Antimicrobial Activity of Nitric Oxide-Releasing Ti-6Al-4V Metal Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
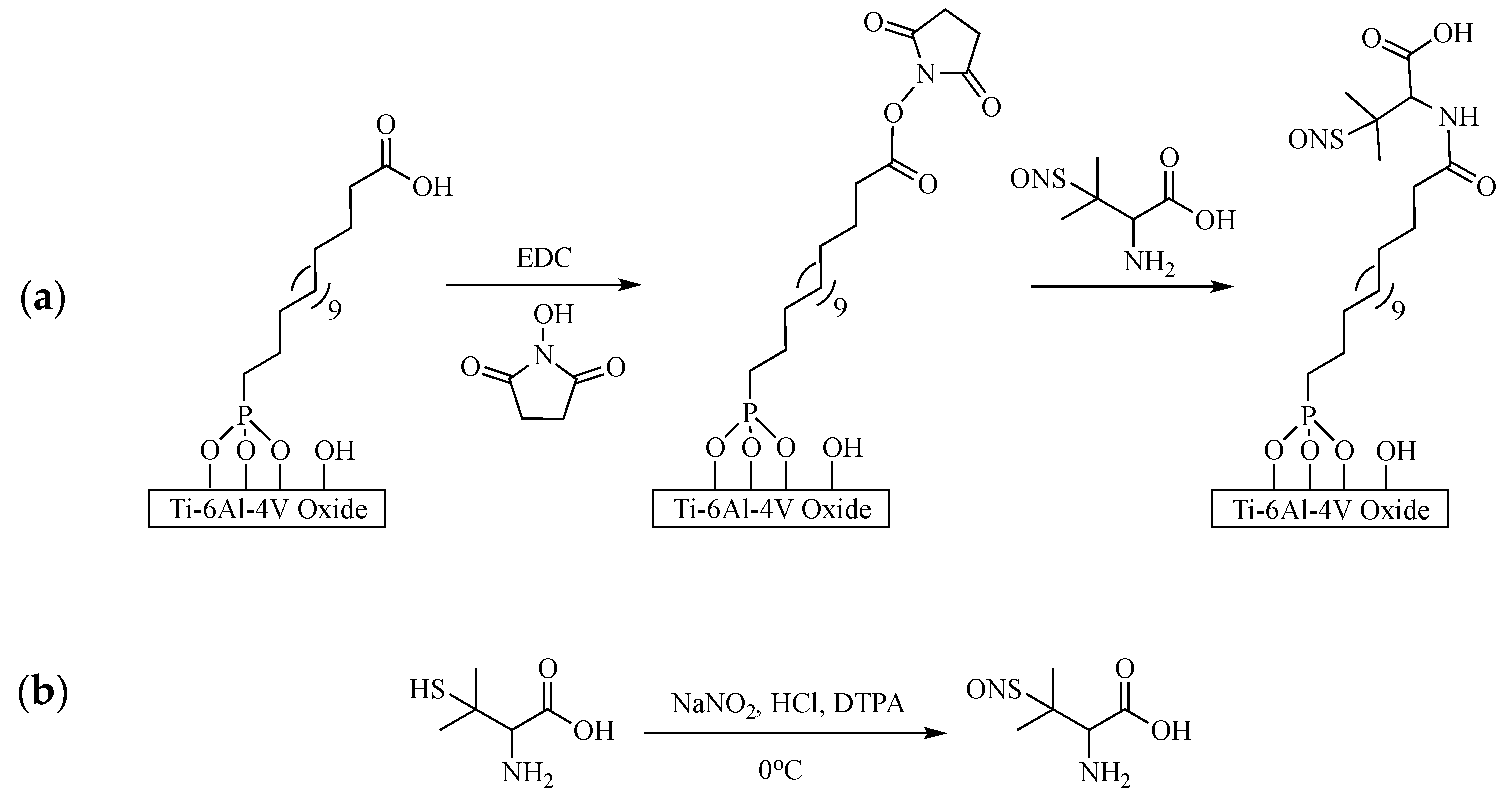
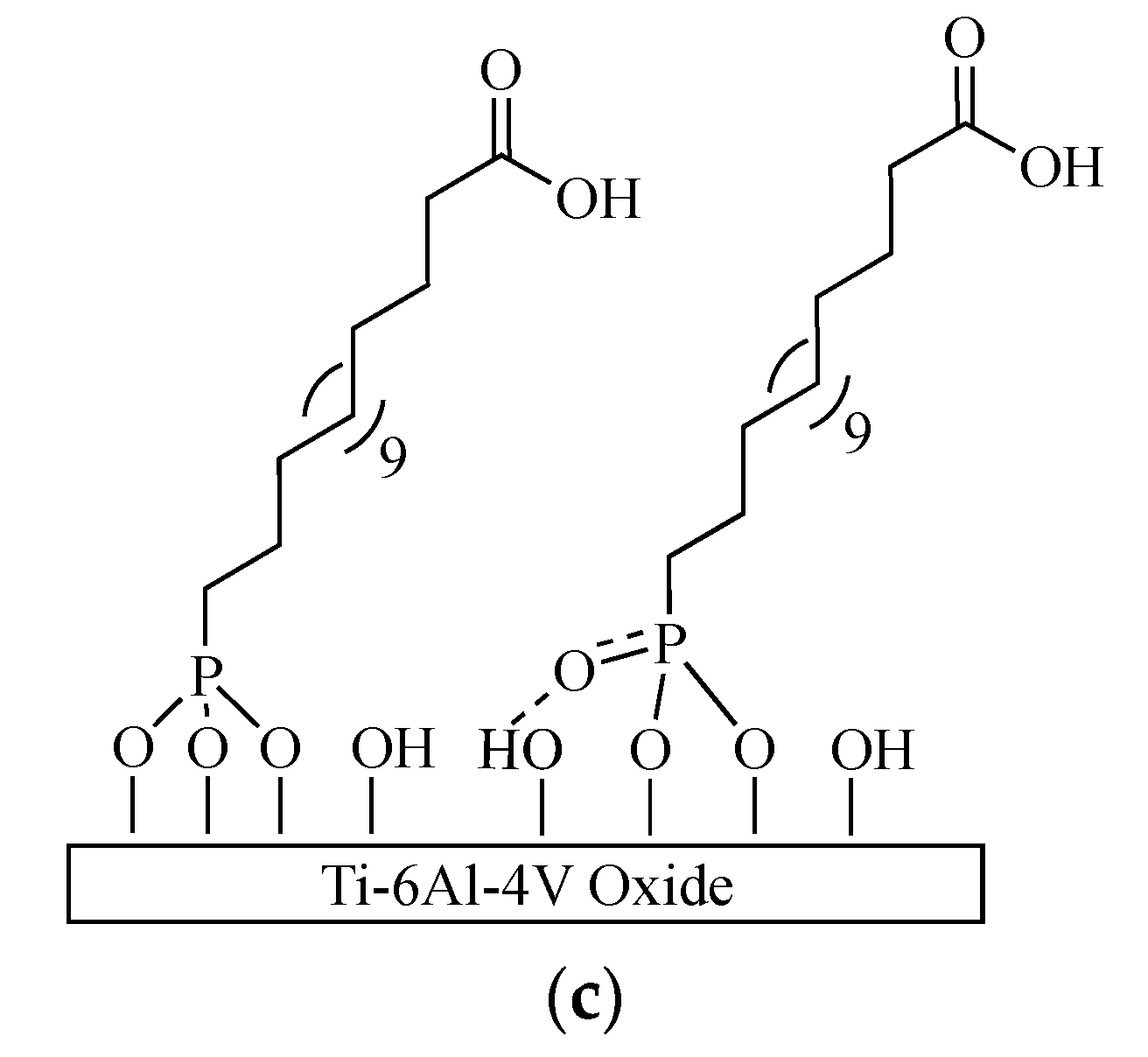
2.2.1. Substrate Preparation and Monolayer Formation
2.2.2. Immobilization of Nitric Oxide Donor
2.2.3. Characterization Techniques
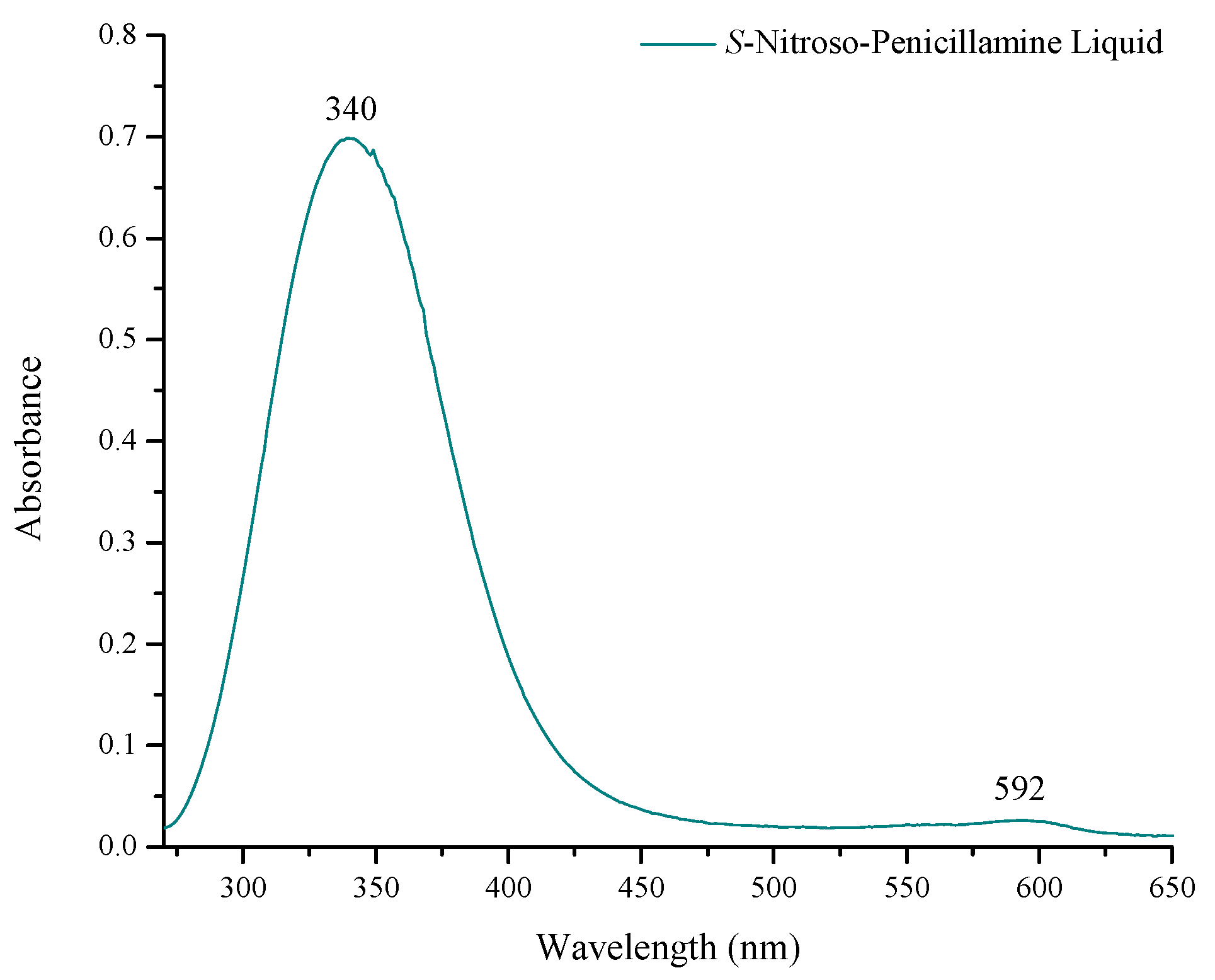
Ultraviolet-Visible Spectrometric Analysis
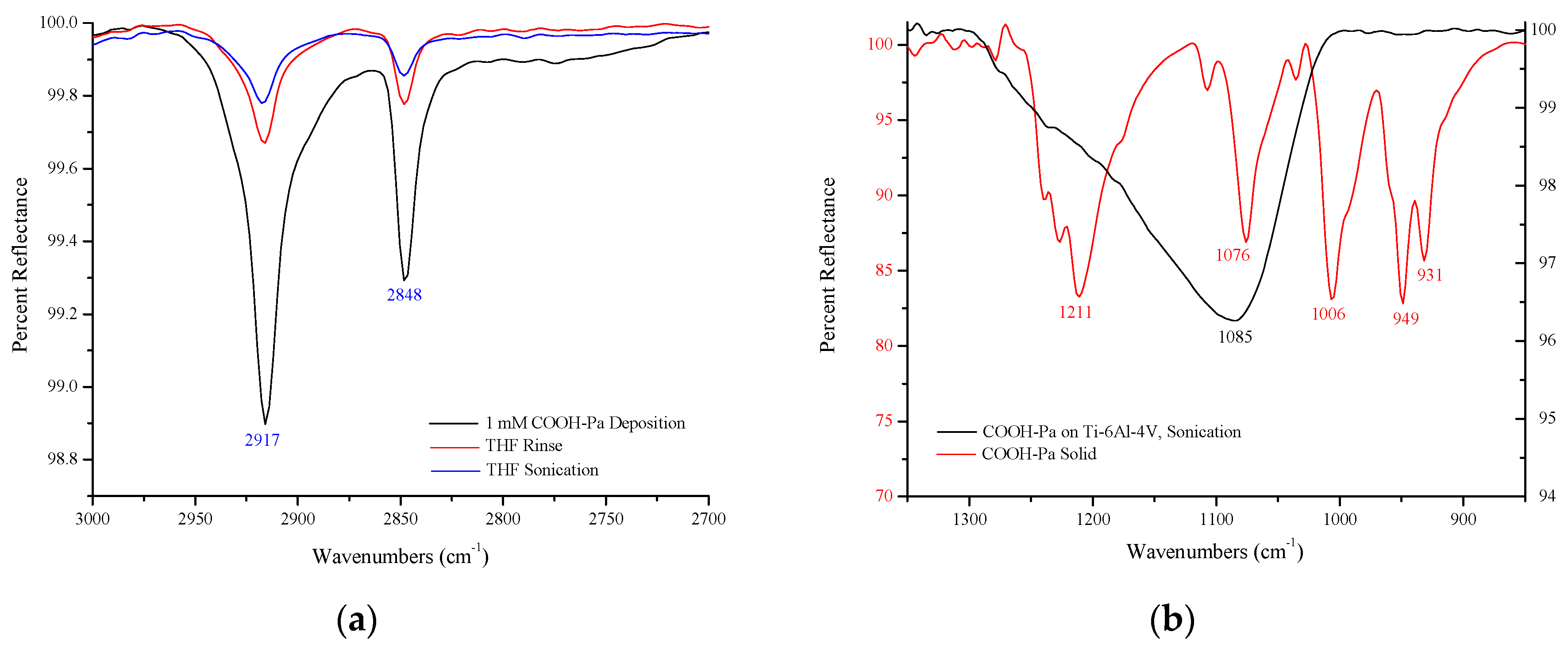
Infrared Spectroscopy
In Vitro Nitric Oxide Release
2.2.4. Bacterial Turbidity
2.2.5. Antibiotic Susceptibility
2.2.6. In Vitro Cytotoxicity of NO-Releasing Ti-6Al-4V
2.2.7. Statistics
3. Results
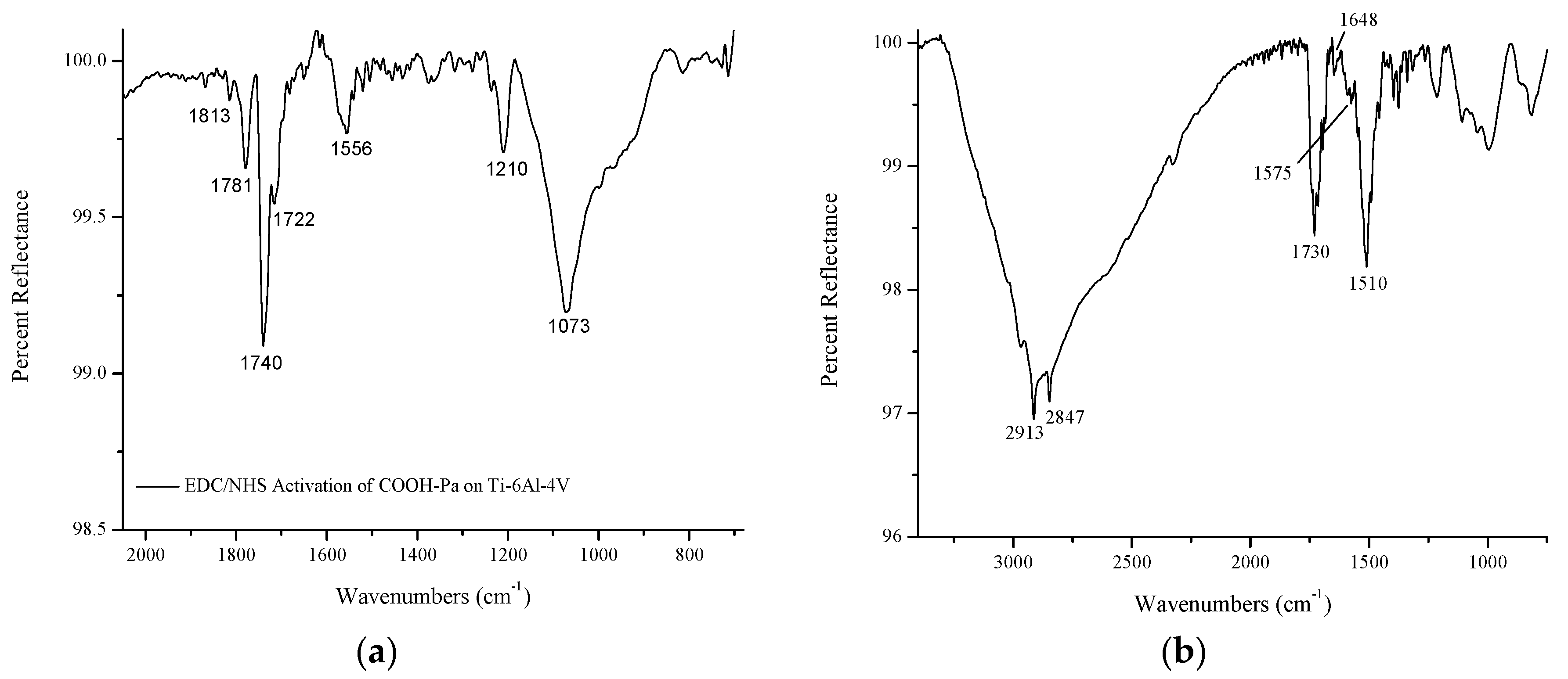
3.1. Characterization of S-Nitroso-Penicillamine-Modified Ti-6Al-4V
3.1.1. Self-Assembled Monolayer Formation
3.1.2. Immobilization of S-Nitroso-Penicillamine
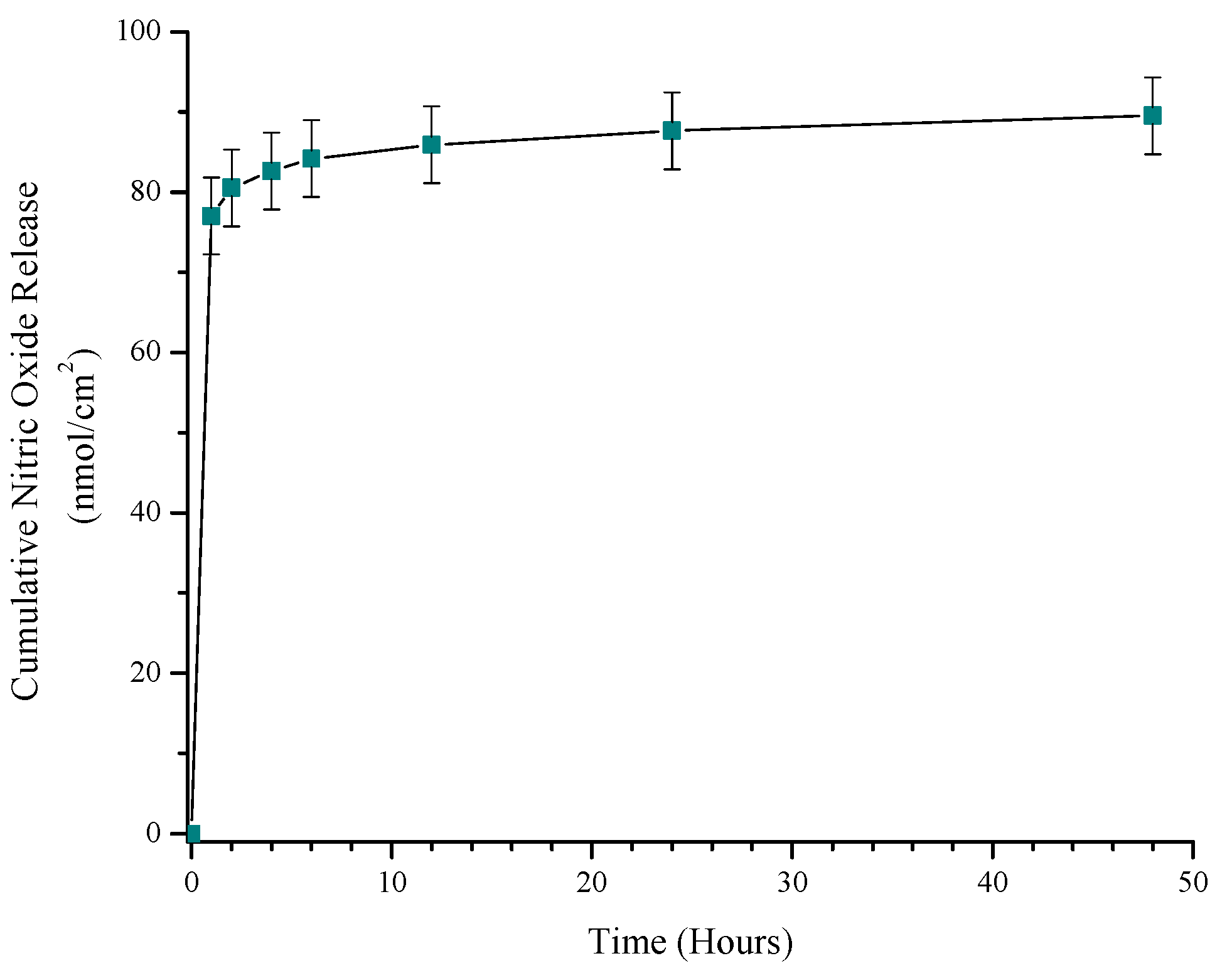
3.2. Quantitation of NO Release from S-Nitroso-Penicillamine-Modified Ti-6Al-4V
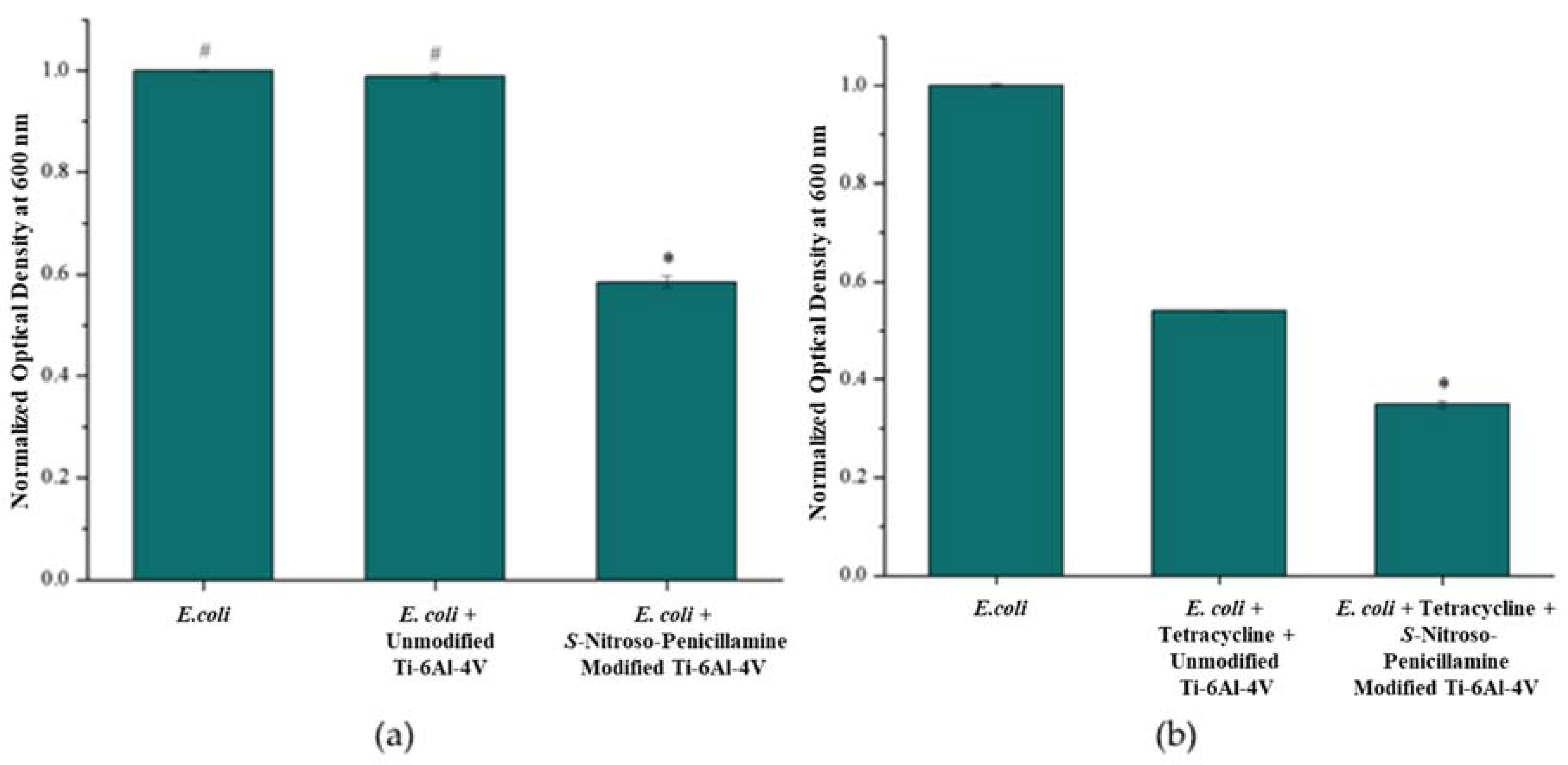
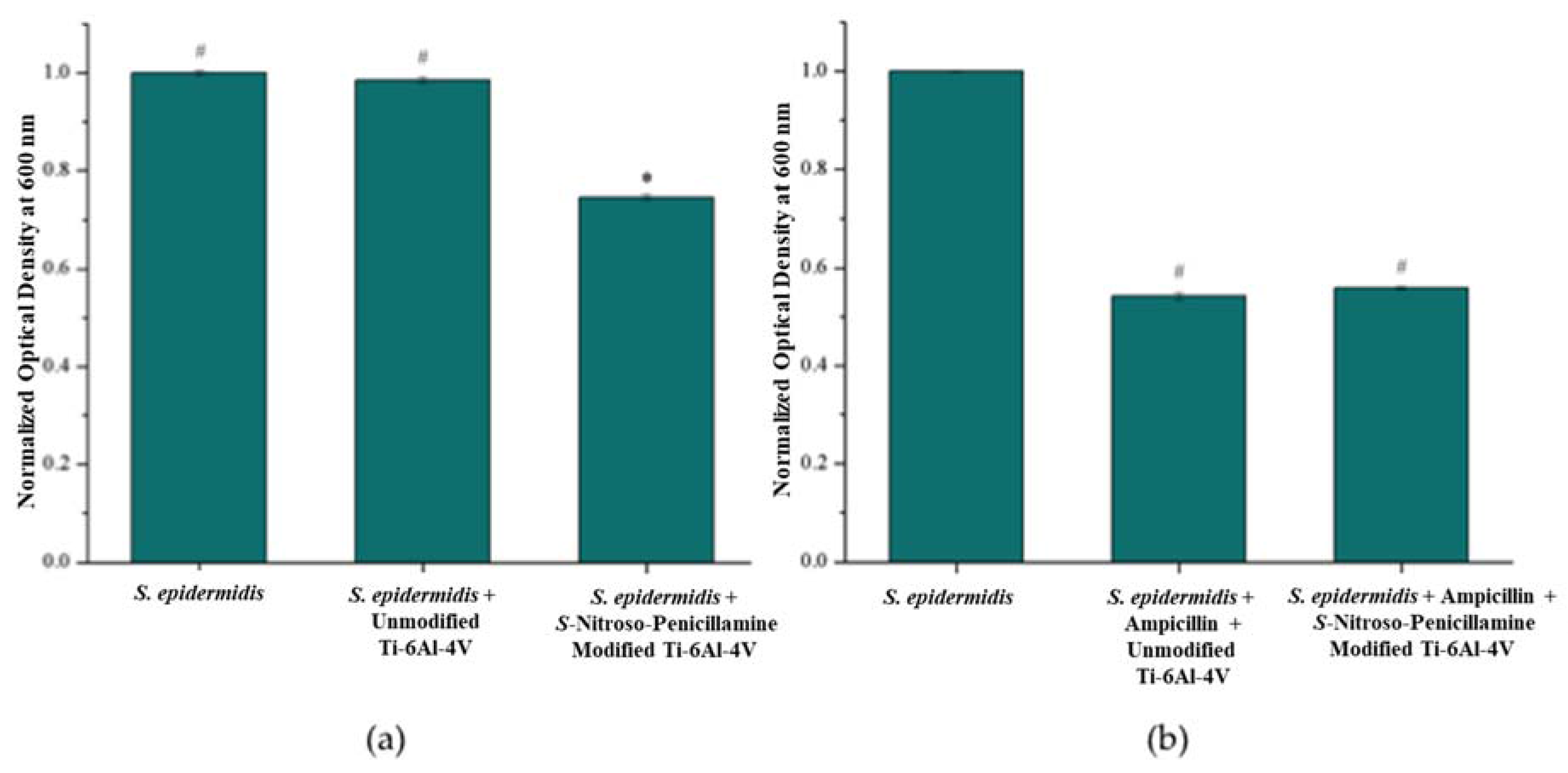
3.3. Antimicrobial Effect of S-Nitroso-Penicillamine-Modified Ti-6Al-4V
3.3.1. Bacterial Turbidity: E. coli and S. epidermidis
3.3.2. Antibiotic Susceptibility
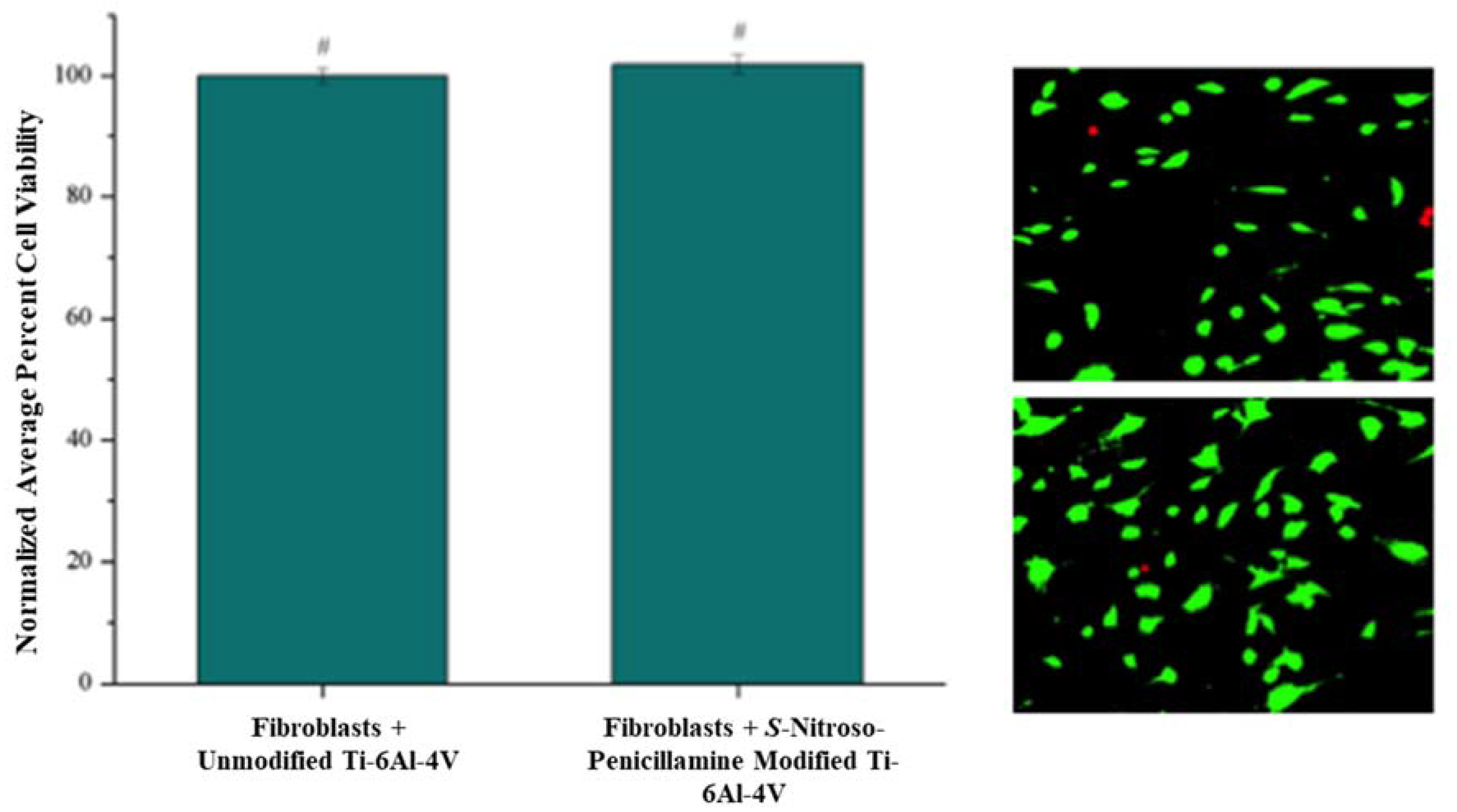
3.4. Cytotoxic Effect of S-Nitroso-Penicillamine-Modified Ti-6Al-4V
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Gawalt, E.S.; Avaltroni, M.J.; Danahy, M.P.; Silverman, B.M.; Hanson, E.L.; Midwood, K.S.; Schwarzbauer, J.E.; Schwartz, J. Bonding organics to Ti alloys: Facilitating human osteoblast attachment and spreading on surgical impant materials. Langmuir 2003, 19, 200–204. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.N.; Lima, J.H.C.; Valiev, R.; Meyers, M.A. Biomedical applications of titanium and its alloys. JOM 2008, 60, 46–49. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Schoenfisch, M.H. Reducing implant-related infections: Active release strategies. Chem. Soc. Rev. 2006, 35, 780–789. [Google Scholar] [CrossRef] [PubMed]
- Trampuz, A.; Widmer, A.F. Infections associated with orthopedic implants. Curr. Opin. Infect. Dis. 2006, 19, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Dohmen, P.M. Antibiotic resistance in common pathogens reinforces the need to minimise surgical site infections. J. Hosp. Infect. 2008, 70, 15–20. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2013.
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009, 30, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Hetrick, E.M.; Shin, J.H.; Stasko, N.A.; Johnson, C.B.; Wespe, D.A.; Holmuhamedov, E.; Schoenfisch, M.H. Bactericidal efficacy of nitric oxide-releasing silica nanoparticles. ACS Nano 2008, 2, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Holt, J.; Hertzberg, B.; Weinhold, P.; Storm, W.; Schoenfisch, M.; Dahners, L. Decreasing bacterial colonization of external fixation pins via nitric oxide release coatings. J. Orthop. Trauma 2011, 25, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Nablo, B.J.; Rothrock, A.R.; Schoenfisch, M.H. Nitric oxide-releasing sol–gels as antibacterial coatings for orthopedic implants. Biomaterials 2005, 26, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Lu, Y.; Broadnax, A.D.; Hunter, R.A.; Carpenter, A.W.; Schoenfisch, M.H. Role of size and shape on biofilm eradication for nitric oxide-releasing silica nanoparticles. ACS Appl. Mater. Interfaces 2013, 5, 9322–9329. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Blecher, K.; Sanchez, D.; Tuckman-Vernon, C.; Gialanella, P.; Friedman, J.M.; Martinez, L.R.; Nosanchuk, J.D. Susceptibility of Gram-positive and -negative bacteria to novel nitric oxide-releasing nanoparticle technology. Virulence 2011, 2, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.J.; Han, G.; Navati, M.S.; Chacko, M.; Gunther, L.; Alfieri, A.; Friedman, J.M. Sustained release nitric oxide releasing nanoparticles: Characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide 2008, 19, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Martinez, L.R.; Han, G.; Chacko, M.; Mihu, M.R.; Jacobson, M.; Gialanella, P.; Friedman, A.J.; Nosanchuk, J.D.; Friedman, J.M. Antimicrobial and healing efficacy of sustained release nitric oxide nanoparticles against staphylococcus aureus skin infection. J. Investig. Dermatol. 2009, 129, 2463–2469. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, A.; Miller, C.C.; McMullin, B.; Ghahary, A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide 2006, 14, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Barraud, N.; Hassett, D.J.; Hwang, S.; Rice, S.A.; Kjelleberg, S.; Webb, J.S. Involvement of nitric oxide in biofilm dispersal of Pseudomonas aeruginosa. J. Bacteriol. 2006, 188, 7344–7353. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; Schoenfisch, M.H. Nitric oxide release: part II. Therapeutic applications. Chem. Soc. Rev. 2012, 41, 3742–3752. [Google Scholar] [CrossRef] [PubMed]
- Reger, N.A.; Meng, W.S.; Gawalt, E.S. Surface modification of PLGA nanoparticles to deliver nitric oxide to inhibit Escherichia coli growth. Appl. Surf. Sci. 2017, 401, 162–171. [Google Scholar] [CrossRef]
- Shin, J.H.; Metzger, S.K.; Schoenfisch, M.H. Synthesis of nitric oxide-releasing silica nanoparticles. J. Am. Chem. Soc. 2007, 129, 4612–4619. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Kebede, N.; Saavedra, J.E.; Nikolaitchik, A.V.; Brady, D.A.; Yourd, E.; Davies, K.M.; Keefer, L.K.; Toscano, J.P. Chemistry of the diazeniumdiolates. 3. Photoreactivity. J. Am. Chem. Soc. 2001, 123, 5465–5472. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Mani, G. A stent for co-delivering paclitaxel and nitric oxide from abluminal and luminal surfaces: Preparation, surface characterization, and in vitro drug release studies. Appl. Surf. Sci. 2013, 279, 216–232. [Google Scholar] [CrossRef]
- Kruszewski, K.M.; Nistico, L.; Longwell, M.J.; Hynes, M.J.; Maurer, J.A.; Hall-Stoodley, L.; Gawalt, E.S. Reducing Staphylococcus aureus biofilm formation on stainless steel 316L using functionalized self-assembled monolayers. Mater. Sci. Eng. C 2013, 33, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Palchesko, R.N.; McGowan, K.A.; Gawalt, E.S. Surface immobilization of active vancomycin on calcium aluminum oxide. Mater. Sci. Eng. C 2011, 31, 637–642. [Google Scholar] [CrossRef]
- Palchesko, R.N.; Buckholtz, G.A.; Romeo, J.D.; Gawalt, E.S. Co-immobilization of active antibiotics and cell adhesion peptides on calcium based biomaterials. Mater. Sci. Eng. C 2014, 40, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fiorini, M.T.; Abell, C.; Williams, D.H. Binding of vancomycin group antibiotics to d-alanine and d-lactate presenting self-assembled monolayers. Bioorgan. Med. Chem. 2000, 8, 2609–2616. [Google Scholar] [CrossRef]
- Humblot, V.; Yala, J.-F.; Thebault, P.; Boukerma, K.; Héquet, A.; Berjeaud, J.-M.; Pradier, C.-M. The antibacterial activity of Magainin I immobilized onto mixed thiols Self-Assembled Monolayers. Biomaterials 2009, 30, 3503–3512. [Google Scholar] [CrossRef] [PubMed]
- Buckholtz, G.A.; Reger, N.A.; Anderton, W.D.; Schimoler, P.J.; Roudebush, S.L.; Meng, W.S.; Miller, M.C.; Gawalt, E.S. Reducing Escherichia coli growth on a composite biomaterial by a surface immobilized antimicrobial peptide. Mater. Sci. Eng. C 2016, 65, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Ullman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef]
- Raman, A.; Quiñones, R.; Barriger, L.; Eastman, R.; Parsi, A.; Gawalt, E.S. Understanding organic film behavior on alloy and metal oxides. Langmuir 2010, 26, 1747–1754. [Google Scholar] [CrossRef] [PubMed]
- Kruszewski, K.M. Inhibiting Bacterial Biofilm Formation on Stainless Steel 316L Using Self-Assembled Monolayers; Duquense University: Pittsburgh, PA, USA, 2012. [Google Scholar]
- Raman, A.; Dubey, M.; Gouzman, I.; Gawalt, E.S. Formation of self-assembled monolayers of alkylphosphonic acid on the native oxide surface of SS316L. Langmuir 2006, 22, 6469–6472. [Google Scholar] [CrossRef] [PubMed]
- NHS and Sulfo-NHS; Thermo Scientific: Rockford, IL, USA, 2009; pp. 1–4.
- Arnelle, D.R.; Stamler, J.S. NO+, NO., and NO− donation by S-Nitrosothiols: Implications for regulation of physiological functions by S-Nitrosylation and acceleration of disulfide formation. Arch. Biochem. Biophys. 1995, 318, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Coneski, P.N.; Rao, K.S.; Schoenfisch, M.H. Degradable nitric oxide-releasing biomaterials via post-polymerization functionalization of cross-linked polyesters. Biomacromolecules 2010, 11, 3208–3215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Z.; Wen, F.; Ren, L.; Li, J.; Teoh, S.H.; Thian, E.S. Gelatin-siloxane nanoparticles to deliver nitric oxide for vascular cell regulation: Synthesis, cytocompatibility, and cellular responses. J. Biomed. Mater. Res. Part A 2015, 103, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.J.; Hogg, N.; Joseph, J.; Kalyanaraman, B. Mechnism of nitric oxide release from S-Nitrosothiols. J. Biol. Chem. 1996, 271, 18596–18603. [Google Scholar] [CrossRef] [PubMed]
- Nitrate/Nitrite Colorimetric Assay Kit; Cayman Chemical Company: Ann Arbor, MI, USA, 2014.
- Brodersen, D.E.; Clemons, W.M., Jr.; Carter, A.P.; Morgan-Warren, R.J.; Wimberly, B.T.; Ramakrishnan, V. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell 2000, 103, 1143–1154. [Google Scholar] [CrossRef]
- Raman, A.; Gawalt, E.S. Reduction of 3T3 fibroblast adhesion on SS316L by methyl-terminated SAMs. Mater. Sci. Eng. C 2010, 30, 1157–1161. [Google Scholar] [CrossRef] [PubMed]
- Quiñones, R.; Raman, A.; Gawalt, E.S. Functionalization of nickel oxide using alkylphosphonic acid self-assembled monolayers. Thin Solid Films 2008, 516, 8774–8781. [Google Scholar] [CrossRef]
- Kruszewski, K.M.; Renk, E.R.; Gawalt, E.S. Self-assembly of organic acid molecules on the metal oxide surface of a cupronickel alloy. Thin Solid Films 2012, 520, 4326–4331. [Google Scholar] [CrossRef]
- Kruszewski, K.M.; Gawalt, E.S. Perfluorocarbon thin films and polymer brushes on stainless steel 316 L for the control of interfacial properties. Langmuir 2011, 27, 8120–8125. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lee, P.I. Controlled nitric oxide delivery platform based on S-Nitrosothiol conjugated interpolymer complexes for diabetic wound healing. Mol. Pharm. 2010, 7, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Damodaran, V.B.; Joslin, J.M.; Wold, K.A.; Lantvit, S.M.; Reynolds, M.M. S-Nitrosated biodegradable polymer for biomedical applications: Synthesis, characterization and impact of thiol structure on the physiochemical properties. J. Mater. Chem. 2012, 22, 5990–6001. [Google Scholar] [CrossRef]
- Damodaran, V.B.; Reynolds, M.M. Biodegradable S-Nitrosothiol tethered multiblock polymer for nitric oxide delivery. J. Mater. Chem. 2011, 21, 5870–5872. [Google Scholar] [CrossRef]
- Sam, S.; Touahir, L.; Salvador Andresa, J.; Allongue, P.; Chazalviel, J.N.; Gouget-Laemmel, A.C.; Henry de Villeneuve, C.; Moraillon, A.; Ozanam, F.; Gabouze, N.; et al. Semiquantitative study of the EDC/NHS activation of acid terminal groups at modified porous silicon surfaces. Langmuir 2010, 26, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Rothrock, A.R.; Donkers, R.L.; Schoenfisch, M.H. Synthesis of nitric oxide-releasing gold nanoparticles. J. Am. Chem. Soc. 2005, 127, 9362–9363. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, M.A.; Stasko, N.A.; Schoenfisch, M.H. Water-soluble nitric oxide-releasing gold nanoparticles. Langmuir 2007, 23, 4938–4943. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-W.; Lee, J.-S.; Lee, C.H. Characterization of nitric oxide-releasing microparticles for the mucosal delivery. J. Biomed. Mater. Res. Part A 2010, 92, 1233–1243. [Google Scholar] [CrossRef] [PubMed]
- Frost, M.C.; Reynolds, M.M.; Meyerhoff, M.E. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials 2005, 26, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Chipinda, I.; Simoyi, R.H. Formation and stability of a nitric oxide donor: S-nitroso-N-acetylpenicillamine. J. Phys. Chem. B 2006, 110, 5052–5061. [Google Scholar] [CrossRef] [PubMed]
- Gusarov, I.; Shatalin, K.; Starodubtseva, M.; Nudler, E. Endogenous nitric oxide protects bacteria against a wide spectrum of antibiotics. Science 2009, 325, 1380–1384. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reger, N.A.; Meng, W.S.; Gawalt, E.S. Antimicrobial Activity of Nitric Oxide-Releasing Ti-6Al-4V Metal Oxide. J. Funct. Biomater. 2017, 8, 20. https://doi.org/10.3390/jfb8020020
Reger NA, Meng WS, Gawalt ES. Antimicrobial Activity of Nitric Oxide-Releasing Ti-6Al-4V Metal Oxide. Journal of Functional Biomaterials. 2017; 8(2):20. https://doi.org/10.3390/jfb8020020
Chicago/Turabian StyleReger, Nina A., Wilson S. Meng, and Ellen S. Gawalt. 2017. "Antimicrobial Activity of Nitric Oxide-Releasing Ti-6Al-4V Metal Oxide" Journal of Functional Biomaterials 8, no. 2: 20. https://doi.org/10.3390/jfb8020020




