Biocompatibility of Bacterial Cellulose Based Biomaterials
Abstract
:1. Introduction
2. Bacterial Cellulose Properties
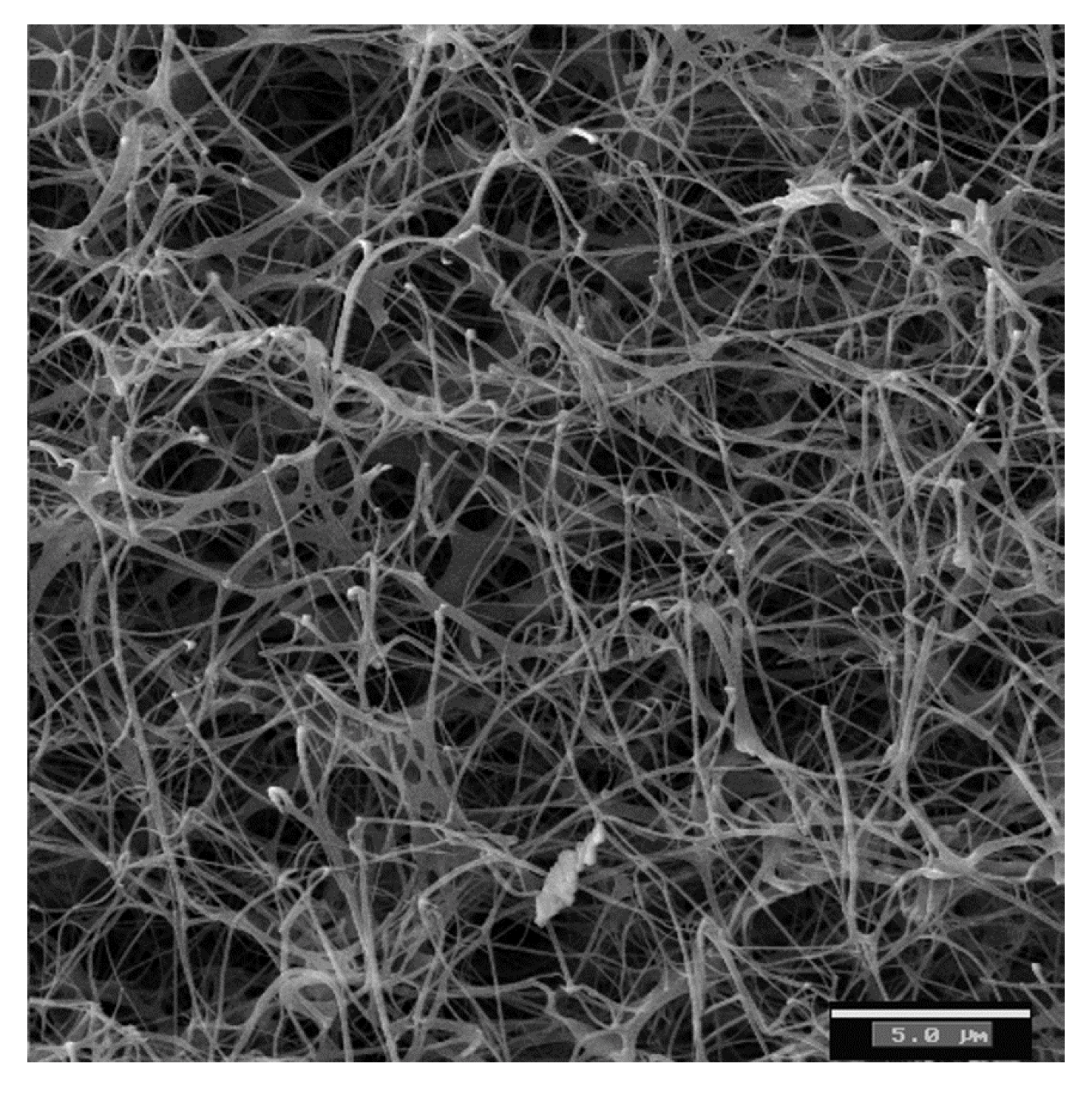

3. Bacterial Cellulose as a Biomaterial
4. Improvement of Biocompatibility of BC Structures
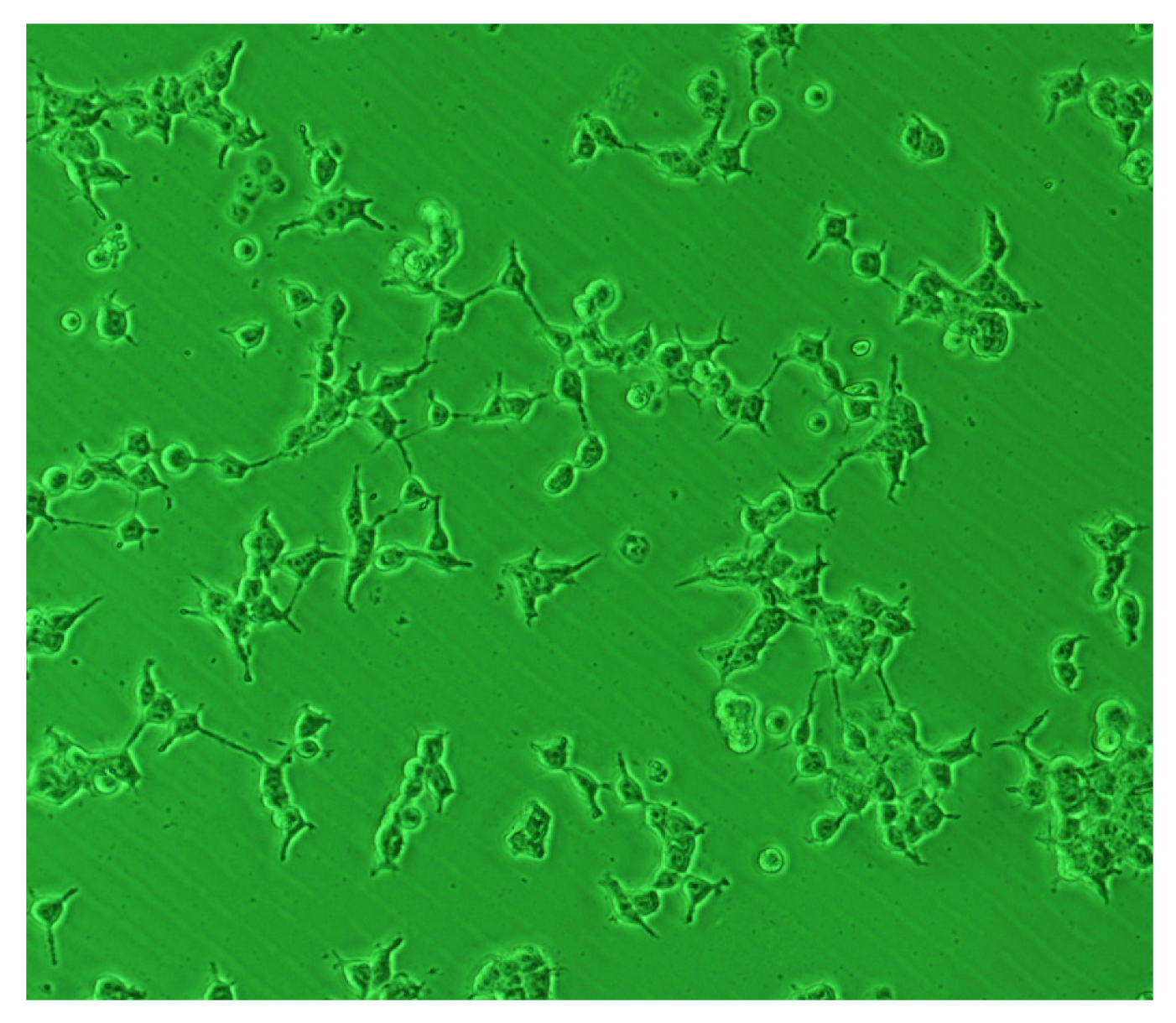
5. BC Based Structures for Biomedical Applications
5.1. Bone Tissue Engineering and Bone Grafting
5.2. Cardiovascular Clinical Applications
5.3. Wound Dressings
5.4. Cartilage Replacement
5.5. Other Biomedical Applications
6. Conclusions
References
- Perez, J.; Muñoz-Dorado, J.; de la Rubia, T.; Martinez, J. Biodegradation and biological treatments of cellulos, hemicellulose and lignin: An overview. Int. Microbiol. 2002, 5, 53–63. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose: A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar]
- Nakagaito, A.N.; Iwamoto, S.; Yano, H. Bacterial cellulose: The ultimate nano-scalar cellulose morphology for the production of high-strength composites. Appl. Phys. A Mat. Sci. Process. 2005, 80, 93–97. [Google Scholar] [CrossRef]
- Bae, S.; Shoda, M. Statistical optimization of culture conditions for bacterial cellulose production using Box-Behnken design. Biotechnol. Bioeng. 2005, 90, 20–28. [Google Scholar]
- Bae, S.; Sugano, Y.; Shoda, M. Improvement of bacterial cellulose production by addition of agar in a jar fermentor. J. Biosci. Bioeng. 2004, 97, 33–38. [Google Scholar]
- Atalla, R.H.; Vanderhart, D.L. Native cellulose: A composite of two distinct crystalline forms. Scienc 1984, 223, 283–285. [Google Scholar]
- Budhiono, A.; Rosidi, B.; Taher, H.; Iguchi, M. Kinetic aspects of bacterial cellulose formation in nata-de-coco culture system. Carbohyd. Polym. 1999, 40, 137–143. [Google Scholar] [CrossRef]
- Sreeramulu, G.; Zhu, Y.; Knol, W. Kombucha fermentation and its antimicrobial activity. J. Agric. Food Chem. 2000, 48, 2589–2594. [Google Scholar]
- Czaja, W.; Krystynowicz, A.; Bielecki, S.; Brownjr, R. Microbial cellulose the natural power to heal wounds. Biomaterials 2006, 27, 145–151. [Google Scholar] [CrossRef]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Troncoso, O.P; Canet-Ferrer, J.; Martinez-Pastor, J. Morphological characterisation of bacterial Cellulose-Starch nanocomposites. Polym. Polym. Composites 2008, 16, 181–185. [Google Scholar]
- Li, J.; Wan, Y.; Li, L.; Liang, H.; Wang, J. Preparation and characterization of 2,3-dialdehyde bacterial cellulose for potential biodegradable tissue engineering scaffolds. Mater. Sci. Eng. C 2009, 29, 1635–1642. [Google Scholar]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Bañó, M.C. Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater. 2009, 5, 1605–1615. [Google Scholar]
- Chen, Y.M.; Xi, T.; Zheng, Y.; Guo, T.; Hou, J.; Wan, Y.; Gao, C. In vitro cytotoxicity of bacterial cellulose scaffolds used for tissue-engineered bone. J. Bioact. Compat. Polym. 2009, 24, S137–S145. [Google Scholar] [CrossRef]
- Mendes, P.N.; Rahal, S.C.; Pereira-Junior, O.P.; Fabris, V.E.; Lenharo, S.L.; de Lima-Neto, J.F.; da Cruz Landim-Alvarenga, F. In vivo and in vitro evaluation of an acetobacter xylinum synthesized microbial cellulose membrane intended for guided tissue repair. Acta Vet. Scand. 2009, 51, 12. [Google Scholar] [CrossRef]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. 2006, 76, 431–438. [Google Scholar]
- Shi, S.; Chen, S.; Zhang, X.; Shen, W.; Li, X.; Hu, W.; Wang, H. Biomimetic mineralization synthesis of calcium-deficient carbonate-containing hydroxyapatite in a three-dimensional network of bacterial cellulose. J. Chem. Technol. Biotechnol. 2009, 84, 285–290. [Google Scholar]
- El-Saied, H.; Basta, A.H.; Gobran, R.H. Research progress in friendly environmental technology for the production of cellulose products (bacterial cellulose and its application). Polym.-Plast. Technol. Eng. 2004, 43, 797–820. [Google Scholar] [CrossRef]
- Klemm, D.; Schumann, D.; Udhardt, U.; Marsch, S. Bacterial synthesized cellulose: Artificial blood vessels for microsurgery. Prog. Polym. Sci. 2001, 26, 1561–1603. [Google Scholar]
- Hirai, A.; Tsuji, M.; Horii, F. TEM study of band-like cellulose assemblies produced by Acetobacter xylinum. Cellulose 2002, 9, 105–113. [Google Scholar]
- Gindl, W.; Keckes, J. Tensile properties of cellulose acetate butyrate composites reinforced with bacterial cellulose. Composites Sci. Technol. 2004, 64, 2407–2413. [Google Scholar]
- Guhados, G.; Wan, W.; Hutter, J.L. Measurement of the elastic modulus of single bacterial cellulose fibers using atomic force microscopy. Langmuir 2005, 21, 6642–6646. [Google Scholar]
- Torres, F.G.; Troncoso, O.P.; Lopez, D.; Grande, C.; Gomez, C.M. Reversible stress softening and stress recovery of cellulose networks. Soft Matter 2009, 5, 4185–4190. [Google Scholar] [CrossRef]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Troncoso, O.P.; Canet-Ferrer, J.; Martínez-Pastor, J. Development of self-assembled bacterial cellulose-starch nanocomposites. Mater. Sci. Eng. C 2009, 29, 1098–1104. [Google Scholar]
- Yamanaka, S.; Watanabe, K.; Kitamura, N.; Iguchi, M.; Mitsuhashi, S.; Nishi, Y.; Uryu, M. The structure and mechanical properties of sheets prepared from bacterial cellulose. J. Mater. Sci. 1989, 24, 3141–3145. [Google Scholar]
- Yano, H.; Sugiyama, J.; Nakagaito, A.N.; Nogi, M.; Matsuura, T.; Hikita, M.; Handa, K. Optically transparent composites reinforced with networks of bacterial nanofibers. Adv. Mater. 2005, 17, 153–155. [Google Scholar] [CrossRef]
- Touzel, J.-P.; Chabbert, B.; Monties, B.; Debeire, P.; Cathala, B. Synthesis and characterization of dehydrogenation polymers in gluconacetobacter xylinus cellulose and Cellulose/Pectin composite. J. Agric. Food Chem. 2003, 51, 981–986. [Google Scholar]
- Mormino, R.; Bungay, H. Composites of bacterial cellulose and paper made with a rotating disk bioreactor. Appl. Microbiol. Biotechnol. 2003, 62, 503–506. [Google Scholar] [CrossRef]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef]
- Bäckdahl, H.; Helenius, G.; Bodin, A.; Nannmark, U.; Johansson, B.R.; Risberg, B.; Gatenholm, P. Mechanical properties of bacterial cellulose and interactions with smooth muscle cells. Biomaterials 2006, 27, 2141–2149. [Google Scholar]
- MacKintosh, F.C.; Käs, J.; Janmey, P.A. Elasticity of semiflexible biopolymer networks. Phys. Rev. Lett. 1995, 75, 4425–4428. [Google Scholar]
- Chaudhuri, O.; Parekh, S.H.; Fletcher, D.A. Reversible stress softening of actin networks. Nature 2007, 445, 295–298. [Google Scholar]
- Gatenholm, P.; Klemm, D. Bacterial nanocellulose as a renewable material for biomedical applications. MRS Bull. 2010, 35, 208–213. [Google Scholar] [CrossRef]
- Williams, D.F. The Williams Dictionary of Biomaterials; Liverpool University Press: Liverpool, UK, 1999. [Google Scholar]
- Schumann, D.A.; Wippermann, J.; Klemm, D.O.; Kramer, F.; Koth, D.; Kosmehl, H.; Wahlers, T.; Salehi-Gelani, S. Artificial vascular implants from bacterial cellulose: preliminary results of small arterial substitutes. Cellulose 2009, 16, 877–885. [Google Scholar] [CrossRef]
- Hutmacher, D.W. Scaffold design and fabrication technologies for engineering tissues: State of the art and future perspectives. J. Biomater. Sci. Polym. Ed. 2001, 12, 107–124. [Google Scholar]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Angelova, N. Rationalizing the design of polymeric biomaterials. Trends in Biotech. 1999, 17, 409–421. [Google Scholar]
- Pértile, R.; Moreira, S.; Andrade, F.; Domingues, L.; Gama, M. Bacterial cellulose modified using recombinant proteins to improve neuronal and mesenchymal cell adhesion. Biotechnol Prog. 2012, 28, 526–532. [Google Scholar] [CrossRef] [Green Version]
- Pertile, R.A.N.; Andrade, F.K.; Alves, C.; Gama, M. Surface modification of bacterial cellulose by nitrogen-containing plasma for improved interaction with cells. Carbohyd. Polym. 2010, 82, 692–698. [Google Scholar]
- Andrade, F.K.; Moreira, S.M.; Domingues, L.; Gama, F.M. Improving the affinity of fibroblasts for bacterial cellulose using carbohydrate-binding modules fused to RGD. J. Biomed. Mater. Res. A 2010, 92, 9–17. [Google Scholar] [Green Version]
- Wang, J.; Wan, Y.Z.; Luo, H.L.; Gao, C.; Huang, Y. Immobilization of gelatin on bacterial cellulose nanofibers surface via crosslinking technique. Mater. Sci. Eng. C 2012, 32, 536–541. [Google Scholar]
- Luo, H.; Xiong, G.; Huang, Y.; He, F.; Wang, Y.; Wan, Y. Preparation and characterization of a novel COL/BC composite for potential tissue engineering scaffolds. Mater. Chem. Phys. 2008, 110, 193–196. [Google Scholar] [CrossRef]
- Cai, Z.; Yang, G. Bacterial cellulose/collagen composite: Characterization and first evaluation of cytocompatibility. J. Appl. Polym. Sci. 2011, 120, 2938–2944. [Google Scholar]
- Kim, J.; Cai, Z.; Lee, H.S.; Choi, G.S.; Lee, D.H.; Jo, C. Preparation and characterization of a bacterial cellulose/Chitosan composite for potential biomedical application. J. Polym. Res. 2011, 18, 739–744. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Shah, N.; Ha, J.H.; Park, J.K. Effect of chitosan penetration on physico-chemical and mechanical properties of bacterial cellulose. Korean J. Chem. Eng. 2011, 28, 1736–1743. [Google Scholar] [CrossRef]
- Cai, Z.; Chen, P.; Jin, H.J.; Kim, J. The effect of chitosan content on the crystallinity, thermal stability, and mechanical properties of bacterial cellulose: Chitosan composites. Proc. Inst. Mech. Eng. C J. Mech. E. 2009, 223, 2225–2230. [Google Scholar] [CrossRef]
- Wan, Y.Z.; Luo, H.; He, F.; Liang, H.; Huang, Y.; Li, X.L. Mechanical, moisture absorption, and biodegradation behaviours of bacterial cellulose fibre-reinforced starch biocomposites. Composites Sci. Technol. 2009, 69, 1212–1217. [Google Scholar] [CrossRef]
- Yin, N.; Chen, S.-y.; Ouyang, Y.; Tang, L.; Yang, J.-x.; Wang, H.-p. Biomimetic mineralization synthesis of hydroxyapatite bacterial cellulose nanocomposites. Prog. Nat. Sci. Mater. Int. 2011, 21, 472–477. [Google Scholar] [CrossRef]
- Wang, J.; Wan, Y.; Han, J.; Lei, X.; Yan, T.; Gao, C. Nanocomposite prepared by immobilising gelatin and hydroxyapatite on bacterial cellulose nanofibres. Micro Nano Lett. 2011, 6, 133–136. [Google Scholar]
- Zimmermann, K.A.; LeBlanc, J.M.; Sheets, K.T.; Fox, R.W.; Gatenholm, P. Biomimetic design of a bacterial cellulose/hydroxyapatite nanocomposite for bone healing applications. Mater. Sci. Eng. C 2011, 31, 43–49. [Google Scholar]
- Wan, Y.Z.; Gao, C.; Luo, H.L.; He, F.; Liang, H.; Li, X.L.; Wang, Y.L. Early growth of Nano-Sized calcium phosphate on phosphorylated bacterial cellulose nanofibers. J. Nanosci. Nanotechnol. 2009, 9, 6494–6500. [Google Scholar] [CrossRef]
- Wan, Y.; Hong, L.; Jia, S.; Huang, Y.; Zhu, Y.; Wang, Y.; Jiang, H. Synthesis and characterization of hydroxyapatite bacterial cellulose nanocomposites. Composites Sci. Technol. 2006, 66, 1825–1832. [Google Scholar]
- Millon, L.E.; Oates, C.J.; Wan, W. Compression properties of polyvinyl alcohol bacterial cellulose nanocomposite. J. Biomed. Mater. Res. Part B 2009, 90, 922–929. [Google Scholar]
- Millon, L.E.; Guhados, G.; Wan, W. Anisotropic polyvinyl alcohol Bacterial cellulose nanocomposite for biomedical applications. J. Biomed. Mater. Res. Part B 2008, 86, 444–452. [Google Scholar]
- Millon, L.E.; Wan, W.K. The polyvinyl alcohol bacterial cellulose system as a new nanocomposite for biomedical applications. J. Biomed. Mater. Res. Part B 2006, 79, 245–253. [Google Scholar]
- Olsson, R.T.; Kraemer, R.; LoÌpez-Rubio, A.; Torres-Giner, S.; Ocio, M.J.; LagaroÌn, J.M. Extraction of microfibrils from bacterial cellulose networks for electrospinning of anisotropic biohybrid fiber yarns. Macromolecules 2010, 43, 4201–4209. [Google Scholar] [CrossRef]
- Hagiwara, Y.; Putra, A.; Kakugo, A.; Furukawa, H.; Gong, J.P. Ligament-like tough double-network hydrogel based on bacterial cellulose. Cellulose 2010, 17, 93–101. [Google Scholar]
- Bäckdahl, H.; Esguerra, M.; Delbro, D.; Risberg, B.; Gatenholm, P. Engineering microporosity in bacterial cellulose scaffolds. J. Tissue Eng. Regen. Med. 2008, 2, 320–330. [Google Scholar]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohyd. Polym. 2012, 88, 596–603. [Google Scholar]
- Kaewnopparat, S.; Sansernluk, K.; Faroongsarng, D. Behavior of freezable bound water in the bacterial cellulose produced by Acetobacter xylinum: An approach using thermoporosimetry. AAPS PharmSciTech 2008, 9, 701–707. [Google Scholar]
- Dahman, Y. Nanostructured biomaterials and biocomposites from bacterial cellulose nanofibers. J. Nanosci. Nanotechnol. 2009, 9, 5105–5122. [Google Scholar] [CrossRef]
- Rambo, C.R.; Recouvreux, D.O.S.; Carminatti, C.A.; Pitlovanciv, A.K.; Antônio, R.V.; Porto, L.M. Template assisted synthesis of porous nanofibrous cellulose membranes for tissue engineering. Mater. Sci. Eng. C 2008, 28, 549–554. [Google Scholar]
- Yoshikawa, H.; Myoui, A. Bone tissue engineering with porous hydroxyapatite ceramics. J. Artif. Organs 2005, 8, 131–136. [Google Scholar] [CrossRef]
- Stoica-Guzun, A.; Stroescu, M.; Jinga, S.; Jipa, I.; Dobre, T.; Dobre, L. Ultrasound influence upon calcium carbonate precipitation on bacterial cellulose membranes. Ultrason. Sonochemistry 2012, 19, 909–915. [Google Scholar] [CrossRef]
- Gao, C.; Xiong, G.Y.; Luo, H.L.; Ren, K.J.; Huang, Y.; Wan, Y.Z. Dynamic interaction between the growing CaP minerals and bacterial cellulose nanofibers during early biomineralization process. Cellulose 2010, 17, 365–373. [Google Scholar]
- Zhang, S.; Xiong, G.; He, F.; Huang, Y.; Wang, Y.; Wan, Y. Characterisation of hydroxyapatite/bacterial cellulose nanocomposites. Polym. Polym. Composites 2009, 17, 353–358. [Google Scholar]
- Hamilton, D.; Vorp, D. Tissue Engineering of Blood Vessel, 2nd ed; Informa HealthCare: London, UK, 2008. [Google Scholar]
- Czaja, W.K.; Young, D.J.; Kawecki, M.; Brown, R.M. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules 2006, 8, 1–12. [Google Scholar]
- Brown, E.E.; Laborie, M.-P.G.; Zhang, J. Glutaraldehyde treatment of bacterial cellulose/fibrin composites: Impact on morphology, tensile and viscoelastic properties. Cellulose 2012, 19, 127–137. [Google Scholar] [CrossRef]
- Mohammadi, H. Nanocomposite biomaterial mimicking aortic heart valve leaflet mechanical behaviour. Proc. Inst. Mech. Eng. H 2011, 225, 718–722. [Google Scholar]
- Fink, H.; Faxalv, L.; Molnár, G.F.; Drotz, K.; Risberg, B.; Lindahl, T.L.; Sellborn, A. Real-time measurements of coagulation on bacterial cellulose and conventional vascular graft materials. Acta Biomater. 2010, 6, 1125–1130. [Google Scholar]
- Andrade, F.K.; Silva, J.P.; Carvalho, M.; Castanheira, E.M.S.; Soares, R.; Gama, M. Studies on the hemocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A 2011, 98, 554–566. [Google Scholar]
- Farah, L.F.X. Process for the Preparation of Cellulose Film, Cellulose Film Produced thereby, Artificial Skin Graft and its Use. U.S. Patent 4,912,049, 27 March 1990. [Google Scholar]
- Fontana, J.D.; Souza, A.M.; Fontana, C.K.; Torriani, I.L.; Moreschi, J.C.; Gallotti, B.J.; Souza, S.J.; Narcisco, G.P.; Bichara, J.A.; Farah, L.F.X. Acetobacter cellulose pellicle as a temporary skin substitute. Appl. Biochem. Biotech. 1990, 24–25, 253–264. [Google Scholar] [CrossRef]
- Legeza, V.I.; Galenko-Yaroshevskii, V.P.; Zinov’ev, E.V.; Paramonov, B.A.; Kreichman, G.S.; Turkovskii, I.I.; Gumenyuk, E.S.; Karnovich, A.G.; Khripunov, A.K. Effects of new wound dressings on healing of thermal burns of the skin in acute radiation disease. Bull. Exp. Biol. Med. 2004, 138, 311–315. [Google Scholar]
- Azuma, C.; Yasuda, K.; Tanabe, Y.; Taniguro, H.; Kanaya, F.; Nakayama, A.; Chen, Y.M.; Gong, J.P.; Osada, Y. Biodegradation of high-toughness double network hydrogels as potential materials for artificial cartilage. J. Biomed. Mater. Res. Part A 2007, 81, 373–380. [Google Scholar]
- Levinson, D.J.; Glonek, T. Microbial Cellulose Contact Lens. U.S. Patent 7,832,857, 16 November 2010. [Google Scholar]
- Shi, Z.; Zang, S.; Jiang, F.; Huang, L.; Lu, D.; Ma, Y.; Yang, G. In situ nano-assembly of bacterial cellulose-polyaniline composites. RSC Adv. 2012, 2, 1040–1046. [Google Scholar]
- Trovatti, E.; Silva, N.H.C.S.; Duarte, I.F.; Rosado, C.F.; Almeida, I.F.; Costa, P.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Biocellulose membranes as supports for dermal release of lidocaine. Biomacromolecules 2011, 12, 4162–4168. [Google Scholar] [CrossRef]
- Park, H.O.; Bang, Y.B.; Joung, H.J.; Kim, B.C.; Kim, H.R. Lactobacillus KCTC 0774BP and Acetobacter KCTC 0773BP for Treatment or Prevention of Obesity and Diabetes Mellitus. U.S. Patent 6,808,703, 26 October 2004. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Torres, F.G.; Commeaux, S.; Troncoso, O.P. Biocompatibility of Bacterial Cellulose Based Biomaterials. J. Funct. Biomater. 2012, 3, 864-878. https://doi.org/10.3390/jfb3040864
Torres FG, Commeaux S, Troncoso OP. Biocompatibility of Bacterial Cellulose Based Biomaterials. Journal of Functional Biomaterials. 2012; 3(4):864-878. https://doi.org/10.3390/jfb3040864
Chicago/Turabian StyleTorres, Fernando G., Solene Commeaux, and Omar P. Troncoso. 2012. "Biocompatibility of Bacterial Cellulose Based Biomaterials" Journal of Functional Biomaterials 3, no. 4: 864-878. https://doi.org/10.3390/jfb3040864



