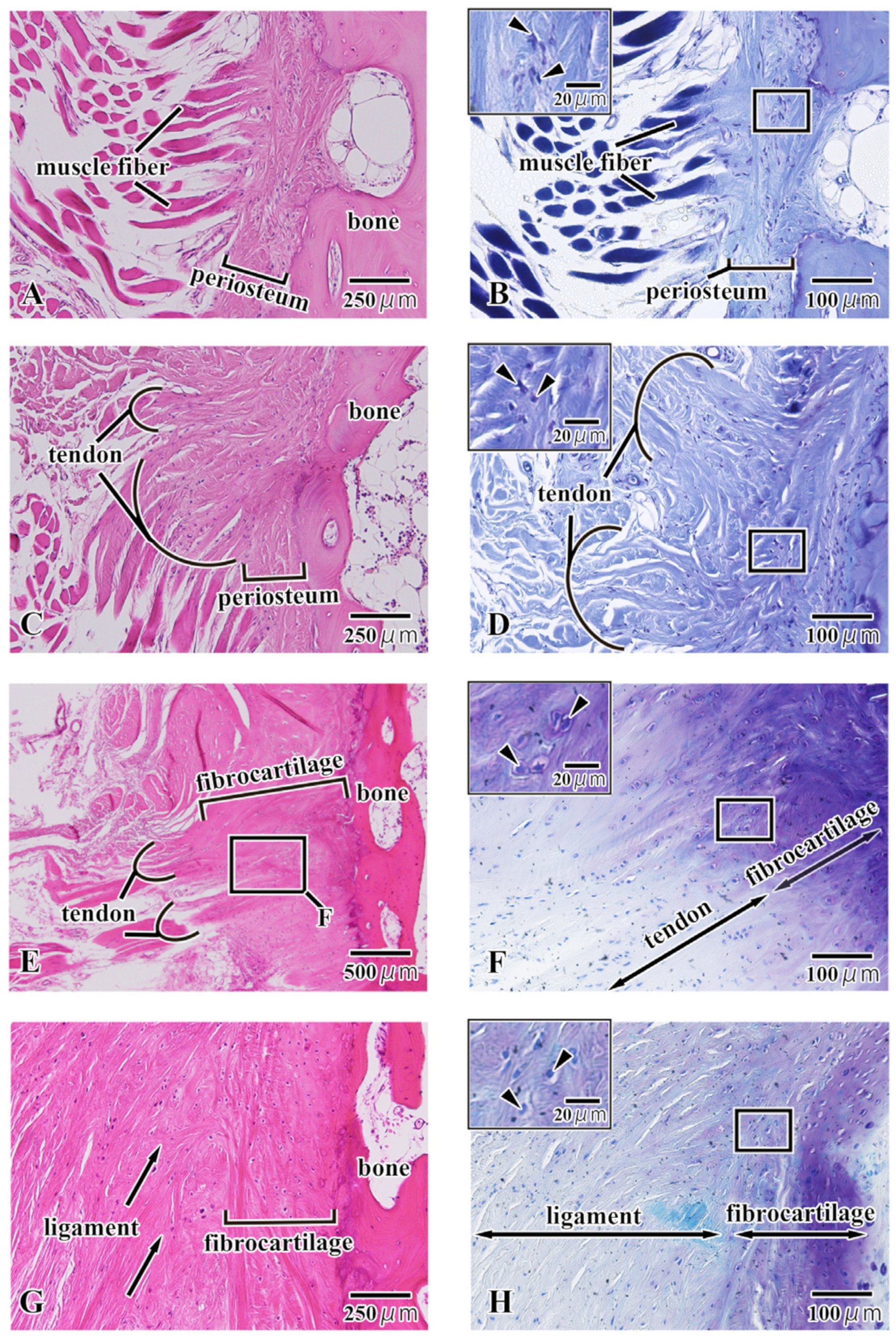
3.2. Histological Observation
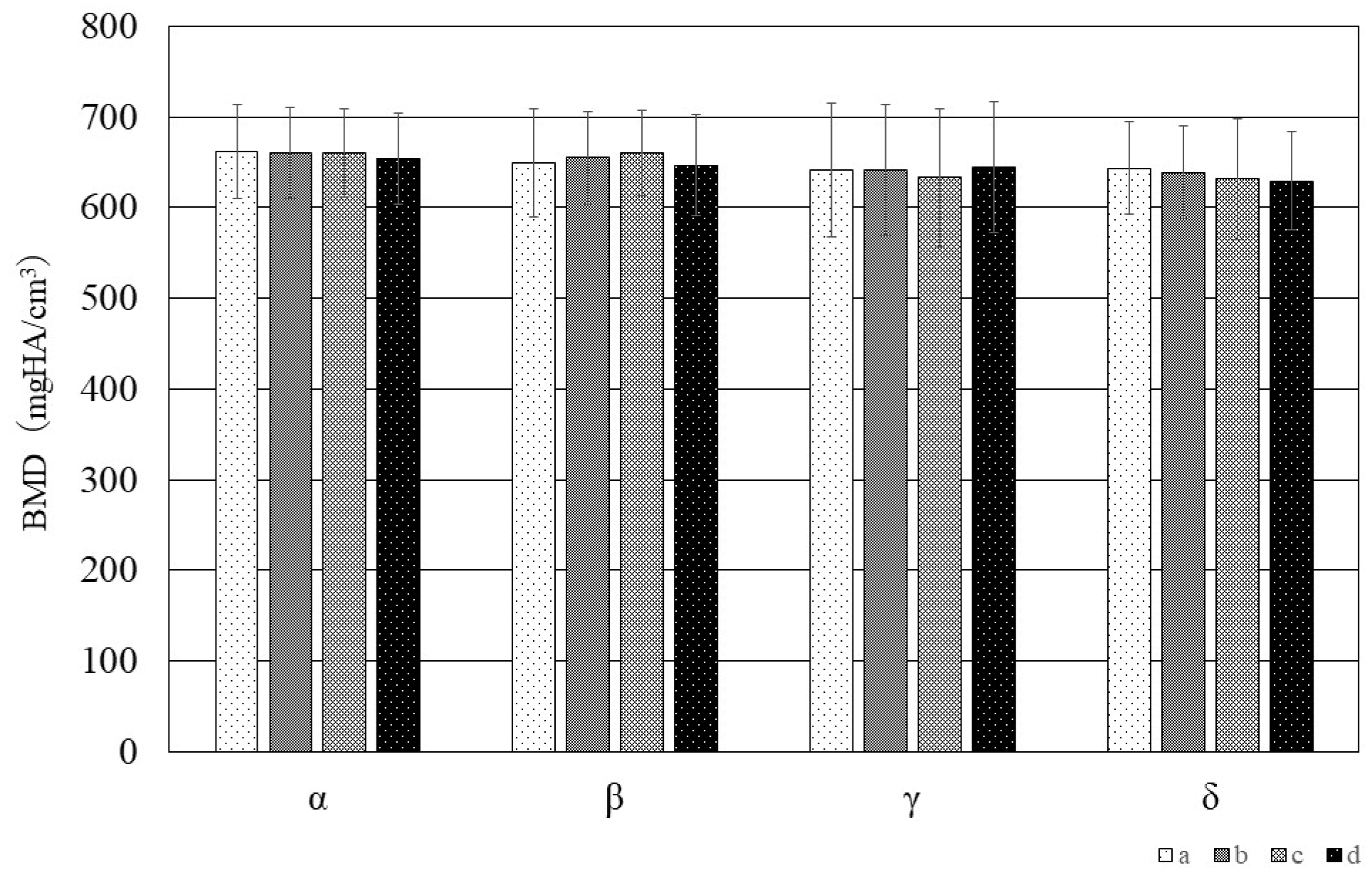
3.3. Bone Mineral Density
3.4. Bap Crystallite Orientation
4. Discussion
4.1. Local Structure of the Hyoid Body from Histological Observations
4.2. BMD of the Hyoid Body
4.3. BAp Crystallite Orientation in the Hyoid Body
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ronald, C.A.; Nathan, J.P. The hyoid bone: An overview. Cranio 2020, 38, 6–14. [Google Scholar]
- Pearson, W.G., Jr.; Langmore, S.E.; Zumwalt, A.C. Evaluating the Structural Properties of Suprahyoid Muscles and their Potential for Moving the Hyoid. Dysphagia 2011, 26, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Gehrking, E.; Klostermann, W.; Wessel, K.; Remmert, S. Electromyography of the infrahyoid muscles—Part 1: Normal findings. Laryngorhinootologie 2001, 80, 662–665. [Google Scholar] [CrossRef]
- Capasso, L.; Michetti, E.; D’Anastasio, R. A homo erectus hyoid bone: Possible implications for origin of the human capability of speech. Coll. Antropol. 2008, 32, 1007–1011. [Google Scholar] [PubMed]
- Shimizu, Y.; Kanetaka, H.; Kim, Y.H.; Okayama, K.; Kano, M.; Kikuchi, M. Age-related morphological changes in the human hyoid bone. Cells Tissues Organs 2005, 180, 185–192. [Google Scholar] [CrossRef]
- NIH Consensus. Development Panel on Osteoporosis Prevention Diagnosis, and Therapy Osteoporosis prevention, diagnosis, and therapy. J. Am. Med. Assoc. 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Sasaki, N.; Matsushima, N.; Ikawa, T.; Yamamura, H.; Fukuda, A. Orientation of bone mineral and its role in the anisotropic mechanical properties of bone-transverse anisotropy. J. Biomech. 1989, 22, 157–164. [Google Scholar] [CrossRef]
- Nakano, T.; Kaibara, K.; Tabata, Y.; Nagata, N.; Enomoto, S.; Marukawa, E.; Umakoshi, Y. Unique alignment and texture of biological apatite crystallites in typical calcified tissues analyzed by microbeam X-ray diffractometer system. Bone 2002, 31, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Miyabe, N.T.; Ishimoto, T.; Takano, N.; Adachi, T.; Iwaki, H.; Kobayashi, A.; Umakoshi, Y. Two-dimensional quantitative analysis of preferential alignment of BAp c-axis for isolated human trabecular bone using microbeam X-ray diffractometer with a transmission optical system. Mater. Trans. 2007, 48, 343–347. [Google Scholar] [CrossRef]
- Furuya, H.; Matsunaga, S.; Tamatsu, Y.; Nakano, T.; Yoshinari, M.; Abe, S.; Ide, Y. Analysis of biological apatite crystal orientation in anterior cortical bone of human mandible using microbeam X-ray diffractometry. Mater. Trans. 2012, 53, 980–984. [Google Scholar] [CrossRef]
- Ishimoto, T.; Nakano, T.; Umakoshi, Y.; Yamamoto, M.; Tabata, Y. Degree of biological apatite c-axis orientation rather than bone mineral density controls mechanical function in bone regenerated using recombinant bone morphogenetic protein-2. J. Bone Miner. Res. 2013, 28, 1170–1179. [Google Scholar] [CrossRef]
- Kasahara, M.; Matsunaga, S.; Someya, T.; Kitamura, K.; Odaka, K.; Ishimoto, T.; Nakano, T.; Abe, S.; Hattori, M. Micro- and nano-bone analyses of the human mandible coronoid process and tendon-bone entheses. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 2799–2806. [Google Scholar] [CrossRef]
- Nakano, T.; Kaibara, K.; Ishimoto, T.; Tabata, Y.; Umakoshi, Y. Biological apatite (BAp) crystallographic orientation and texture as a new index for assessing the microstructure and function of bone regenerated by tissue engineering. Bone 2012, 51, 741–747. [Google Scholar] [CrossRef]
- Shaw, H.M.; Benjamin, M. Structure-function relationships of entheses in relation to mechanical load and exercise. Scand. J. Med. Sci. Sports 2007, 17, 303–315. [Google Scholar] [CrossRef]
- Apostolakos, J.; Durant, T.J.; Dwyer, C.R.; Russell, R.P.; Weinreb, J.H.; Alaee, F.; Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Mazzocca, A.D. The enthesis: A review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014, 4, 33–42. [Google Scholar] [CrossRef]
- Lu, H.H.; Thomopoulos, S. Functional attachment of soft tissues to bone: Development, healing, and tissue engineering. Annu. Rev. Biomed. Eng. 2013, 15, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Doschak, M.R.; Zernicke, R.F. Structure, function and adaptation of bone-tendon and bone-ligament complexes. J. Musculoskelet. Neuronal Interact. 2005, 5, 35–40. [Google Scholar]
- Benjamin, M.; Ralphs, J.R. The skeletal attachment of tendons—Tendon “entheses”. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 133, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Ralphs, J.R. Fibrocartilage in tendons and ligaments—An adaptation to compressive load. J. Anat. 1998, 193, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Hems, T.; Tillmann, B. Tendon entheses of the human masticatory muscles. Anat. Embryol. 2000, 202, 201–208. [Google Scholar] [CrossRef]
- Chancey, V.C.; Nightingale, R.W.; Van Ee, C.A.; Knaub, K.E.; Myers, B.S. Improved estimation of human neck tensile tolerance: Reducing the range of reported tolerance using anthropometrically correct muscles and optimized physiologic initial conditions. Stapp Car Crash J. 2003, 47, 135–153. [Google Scholar] [PubMed]
- Roberts, J.L.; Reed, W.R.; Thach, B.T. Pharyngeal airway-stabilizing function of sternohyoid and sternothyroid muscles in the rabbit. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1984, 57, 1790–1795. [Google Scholar] [CrossRef] [PubMed]
- Leelamanit, V.; Limsakul, C.; Geater, A. Synchronized electrical stimulation in treating pharyngeal dysphagia. Laryngoscope 2002, 112, 2204–2210. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Palmer, J.B. Anatomy and physiology of feeding and swallowing: Normal and abnormal. Phys. Med. Rehabil. Clin. N. Am. 2008, 19, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Fink, B.R.; Martin, R.W.; Rohrmann, C.A. Biomechanics of the human epiglottis. Acta Otolaryngol. 1979, 87, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Reidenbach, M.M. The periepiglottic space: Tomographic relations and histological organisation. J. Anat. 1996, 188, 173–182. [Google Scholar]
- Kitamura, K.; Watanabe, T.; Yamamoto, M.; Ishikawa, N.; Kasahara, N.; Abe, S.; Yamamoto, H. A Newly Discovered Tendon Between the Genioglossus Muscle and Epiglottic Cartilage Identified by Histological Observation of the Pre-Epiglottic Space. Dysphagia 2023, 38, 315–329. [Google Scholar] [CrossRef]
- Vandaele, D.J.; Perlman, A.L.; Cassell, M.D. Intrinsic fibre architecture and attachments of the human epiglottis and their contributions to the mechanism of deglutition. J. Anat. 1995, 186, 1–15. [Google Scholar]
- Wang, X.; Wang, C.; Zhang, S.; Wang, W.; Li, X.; Gao, S.; Li, K.; Chen, J.; Wang, H.; Chen, L.; et al. Microstructure of the hyoid bone based on micro-computed tomography findings. Medicine 2020, 99, e22246. [Google Scholar] [CrossRef]
- Picard, S.; Lapointe, N.; Brown, J.; Guertin, P. Histomorphometric and densitometric changes in the femora of spinal cord transected mice. Anat. Rec. 2008, 291, 303–307. [Google Scholar] [CrossRef]
- Tsai, C.; Huang, R.; Lee, C.; Hsiao, W.; Yang, L. Morphologic and bony structural changes in the mandible after a unilateral injection of botulinum Neurotoxin in adult rats. J. Oral. Maxillofac. Surg. 2010, 68, 1081–1087. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasahara, M.; Someya, T.; Kitamura, K.; Watanabe, G.; Matsunaga, S.; Abe, S.; Hattori, M. Analysis of Bone Mineral Density and Bone Quality of Cortical Bone in the Human Hyoid Body and Histological Observation of the Entheses. J. Funct. Biomater. 2024, 15, 56. https://doi.org/10.3390/jfb15030056
Kasahara M, Someya T, Kitamura K, Watanabe G, Matsunaga S, Abe S, Hattori M. Analysis of Bone Mineral Density and Bone Quality of Cortical Bone in the Human Hyoid Body and Histological Observation of the Entheses. Journal of Functional Biomaterials. 2024; 15(3):56. https://doi.org/10.3390/jfb15030056
Chicago/Turabian StyleKasahara, Masaaki, Tomoko Someya, Kei Kitamura, Genji Watanabe, Satoru Matsunaga, Shinichi Abe, and Masayuki Hattori. 2024. "Analysis of Bone Mineral Density and Bone Quality of Cortical Bone in the Human Hyoid Body and Histological Observation of the Entheses" Journal of Functional Biomaterials 15, no. 3: 56. https://doi.org/10.3390/jfb15030056




