Deferoxamine-Loaded Chitosan-Based Hydrogel on Bone Implants Showing Enhanced Bond Strength and Pro-Angiogenic Effects
Abstract
:1. Introduction
2. Materials and Methods
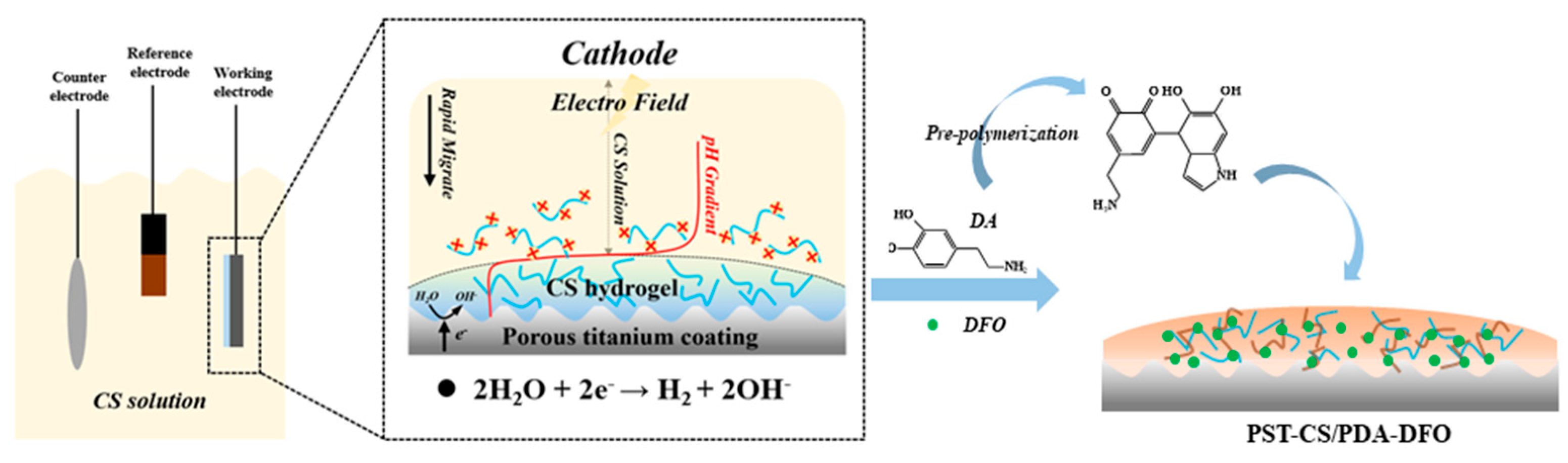
2.1. Coating Preparation
2.2. Surface Characterization
2.3. DFO Release from Hydrogel Coating
2.4. Cell Culture
2.5. Cell Morphology Observation
2.6. Cell Proliferation Assay
2.7. NO and VEGF Secretion
2.8. Statistical Analysis
3. Results and Discussion
3.1. Surface Characterization
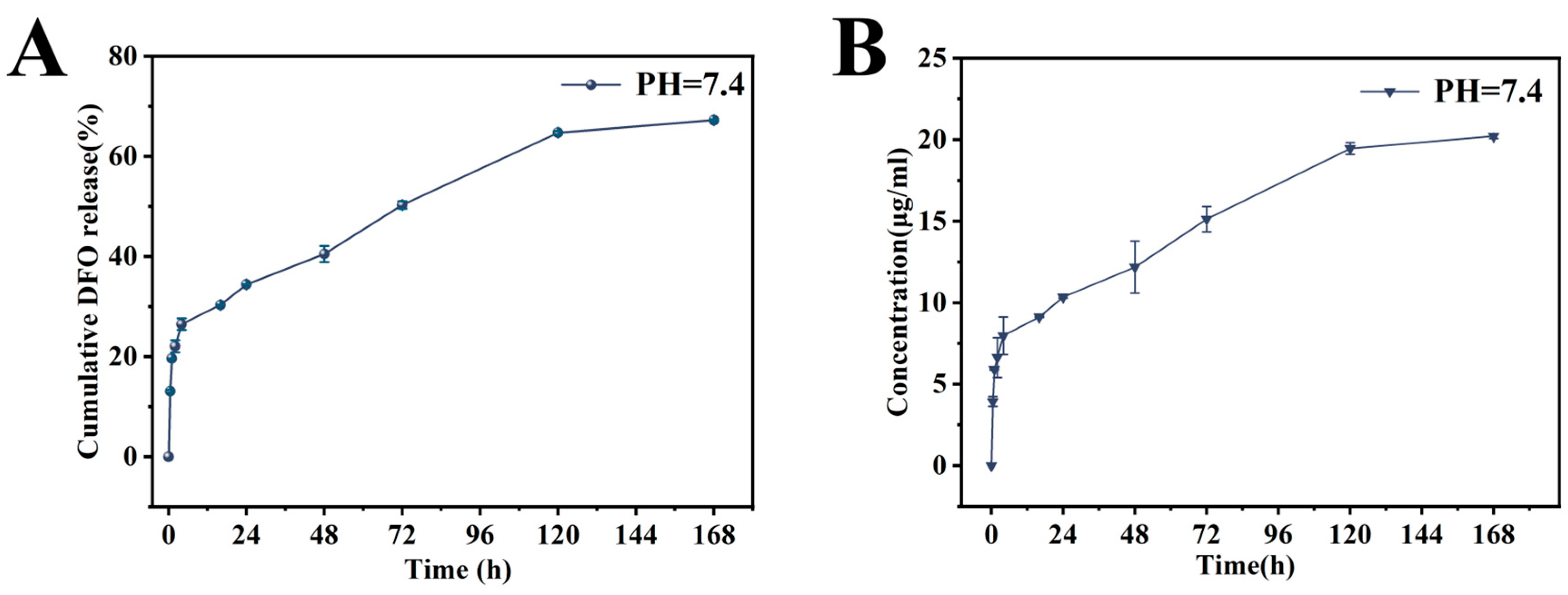
3.2. DFO Release from the PST-CS/PDA-DFO Coating
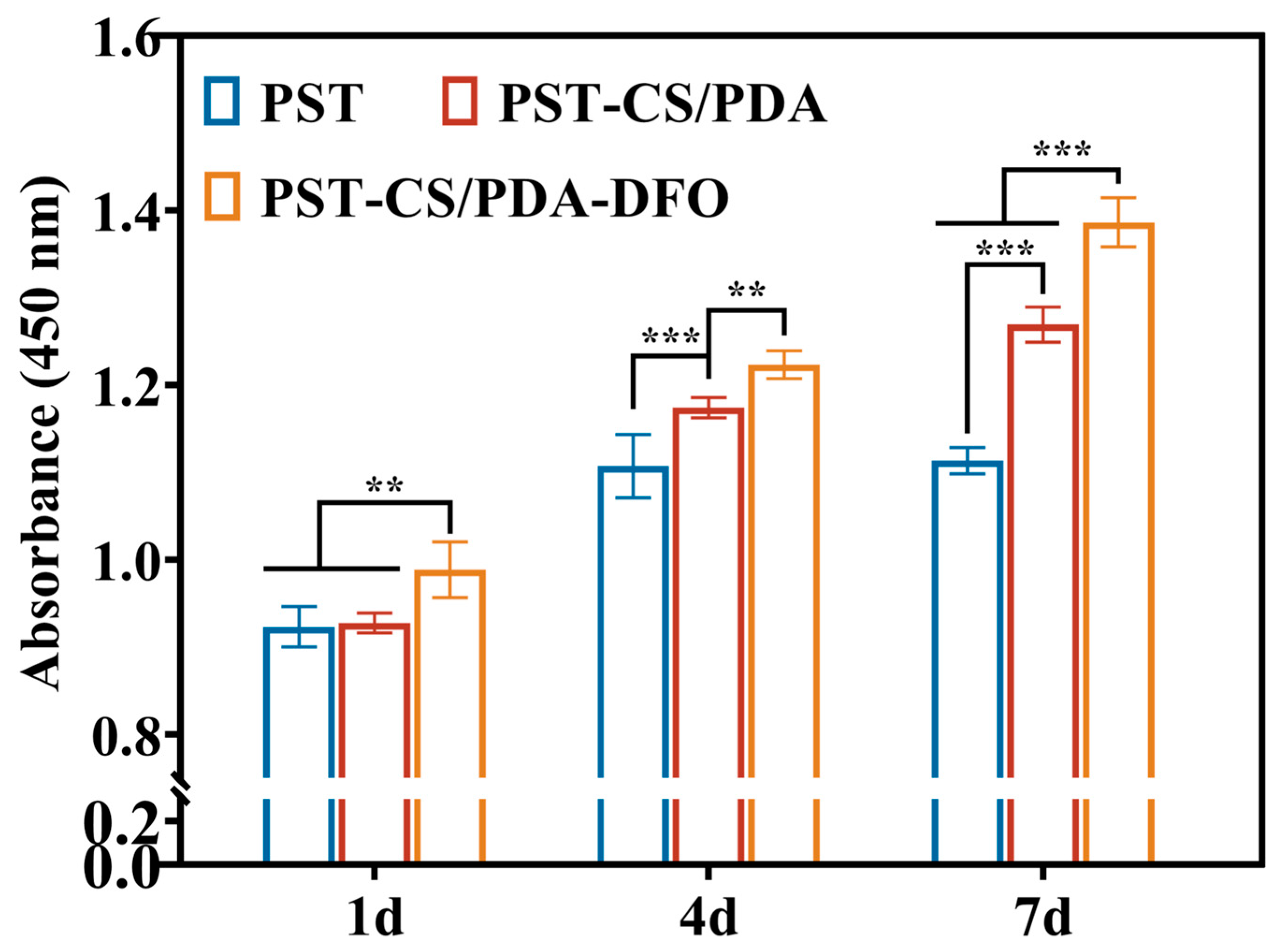
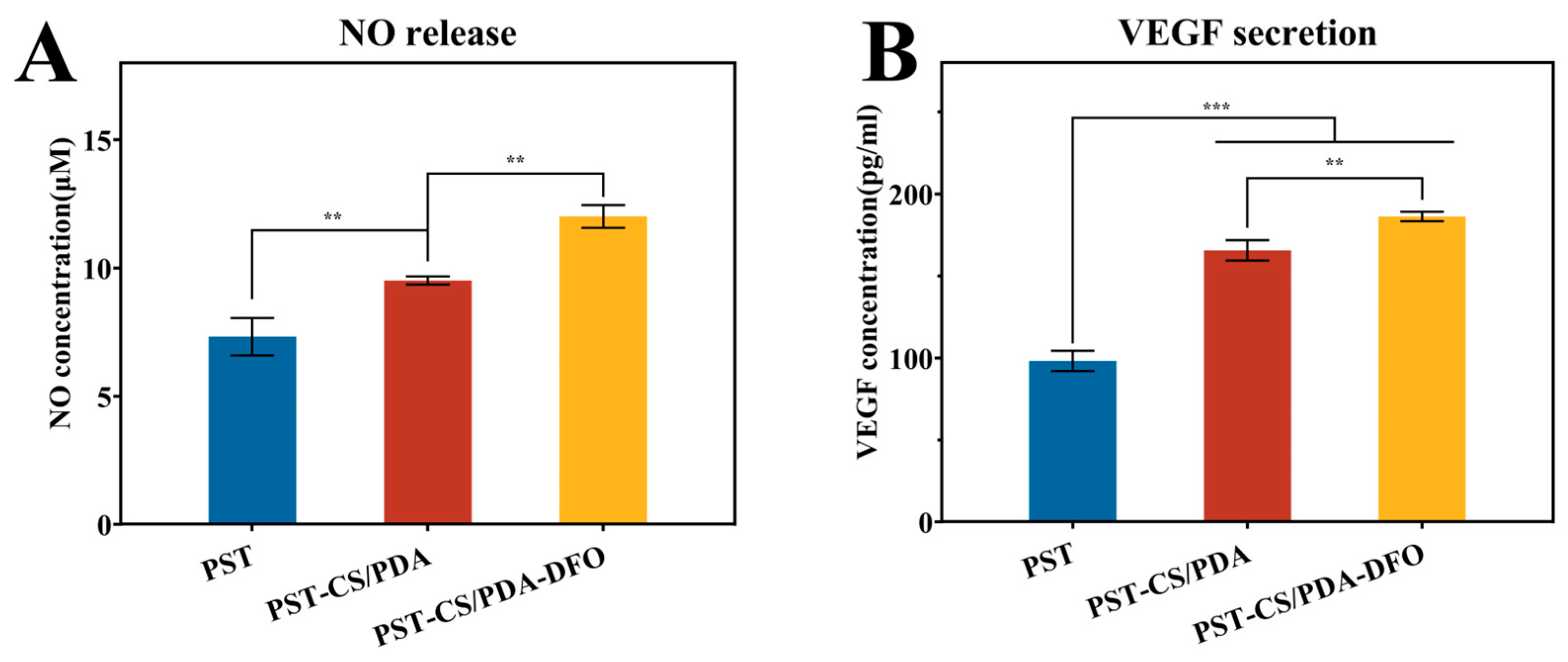
3.3. Combined Effects of CS/PDA Hydrogel and DFO on the HUVEC Behaviors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, K.T.; Huang, J.W.; Lin, W.T.; Kuo, T.Y.; Chien, C.S.; Chang, C.P.; Lin, Y.D. Effects of Micro-Arc Oxidation Discharge Parameters on Formation and Biomedical Properties of Hydroxyapatite-Containing Flower-like Structure Coatings. Materials 2022, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Abdollahi, F.; Saghatchi, M.; Paryab, A.; Malek Khachatourian, A.; Stephens, E.D.; Toprak, M.S.; Badv, M. Angiogenesis in bone tissue engineering via ceramic scaffolds: A review of concepts and recent advancements. Biomater. Adv. 2024, 159, 213828. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lu, X.; Razanau, I.; Wu, X.; Hu, T.; Liu, S.; Xie, Y.; Huang, L.; Zheng, X. The enhanced angiogenic responses to ionic dissolution products from a boron-incorporated calcium silicate coating. Mater. Sci. Eng. C 2019, 101, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Ran, Q.; Yu, Y.; Chen, W.; Shen, X.; Mu, C.; Yuan, Z.; Tao, B.; Hu, Y.; Yang, W.; Cai, K. Deferoxamine loaded titania nanotubes substrates regulate osteogenic and angiogenic differentiation of MSCs via activation of HIF-1α signaling. Mater. Sci. Eng. C 2018, 91, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Li, J.; Yu, Z.; Dang, X.; Wang, K. The hypoxia-inducible factor pathway, prolyl hydroxylase domain protein inhibitors, and their roles in bone repair and regeneration. BioMed Res. Int. 2014, 2014, 239356. [Google Scholar] [CrossRef]
- Maes, C.; Carmeliet, G.; Schipani, E. Hypoxia-driven pathways in bone development, regeneration and disease. Nat. Rev. Rheumatol. 2012, 8, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guo, L.; Wicks, J.; Ling, C.; Zhao, X.; Yan, Y.; Qi, J.; Cui, W.; Deng, L. Quickly promoting angiogenesis by using a DFO-loaded photo-crosslinked gelatin hydrogel for diabetic skin regeneration. J. Mater. Chem. B 2016, 4, 3770–3781. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, D.; Yang, Y.; Gao, P.; Fan, W.; Zhang, X.; Tang, Y.; Yang, W.; Cai, K. Caffeic Acid-Deferoxamine Self-Polymerization Coating on Ti Implant Promotes Osteointegration by Synergetic Regulation of Multi-Pathways in ONFH Mechanism. Adv. Funct. Mater. 2023, 33, 2212016. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, Y.; Tao, J.; Baumgarten, K.M.; Sun, H. Hypoxia-Mimicking Nanofibrous Scaffolds Promote Endogenous Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 32450–32459. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, Y.; Guo, P.; Duan, T.; Cheng, W.; Guo, Y.; Zheng, X.; Lu, G.; Lu, Q.; Kaplan, D.L. Injectable Desferrioxamine-Laden Silk Nanofiber Hydrogels for Accelerating Diabetic Wound Healing. ACS Biomater. 2021, 7, 1147–1158. [Google Scholar] [CrossRef]
- Yang, C.; Wang, M.; Wang, W.; Liu, H.; Deng, H.; Du, Y.; Shi, X. Electrodeposition induced covalent cross-linking of chitosan for electrofabrication of hydrogel contact lenses. Carbohydr. Polym. 2022, 292, 119678. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, W.; Li, L.; Yu, F.; Li, J.; Liu, L.; Fang, B.; Xia, L. Photothermally triggered biomimetic drug delivery of Teriparatide via reduced graphene oxide loaded chitosan hydrogel for osteoporotic bone regeneration. Chem. Eng. J. 2021, 413, 127413. [Google Scholar] [CrossRef]
- Liu, F.; Cheng, X.; Xiao, L.; Wang, Q.; Yan, K.; Su, Z.; Wang, L.; Ma, C.; Wang, Y. Inside-outside Ag nanoparticles-loaded polylactic acid electrospun fiber for long-term antibacterial and bone regeneration. Int. J. Biol. Macromol. 2021, 167, 1338–1348. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Liu, H.; Deng, H.; Xiao, L.; Qin, C.; Du, Y.; Shi, X. A study of chitosan hydrogel with embedded mesoporous silica nanoparticles loaded by ibuprofen as a dual stimuli-responsive drug release system for surface coating of titanium implants. Colloids Surf. B 2014, 123, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ma, D.; Wu, D.; Qiu, X.; Yang, S.; Wang, Y.; Xiao, L.; Ji, X.; Zhang, W.; Han, S.; et al. A pH-responsive, injectable and self-healing chitosan-coumarin hydrogel based on Schiff base and hydrogen bonds. Int. J. Biol. Macromol. 2024, 255, 128122. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liu, H.; Zhang, C.; Lei, Y.; Lei, M.; Xu, M.; Jin, D.; Li, P.; Yin, M.; Payne, G.F.; et al. Electrofabrication of functional materials: Chloramine-based antimicrobial film for infectious wound treatment. Acta Biomater. 2018, 73, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.; Qu, X.; Liu, H.; Liu, Y.; Wang, S.; Wu, S.; Bentley, W.E.; Payne, G.F.; Liu, C. Programmable Electrofabrication of Porous Janus Films with Tunable Janus Balance for Anisotropic Cell Guidance and Tissue Regeneration. Adv. Funct. Mater. 2019, 29, 1900065. [Google Scholar] [CrossRef]
- Li, J.; Wu, S.; Kim, E.; Yan, K.; Liu, H.; Liu, C.; Dong, H.; Qu, X.; Shi, X.; Shen, J.; et al. Electrobiofabrication: Electrically based fabrication with biologically derived materials. Biofabrication 2019, 11, 032002. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hu, J.; Hu, J.; Cheng, Y.; Chen, X.; Gu, Z.; Li, Y. Bioinspired polydopamine hydrogels: Strategies and applications. Prog. Polym. Sci. 2023, 146, 101740. [Google Scholar] [CrossRef]
- Pacelli, S.; Rampetsreiter, K.; Modaresi, S.; Subham, S.; Chakravarti, A.R.; Lohfeld, S.; Detamore, M.S.; Paul, A. Fabrication of a Double-Cross-Linked Interpenetrating Polymeric Network (IPN) Hydrogel Surface Modified with Polydopamine to Modulate the Osteogenic Differentiation of Adipose-Derived Stem Cells. ACS Appl. Mater. Interfaces 2018, 10, 24955–24962. [Google Scholar] [CrossRef]
- Liu, T.; Feng, Z.; Li, Z.; Lin, Z.; Chen, L.; Li, B.; Chen, Z.; Wu, Z.; Zeng, J.; Zhang, J.; et al. Carboxymethyl chitosan/sodium alginate hydrogels with polydopamine coatings as promising dressings for eliminating biofilm and multidrug-resistant bacteria induced wound healing. Int. J. Biol. Macromol. 2023, 225, 923–937. [Google Scholar] [CrossRef]
- Gan, Y.; Lin, C.; Zhu, H.; Cheng, X.; Liu, C.; Shi, J. An injectable self-healing CS/PDA–AgNPs hybrid hydrogel for mild and highly-efficient photothermal sterilization. New J. Chem. 2022, 46, 8043–8052. [Google Scholar] [CrossRef]
- Ghahremani, P.; Mostafatabar, A.H.; Bahlakeh, G.; Ramezanzadeh, B. Rational design of a novel multi-functional carbon-based nano-carrier based on multi-walled-CNT-oxide/polydopamine/chitosan for epoxy composite with robust pH-sensitive active anti-corrosion properties. Carbon 2022, 189, 113–141. [Google Scholar] [CrossRef]
- Guo, D.M.; An, Q.D.; Xiao, Z.Y.; Zhai, S.R.; Yang, D.J. Efficient removal of Pb(II), Cr(VI) and organic dyes by polydopamine modified chitosan aerogels. Carbohydr. Polym. 2018, 202, 306–314. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Hou, C.; Liu, M. Mussel-inspired synthesis of magnetic polydopamine–chitosan nanoparticles as biosorbent for dyes and metals removal. J. Taiwan Inst. Chem. Eng. 2016, 61, 292–298. [Google Scholar] [CrossRef]
- Liu, H.; Wen, W.; Chen, S.; Zhou, C.; Luo, B. Preparation of Icariin and Deferoxamine Functionalized Poly(l-lactide)/chitosan Micro/Nanofibrous Membranes with Synergistic Enhanced Osteogenesis and Angiogenesis. ACS Appl. Bio Mater. 2018, 1, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Luo, B.; Wen, W.; Zhou, C.; Tian, L.; Ramakrishna, S. Deferoxamine immobilized poly(D,L-lactide) membrane via polydopamine adhesive coating: The influence on mouse embryo osteoblast precursor cells and human umbilical vein endothelial cells. Mater. Sci. Eng. C 2017, 70, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Eren, E.D.; Guisong, G.; Mingming, L.; Bingchun, Z.; Ke, Y.; Shanshan, C. A novel chitosan and polydopamine interlinked bioactive coating for metallic biomaterials. J. Mater. Sci. Mater. Med. 2022, 33, 65. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Li, X.; Gong, Z.; Fan, B.; Wang, M.; Peng, J.; Lu, K.; Gao, S. High efficiency activation of peroxydisulfate by an Fe3O4@PDAn-CuxO core–shell composite for triclosan degradation: The role of oxygen vacancy. Chem. Eng. J. 2023, 461, 141719. [Google Scholar] [CrossRef]
- He, X.; Li, W.; Liu, K.; Wen, W.; Lu, L.; Liu, M.; Zhou, C.; Luo, B. Anisotropic and robust hydrogels combined osteogenic and angiogenic activity as artificial periosteum. Compos. B. Eng. 2022, 233, 109627. [Google Scholar] [CrossRef]
- Liu, J.; Qu, S.; Suo, Z.; Yang, W. Functional hydrogel coatings. Natl. Sci. Rev. 2021, 8, nwaa254. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Sun, H.; Guo, Z.; Sun, X.; Yu, Q.; Wu, X.; Yu, C.; Zhang, H.; Yao, F.; Li, J. A starch-based zwitterionic hydrogel coating for blood-contacting devices with durability and bio-functionality. Chem. Eng. J. 2021, 421, 129702. [Google Scholar] [CrossRef]
- Shao, Z.; Yin, T.; Jiang, J.; He, Y.; Xiang, T.; Zhou, S. Wound microenvironment self-adaptive hydrogel with efficient angiogenesis for promoting diabetic wound healing. Bioact. Mater. 2023, 20, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, Y.; Yang, Z.; Aimaijiang, M.; Ma, R.; Yang, Y.; Zhang, Y.; Zhou, Y. Hypoxia-Inducible Factors Signaling in Osteogenesis and Skeletal Repair. Int. J. Mol. Sci. 2022, 23, 11201. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Li, K.; Yi, D.; Ding, Y.; Gao, Y.; Zheng, X. Deferoxamine-Loaded Chitosan-Based Hydrogel on Bone Implants Showing Enhanced Bond Strength and Pro-Angiogenic Effects. J. Funct. Biomater. 2024, 15, 112. https://doi.org/10.3390/jfb15040112
Liu H, Li K, Yi D, Ding Y, Gao Y, Zheng X. Deferoxamine-Loaded Chitosan-Based Hydrogel on Bone Implants Showing Enhanced Bond Strength and Pro-Angiogenic Effects. Journal of Functional Biomaterials. 2024; 15(4):112. https://doi.org/10.3390/jfb15040112
Chicago/Turabian StyleLiu, Huan, Kai Li, Deliang Yi, Yi Ding, Yanfeng Gao, and Xuebin Zheng. 2024. "Deferoxamine-Loaded Chitosan-Based Hydrogel on Bone Implants Showing Enhanced Bond Strength and Pro-Angiogenic Effects" Journal of Functional Biomaterials 15, no. 4: 112. https://doi.org/10.3390/jfb15040112




