Water-Soluble Quaternary and Protonable Basic Chitotriazolans: Synthesis by Click Chemistry Conversion of Chitosan Azides and Investigation of Antibacterial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Methods
2.2.1. Synthesis of Chitosan Azide
2.2.2. Synthesis of Different Alkynes (1a–9a)
2.2.3. General Synthesis Procedure for Chitotriazolan Derivatives 1–9
2.3. Characterization
2.3.1. 1H NMR and FTIR Spectra
2.3.2. Gel Permeation Chromatography
2.4. Degree of Substitution
2.5. Solubility Test in Water
2.6. Zeta Potential Analysis
2.7. In Vitro Antibacterial Assay
3. Results and Discussion
3.1. Characterization by IR and NMR Spectroscopy
3.2. Solubility Analysis in Water
3.3. Investigation of Antibacterial Activity of Chitotriazolan Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Másson, M. Chapter 33—Chitin and chitosan. In Handbook of Hydrocolloids, 3rd ed.; Phillips, G.O., Williams, P.A., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 1039–1072. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Rathinam, S. N-alkyl, N-acyl, and Triazolyl Derivatives of Chitosan: Synthesis and Antibacterial Properties. Ph.D. Thesis, University of Iceland, Reykjavík, Iceland, 2022. [Google Scholar]
- dos Santos, J.E.; Dockal, E.R.; Cavalheiro, É.T.G. Synthesis and characterization of schiff bases from chitosan and salicylaldehyde derivatives. Carbohydr. Polym. 2005, 60, 277–282. [Google Scholar] [CrossRef]
- Anush, S.M.; Vishalakshi, B.; Kalluraya, B.; Manju, N. Synthesis of pyrazole-based schiff bases of chitosan: Evaluation of antimicrobial activity. Int. J. Biol. Macromol. 2018, 119, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Sholkamy, E.N.; Alobaidi, A.S.; Al-Muhanna, M.K.; Barakat, A. Synthesis of schiff bases based on chitosan and heterocyclic moiety: Evaluation of antimicrobial activity. ACS Omega 2023, 8, 47304–47312. [Google Scholar] [CrossRef]
- Piegat, A.; Goszczyńska, A.; Idzik, T.; Niemczyk, A. The importance of reaction conditions on the chemical structure of n,o-acylated chitosan derivatives. Molecules 2019, 24, 3047. [Google Scholar] [CrossRef]
- Reis, B.; Gerlach, N.; Steinbach, C.; Haro Carrasco, K.; Oelmann, M.; Schwarz, S.; Müller, M.; Schwarz, D. A complementary and revised view on the n-acylation of chitosan with hexanoyl chloride. Mar. Drugs 2021, 19, 385. [Google Scholar] [CrossRef]
- Rathinam, S.; Ólafsdóttir, S.; Jónsdóttir, S.; Hjálmarsdóttir, M.Á.; Másson, M. Selective synthesis of n,n,n-trimethylated chitosan derivatives at different degree of substitution and investigation of structure-activity relationship for activity against p. Aeruginosa and mrsa. Int. J. Biol. Macromol. 2020, 160, 548–557. [Google Scholar] [CrossRef]
- Rathinam, S.; Solodova, S.; Kristjánsdóttir, I.; Hjálmarsdóttir, M.Á.; Másson, M. The antibacterial structure-activity relationship for common chitosan derivatives. Int. J. Biol. Macromol. 2020, 165, 1686–1693. [Google Scholar] [CrossRef]
- Jin, H.; Wang, Z. Advances in alkylated chitosan and its applications for hemostasis. Macromol 2022, 2, 346–360. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Chelation capability of chitosan and chitosan derivatives: Recent developments in sustainable corrosion inhibition and metal decontamination applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100184. [Google Scholar] [CrossRef]
- Sharef, H.Y.; Fakhre, N.A. Rapid adsorption of some heavy metals using extracted chitosan anchored with new aldehyde to form a schiff base. PLoS ONE 2022, 17, e0274123. [Google Scholar] [CrossRef]
- Luan, F.; Wei, L.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Antioxidant activity and antifungal activity of chitosan derivatives with propane sulfonate groups. Polymers 2018, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shi, C.; Wang, X.; Fang, Q.; Huang, J. Synthesis, characterization, and antimicrobial activities of sulfonated chitosan. Carbohydr. Polym. 2017, 155, 321–328. [Google Scholar] [CrossRef] [PubMed]
- An, N.T.; Dung, P.L.; Thien, D.T.; Dong, N.T.; Nhi, T.T.Y. An improved method for synthesizing n,n′-dicarboxymethylchitosan. Carbohydr. Polym. 2008, 73, 261–264. [Google Scholar] [CrossRef]
- Fonseca-Santos, B.; Chorilli, M. An overview of carboxymethyl derivatives of chitosan: Their use as biomaterials and drug delivery systems. Mater. Sci. Eng. C 2017, 77, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Islam, R.; Mahmudul Hassan, S.M.; Karim, M.R.; Rahman, M.M.; Rahman, S.; Nur Hossain, M.; Islam, D.; Aftab Ali Shaikh, M.; Georghiou, P.E. Carboxymethyl chitin and chitosan derivatives: Synthesis, characterization and antibacterial activity. Carbohydr. Polym. Technol. Appl. 2023, 5, 100283. [Google Scholar] [CrossRef]
- Diao, Y.; Yu, X.; Zhang, C.; Jing, Y. Quercetin-grafted chitosan prepared by free radical grafting: Characterization and evaluation of antioxidant and antibacterial properties. J. Food Sci. Technol. 2020, 57, 2259–2268. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, E.; Winnicka, K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise huisgen cycloaddition process: Copper(i)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Thakur, K.; Khare, N.K. Synthesis of glycoconjugate mimics by ‘click chemistry’. Carbohydr. Res. 2019, 484, 107775. [Google Scholar] [CrossRef]
- Xu, Z.; Bratlie, K.M. Click chemistry and material selection for in situ fabrication of hydrogels in tissue engineering applications. ACS Biomater. Sci. Eng. 2018, 4, 2276–2291. [Google Scholar] [CrossRef] [PubMed]
- Binder, W.H.; Sachsenhofer, R. ‘Click’ chemistry in polymer and material science: An update. Macromol. Rapid Commun. 2008, 29, 952–981. [Google Scholar] [CrossRef]
- Dolci, M.; Toulemon, D.; Chaffar, Z.; Bubendorff, J.-L.; Tielens, F.; Calatayud, M.; Zafeiratos, S.; Begin-Colin, S.; Pichon, B.P. Nanoparticle assembling through click chemistry directed by mixed sams for magnetic applications. ACS Appl. Nano Mater. 2019, 2, 554–565. [Google Scholar] [CrossRef]
- Yi, G.; Son, J.; Yoo, J.; Park, C.; Koo, H. Application of click chemistry in nanoparticle modification and its targeted delivery. Biomater. Res. 2018, 22, 13. [Google Scholar] [CrossRef] [PubMed]
- Zheng, T.; Rouhanifard, S.H.; Jalloh, A.S.; Wu, P. Click triazoles for bioconjugation. Top. Heterocycl. Chem. 2012, 28, 163–183. [Google Scholar] [PubMed]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine ligation: Fast bioconjugation based on inverse-electron-demand diels−alder reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, Z.; Chen, L.; Gu, W.; Li, Y. Synthesis of 6-n,n,n-trimethyltriazole chitosan via “click chemistry” and evaluation for gene delivery. Biomacromolecules 2009, 10, 2175–2182. [Google Scholar] [CrossRef]
- Sarwar, A.; Katas, H.; Samsudin, S.N.; Zin, N.M. Regioselective sequential modification of chitosan via azide-alkyne click reaction: Synthesis, characterization, and antimicrobial activity of chitosan derivatives and nanoparticles. PLoS ONE 2015, 10, e0123084. [Google Scholar] [CrossRef]
- Barbosa, M.; Vale, N.; Costa, F.M.T.A.; Martins, M.C.L.; Gomes, P. Tethering antimicrobial peptides onto chitosan: Optimization of azide-alkyne “click” reaction conditions. Carbohydr. Polym. 2017, 165, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Sahariah, P.; Sørensen, K.K.; Hjálmarsdóttir, M.Á.; Sigurjónsson, Ó.E.; Jensen, K.J.; Másson, M.; Thygesen, M.B. Antimicrobial peptide shows enhanced activity and reduced toxicity upon grafting to chitosan polymers. Chem. Commun. 2015, 51, 11611–11614. [Google Scholar] [CrossRef]
- Zhang, F.; Bernet, B.; Bonnet, V.; Dangles, O.; Sarabia, F.; Vasella, A. 2-azido-2-deoxycellulose: Synthesis and 1,3-dipolar cycloaddition. Helv. Chim. Acta 2008, 91, 608–617. [Google Scholar] [CrossRef]
- Rathinam, S.; Hjálmarsdóttir, M.Á.; Thygesen, M.B.; Másson, M. Chitotriazolan (poly(β(1–4)-2-(1h-1,2,3-triazol-1-yl)-2-deoxy-d-glucose)) derivatives: Synthesis, characterization, and evaluation of antibacterial activity. Carbohydr. Polym. 2021, 267, 118162. [Google Scholar] [CrossRef]
- Kulbokaite, R.; Ciuta, G.; Netopilik, M.; Makuska, R. N-peg’ylation of chitosan via “click chemistry” reactions. React. Funct. Polym. 2009, 69, 771–778. [Google Scholar] [CrossRef]
- Lunkov, A.; Shagdarova, B.; Lyalina, T.; Dubinnyi, M.A.; Karpova, N.; Lopatin, S.; Il’ina, A.; Varlamov, V. Simple method for ultrasound assisted «click» modification of azido-chitosan derivatives by cuaac. Carbohydr. Polym. 2022, 282, 119109. [Google Scholar] [CrossRef] [PubMed]
- Kritchenkov, A.S.; Egorov, A.R.; Dysin, A.P.; Volkova, O.V.; Zabodalova, L.A.; Suchkova, E.P.; Kurliuk, A.V.; Shakola, T.V. Ultrasound-assisted cu(i)-catalyzed azide-alkyne click cycloaddition as polymer-analogous transformation in chitosan chemistry. High antibacterial and transfection activity of novel triazol betaine chitosan derivatives and their nanoparticles. Int. J. Biol. Macromol. 2019, 137, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Luan, F.; Wei, L.; Zhang, J.; Tan, W.; Chen, Y.; Dong, F.; Li, Q.; Guo, Z. Preparation and characterization of quaternized chitosan derivatives and assessment of their antioxidant activity. Molecules 2018, 23, 516. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Zhao, X.; Dong, F.; Li, Q.; Guo, Z. Synthesis and antioxidant action of chitosan derivatives with amino-containing groups via azide-alkyne click reaction and n-methylation. Carbohydr. Polym. 2018, 199, 583–592. [Google Scholar] [CrossRef]
- Li, Q.; Tan, W.; Zhang, C.; Gu, G.; Guo, Z. Synthesis of water soluble chitosan derivatives with halogeno-1,2,3-triazole and their antifungal activity. Int. J. Biol. Macromol. 2016, 91, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Wada, M.; Morimoto, M.; Saimoto, H. A short synthesis of highly soluble chemoselective chitosan derivatives via “click chemistry”. Carbohydr. Polym. 2012, 90, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tan, W.; Zhang, C.; Gu, G.; Guo, Z. Novel triazolyl-functionalized chitosan derivatives with different chain lengths of aliphatic alcohol substituent: Design, synthesis, and antifungal activity. Carbohydr. Res. 2015, 418, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Ifuku, S.; Matsumoto, C.; Wada, M.; Morimoto, M.; Saimoto, H. Preparation of highly regioselective amphiprotic chitosan derivative via “click chemistry”. Int. J. Biol. Macromol. 2013, 52, 72–76. [Google Scholar] [CrossRef]
- Ifuku, S.; Wada, M.; Morimoto, M.; Saimoto, H. Preparation of highly regioselective chitosan derivatives via “click chemistry”. Carbohydr. Polym. 2011, 85, 653–657. [Google Scholar] [CrossRef]
- Li, Q.; Sun, X.; Gu, G.; Guo, Z. Novel water soluble chitosan derivatives with 1,2,3-triazolium and their free radical-scavenging activity. Mar. Drugs 2018, 16, 107. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Dong, F.; Zhang, J.; Luan, F.; Wei, L.; Chen, Y.; Guo, Z. Novel cationic chitosan derivative bearing 1,2,3-triazolium and pyridinium: Synthesis, characterization, and antifungal property. Carbohydr. Polym. 2018, 182, 180–187. [Google Scholar] [CrossRef]
- Tan, W.; Zhang, J.; Mi, Y.; Dong, F.; Li, Q.; Guo, Z. Synthesis, characterization, and evaluation of antifungal and antioxidant properties of cationic chitosan derivative via azide-alkyne click reaction. Int. J. Biol. Macromol. 2018, 120, 318–324. [Google Scholar] [CrossRef]
- Rathinam, S.; Hjálmarsdóttir, M.Á.; Thygesen, M.B.; Másson, M. Water-soluble chitotriazolans derived from cationic, neutral, and anionic common chitosan derivatives: Synthesis, characterization, and antibacterial activity. Eur. Polym. J. 2023, 196, 112311. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Z.; Mu, H.; Wang, X.; Li, Y.; Yan, J.; Wang, Z. A novel approach to 1,2,3-triazole grafted chitosans via modified wolff’s cyclocondensation. Eur. Polym. J. 2018, 98, 492–498. [Google Scholar] [CrossRef]
- D, P.M.; Chawla, R.; Dutta, P.K. ‘Click’ synthesized non-substituted triazole modified chitosan from cac2 as a novel antibacterial and antioxidant polymer. J. Polym. Res. 2022, 29, 179. [Google Scholar] [CrossRef]
- Gaitor, J.C.; Paul, L.M.; Reardon, M.M.; Hmissa, T.; Minkowicz, S.; Regner, M.; Sheng, Y.; Michael, S.F.; Isern, S.; Mirjafari, A. Ionic liquids with thioether motifs as synthetic cationic lipids for gene delivery. Chem. Commun. 2017, 53, 8328–8331. [Google Scholar] [CrossRef] [PubMed]
- Kanitskaya, L.V.; Elokhina, V.N.; Fedorov, S.V.; Shulunova, A.M.; Nakhmanovich, A.S.; Turchaninov, V.K.; Lopyrev, V.A. Spectroscopic study of reaction of propargyl bromide with pyridine. Russ. J. Gen. Chem. 2002, 72, 778–784. [Google Scholar] [CrossRef]
- Doebelin, C.; Wagner, P.; Bertin, I.; Simonin, F.; Schmitt, M.; Bihel, F.; Bourguignon, J.-J. Trisubstitution of pyridine through sequential and regioselective palladium cross-coupling reactions affording analogs of known gpr54 antagonists. RSC Adv. 2013, 3, 10296–10300. [Google Scholar] [CrossRef]
- Karoli, T.; Mamidyala, S.K.; Zuegg, J.; Fry, S.R.; Tee, E.H.L.; Bradford, T.A.; Madala, P.K.; Huang, J.X.; Ramu, S.; Butler, M.S.; et al. Structure aided design of chimeric antibiotics. Bioorganic Med. Chem. Lett. 2012, 22, 2428–2433. [Google Scholar] [CrossRef] [PubMed]
- CLSI standard M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2009.
- Holappa, J.; Nevalainen, T.; Soininen, P.; Másson, M.; Järvinen, T. Synthesis of novel quaternary chitosan derivatives via n-chloroacyl-6-o-triphenylmethylchitosans. Biomacromolecules 2006, 7, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Holappa, J.; Nevalainen, T.; Savolainen, J.; Soininen, P.; Elomaa, M.; Safin, R.; Suvanto, S.; Pakkanen, T.; Másson, M.; Loftsson, T.; et al. Synthesis and characterization of chitosan n-betainates having various degrees of substitution. Macromolecules 2004, 37, 2784–2789. [Google Scholar] [CrossRef]
- Rúnarsson, Ö.V.; Holappa, J.; Malainer, C.; Steinsson, H.; Hjálmarsdóttir, M.; Nevalainen, T.; Másson, M. Antibacterial activity of n-quaternary chitosan derivatives: Synthesis, characterization and structure activity relationship (sar) investigations. Eur. Polym. J. 2010, 46, 1251–1267. [Google Scholar] [CrossRef]
- Nagy, V.; Sahariah, P.; Hjálmarsdóttir, M.Á.; Másson, M. Chitosan-hydroxycinnamic acid conjugates: Optimization of the synthesis and investigation of the structure activity relationship. Carbohydr. Polym. 2022, 277, 118896. [Google Scholar] [CrossRef]
- Holappa, J.; Nevalainen, T.; Safin, R.; Soininen, P.; Asplund, T.; Luttikhedde, T.; Másson, M.; Järvinen, T. Novel water-soluble quaternary piperazine derivatives of chitosan: Synthesis and characterization. Macromol. Biosci. 2006, 6, 139–144. [Google Scholar] [CrossRef]
- Si, Z.; Hou, Z.; Vikhe, Y.S.; Thappeta, K.R.V.; Marimuthu, K.; De, P.P.; Ng, O.T.; Li, P.; Zhu, Y.; Pethe, K.; et al. Antimicrobial effect of a novel chitosan derivative and its synergistic effect with antibiotics. ACS Appl. Mater. Interfaces 2021, 13, 3237–3245. [Google Scholar] [CrossRef] [PubMed]
- Hoque, J.; Adhikary, U.; Yadav, V.; Samaddar, S.; Konai, M.M.; Prakash, R.G.; Paramanandham, K.; Shome, B.R.; Sanyal, K.; Haldar, J. Chitosan derivatives active against multidrug-resistant bacteria and pathogenic fungi: In vivo evaluation as topical antimicrobials. Mol. Pharm. 2016, 13, 3578–3589. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Másson, M. Antimicrobial properties of chitosan and its derivatives. In Chitosan for Biomaterials iii: Structure-Property Relationships; Jayakumar, R., Prabaharan, M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 131–168. [Google Scholar]

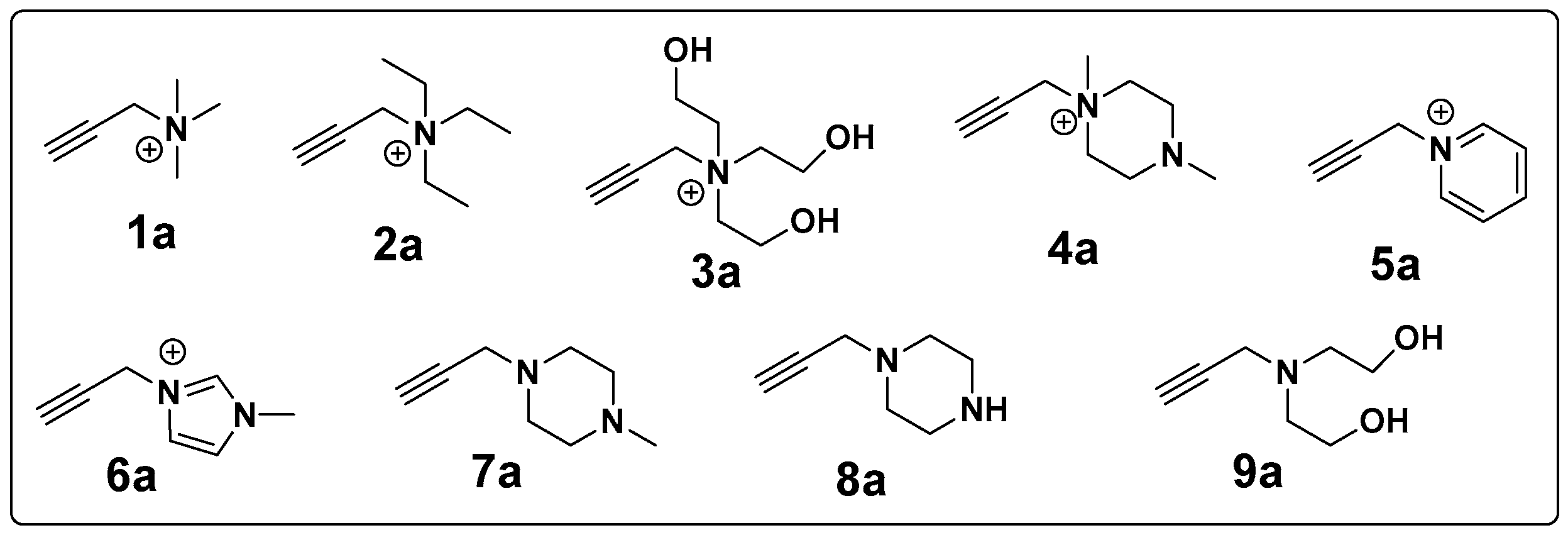
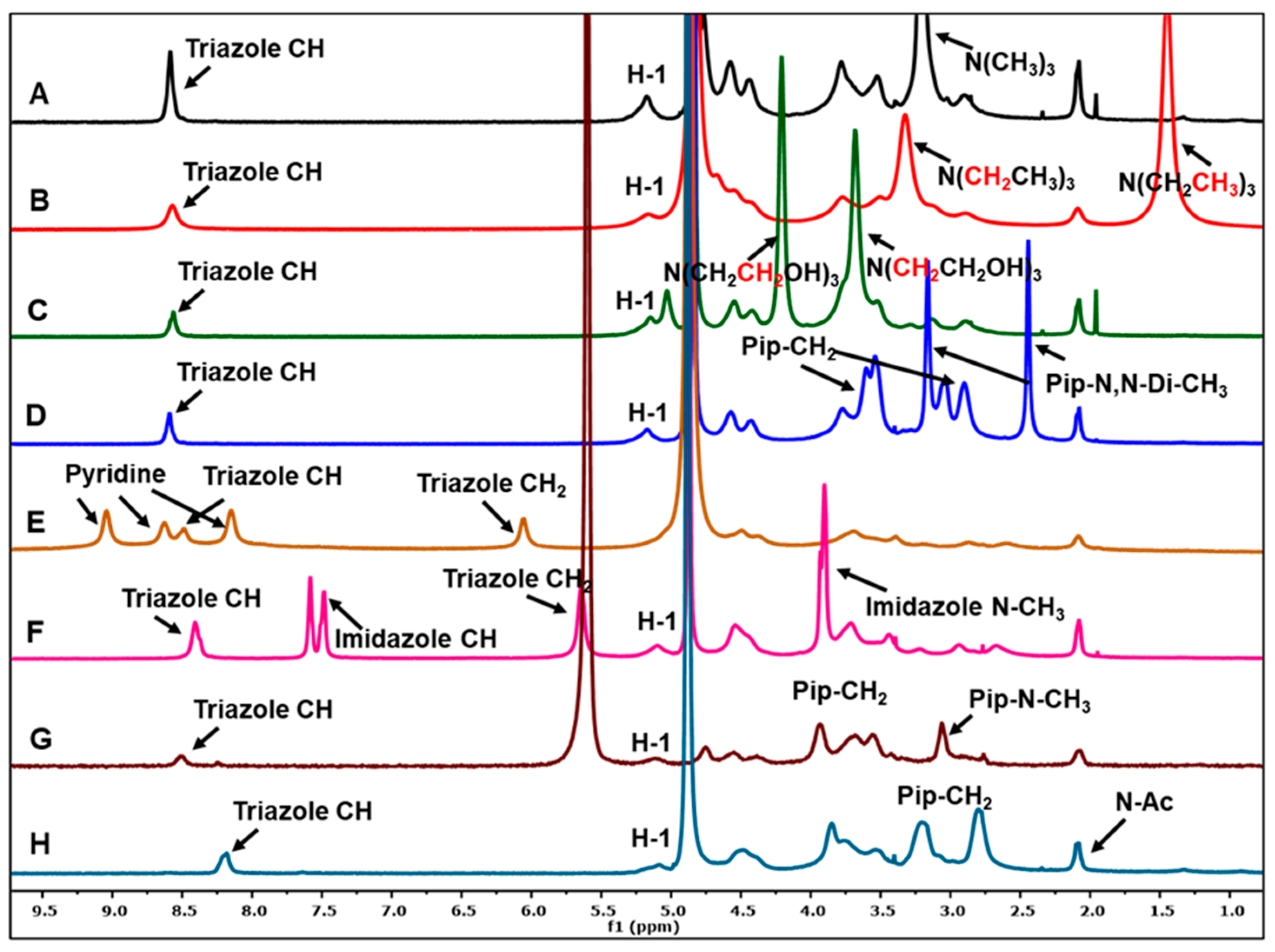
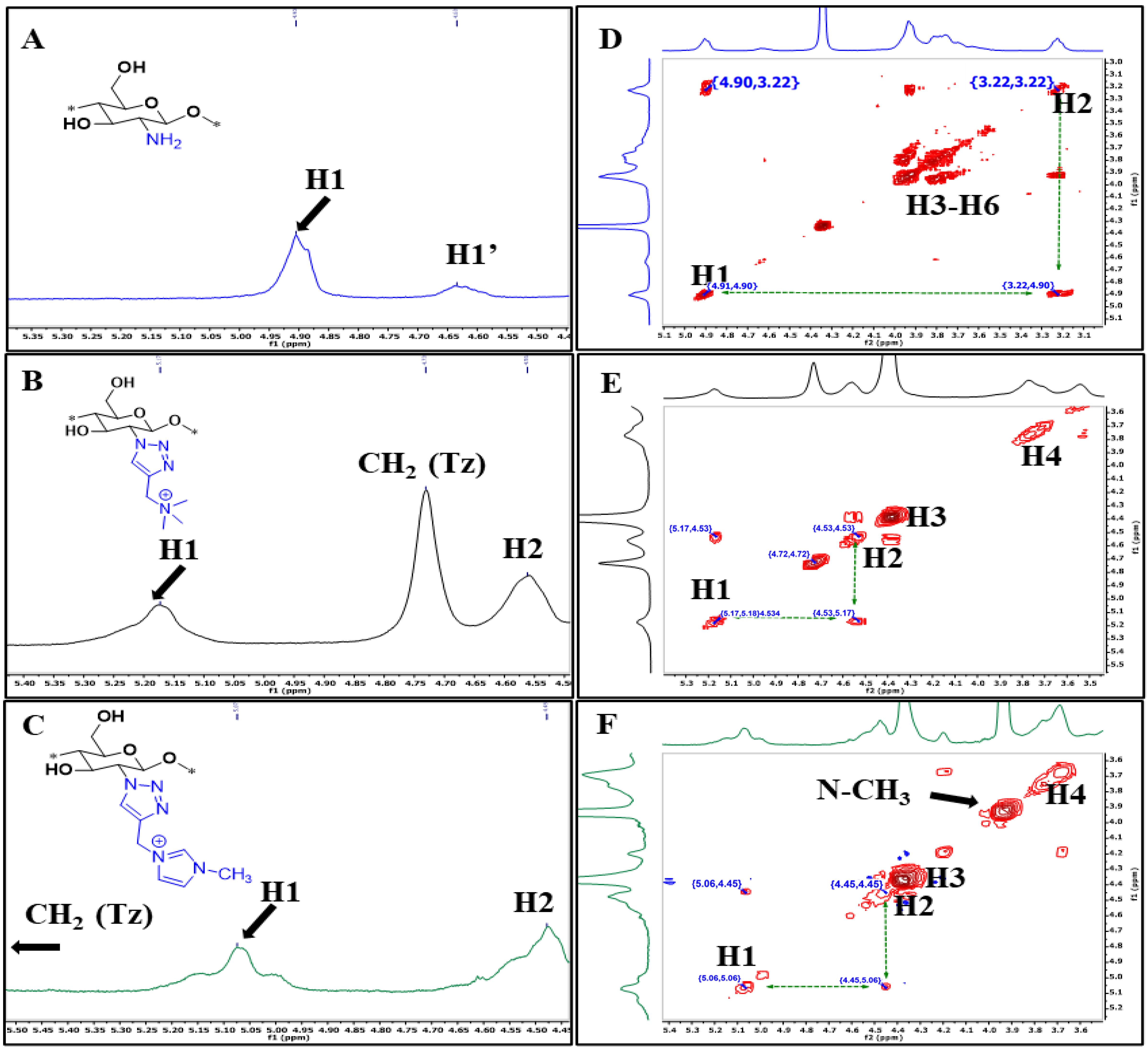

| Chitosan Derivatives | Yield (%) | DS (Based on Triazole CH and Acetyl Peak) | MW (Da) | Polydispersity Index (D) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| 1 | 82 | (0.74) | 7.055 × 104 | 2.30 | 46.4 |
| 2 | 91 | (0.77) | 1.046 × 105 | 3.92 | 51.5 |
| 3 | 80 | (0.44) | 3.225 × 105 | 3.93 | 47.0 |
| 4 | 88 | (0.53) | 1.709 × 105 | 1.80 | 35.1 |
| 5 | 94 | (0.73) | 1.164 × 105 | 1.23 | 39.5 |
| 6 | 84 | (0.70) | 1.330 × 105 | 2.77 | 52.2 |
| 7 | 75 | (0.54) | 2.721 × 104 | 1.18 | 16.3 |
| 8 | 60 | (0.57) | 1.699 × 104 | 1.00 | 32.7 |
| 9 | 61 | NA * | 4.724 × 105 | 1.23 | 15.6 |
| Chitosan Derivatives | Structures | S. aureus µg/mL | E. faecalis µg/mL | E. coli µg/mL | P. aeruginosa µg/mL |
|---|---|---|---|---|---|
| 1 | 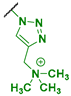 | 512 | 8192 | 512 | 1024 * |
| 2 |  | 8192 | 8192 | 512 | 1024 * |
| 3 |  | 256 | 8192 | 4096 | 512 |
| 4 | 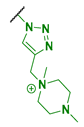 | 4096 | 8192 | 1024 | 4096 |
| 5 | 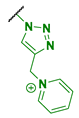 | 512 | 2048 | 512 | 1024 |
| 6 |  | 256 | 4096 | 256 | 256 |
| 7 |  | 8192 | 8192 | 8192 | ≥8192 |
| 8 | 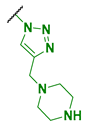 | ≥8192 | ≥8192 | ≥8192 | 8192 * |
| 9 |  | ≥8192 | ≥8192 | ≥8192 | ≥8192 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rathinam, S.; Magdadaro, R.; Hjálmarsdóttir, M.Á.; Másson, M. Water-Soluble Quaternary and Protonable Basic Chitotriazolans: Synthesis by Click Chemistry Conversion of Chitosan Azides and Investigation of Antibacterial Activity. J. Funct. Biomater. 2024, 15, 63. https://doi.org/10.3390/jfb15030063
Rathinam S, Magdadaro R, Hjálmarsdóttir MÁ, Másson M. Water-Soluble Quaternary and Protonable Basic Chitotriazolans: Synthesis by Click Chemistry Conversion of Chitosan Azides and Investigation of Antibacterial Activity. Journal of Functional Biomaterials. 2024; 15(3):63. https://doi.org/10.3390/jfb15030063
Chicago/Turabian StyleRathinam, Sankar, Romano Magdadaro, Martha Á. Hjálmarsdóttir, and Már Másson. 2024. "Water-Soluble Quaternary and Protonable Basic Chitotriazolans: Synthesis by Click Chemistry Conversion of Chitosan Azides and Investigation of Antibacterial Activity" Journal of Functional Biomaterials 15, no. 3: 63. https://doi.org/10.3390/jfb15030063





