Novel Biomaterials Used in Medical 3D Printing Techniques
Abstract
:1. Introduction
2. Commonly Used 3D Printing Technologies in the Medical Field
2.1. Fused Deposition Modeling (FDM) or Free Form Fabriction (FFF)
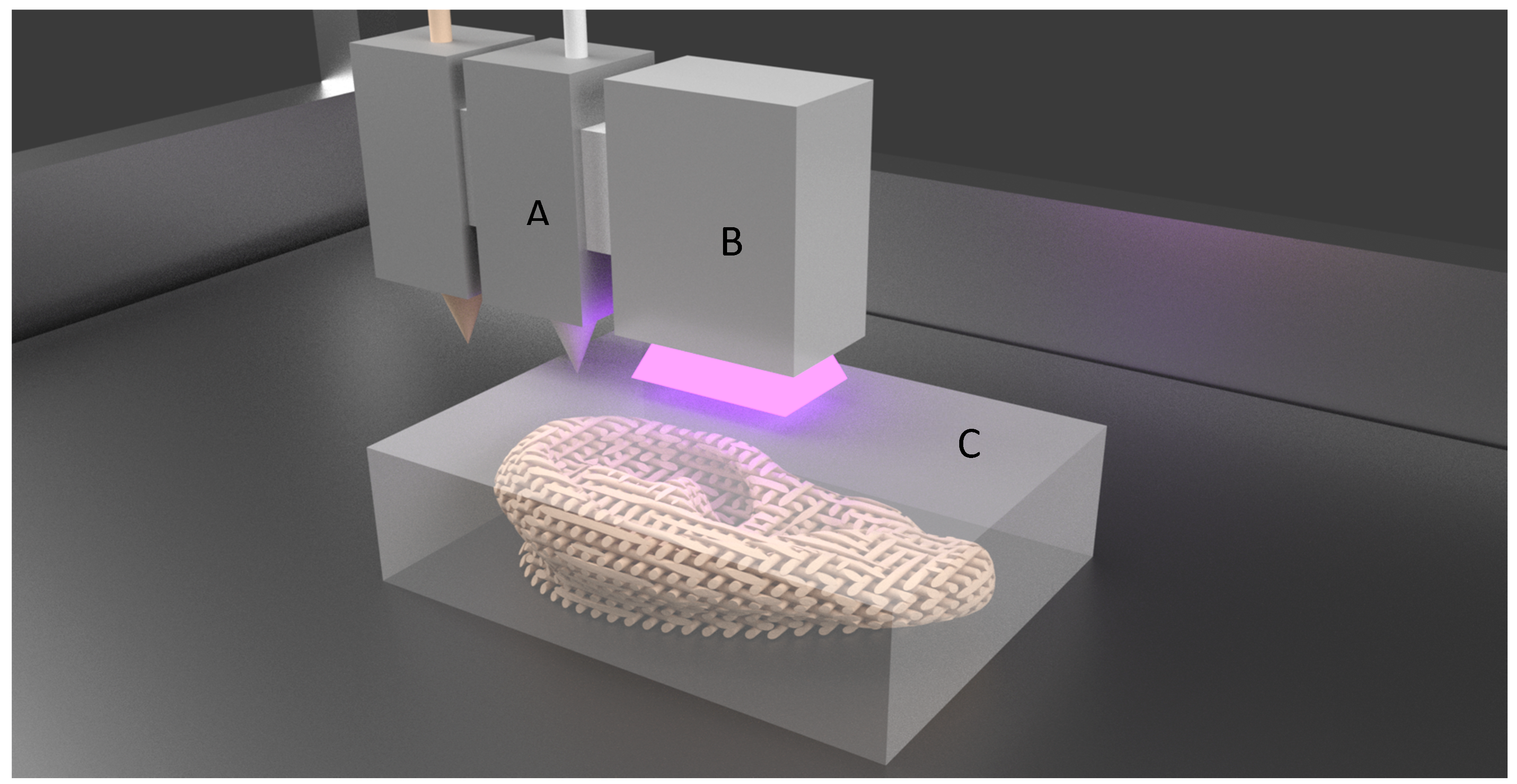
2.2. Extrusion Based Bioprinting
2.3. Material Sintering
2.4. Inkjet or Binder Jet Printing
2.5. Polyjet Printing
2.6. Laminated Object Manufacturing
3. Limitations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Belhabib, S.; Guessasma, S. Compression performance of hollow structures: From topology optimisation to design 3D printing. Int. J. Mech. Sci. 2017, 133, 728–739. [Google Scholar] [CrossRef]
- Guessasma, S.; Nouri, H.; Roger, F. Microstructural and Mechanical Implications of Microscaled Assembly in Droplet-based Multi-Material Additive Manufacturing. Polymers 2017, 9, 372. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Guessasma, S.; Zhu, J.; Zhang, W.; Nouri, H.; Belhabib, S. Microstructural defects induced by stereolithography and related compressive behaviour of polymers. J. Mater. Process. Technol. 2018, 251, 37–46. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Godbey, W.T.; Atala, A. In vitro systems for tissue engineering. Ann. N. Y. Acad. Sci. 2002, 961, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Wang, S.J.; Zhao, X.R.; Zhu, Y.F.; Yu, J.K. 3D-printed poly (ϵ-caprolactone) scaffold integrated with cell-laden chitosan hydrogels for bone tissue engineering. Sci. Rep. 2017, 7, 13412. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-H.; Won, J.-Y.; Park, J.-H.; Bae, J.-H.; Ahn, G.; Kim, C.-H.; Lim, D.-H.; Cho, D.-W.; Yun, W.-S.; Bae, E.-B.; et al. Effects of 3D-Printed Polycaprolactone/β-Tricalcium Phosphate Membranes on Guided Bone Regeneration. Int. J. Mol. Sci. 2017, 18, 899. [Google Scholar] [CrossRef] [PubMed]
- Mills, D.; Tappa, K.; Jammalamadaka, U.; Weisman, J.; Woerner, J. The Use of 3D Printing in the Fabrication of Nasal Stents. Inventions 2017, 3, 1. [Google Scholar] [CrossRef]
- Weisman, J.A.; Nicholson, J.C.; Tappa, K.; Jammalamadaka, U.; Wilson, C.G.; Mills, D.K. Antibiotic and chemotherapeutic enhanced three-dimensional printer filaments and constructs for biomedical applications. Int. J. Nanomed. 2015, 10, 357–370. [Google Scholar]
- Tappa, K.; Jammalamadaka, U.; Ballard, D.H.; Bruno, T.; Israel, M.R.; Vemula, H.; Meacham, J.M.; Mills, D.K.; Woodard, P.K.; Weisman, J.A. Medication eluting devices for the field of OBGYN (MEDOBGYN): 3D printed biodegradable hormone eluting constructs; a proof of concept study. PLoS ONE 2017, 12, e0182929. [Google Scholar] [CrossRef] [PubMed]
- Horst, D.J.; Tebcherani, S.M.; Kubaski, E.T.; De Almeida Vieira, R. Bioactive Potential of 3D-Printed Oleo-Gum-Resin Disks: B. papyrifera; C. myrrha; and S. benzoin Loading Nanooxides—TiO2, P25, Cu2O; and MoO3. Bioinorg. Chem. Appl. 2017, 2017, 6398167. [Google Scholar] [CrossRef] [PubMed]
- Weisman, J.; Jammalamadaka, U.; Tappa, K.; Mills, D. Doped Halloysite Nanotubes for Use in the 3D Printing of Medical Devices. Bioengineering 2017, 4, 96. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Det-Amornrat, U.; Wang, J.; Basit, A.W.; Gaisford, S. 3D scanning and 3D printing as innovative technologies for fabricating personalized topical drug delivery systems. J. Control. Release 2016, 234, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Goyanes, A.; Wang, J.; Buanz, A.; Martínez-Pacheco, R.; Telford, R.; Gaisford, S.; Basit, A.W. 3D Printing of Medicines: Engineering Novel Oral Devices with Unique Design and Drug Release Characteristics. Mol. Pharm. 2015, 12, 4077–4084. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wang, H.; Xue, Y.; Yuan, L.; Zhou, X.; Zhao, Z.; Dong, E.; Liu, B.; Liu, W.; Cromeens, B.; et al. Freeform fabrication of tissue-simulating phantom for potential use of surgical planning in conjoined twins separation surgery. Sci. Rep. 2017, 7, 11048. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-J.; Ren, J.-A.; Wang, G.-F.; Li, Z.-A.; Wu, X.-W.; Ren, H.-J.; Liu, S. 3D-printed “fistula stent” designed for management of enterocutaneous fistula: An advanced strategy. World J. Gastroenterol. 2017, 23, 7489–7494. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.R.; Serra, T.; Oliveira, M.I.; Planell, J.A.; Barbosa, M.A.; Navarro, M. Impact of 3-D printed PLA- and chitosan-based scaffolds on human monocyte/macrophage responses: Unraveling the effect of 3-D structures on inflammation. Acta Biomater. 2014, 10, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.; Puetzer, J.L.; Mason, B.N.; Reinhart-King, C.A.; Bonassar, L.J. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805. [Google Scholar] [CrossRef]
- Laronda, M.M.; Rutz, A.L.; Xiao, S.; Whelan, K.A.; Duncan, F.E.; Roth, E.W.; Woodruff, T.K.; Shah, R.N. A bioprosthetic ovary created using 3D printed microporous scaffolds restores ovarian function in sterilized mice. Nat. Commun. 2017, 8, 15261. [Google Scholar] [CrossRef] [PubMed]
- Markstedt, K.; Mantas, A.; Tournier, I.; Martínez Ávila, H.; Hägg, D.; Gatenholm, P. 3D Bioprinting Human Chondrocytes with Nanocellulose–Alginate Bioink for Cartilage Tissue Engineering Applications. Biomacromolecules 2015, 16, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.; Hägg, D.A.; Forsman, A.; Ekholm, J.; Nimkingratana, P.; Brantsing, C.; Kalogeropoulos, T.; Zaunz, S.; Concaro, S.; Brittberg, M.; et al. Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Sci. Rep. 2017, 7, 658. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.; Parisi, C.; Di Silvio, L.; Dini, D.; Forte, A.E. Cryogenic 3D Printing of Super Soft Hydrogels. Sci. Rep. 2017, 7, 16293. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Hong, J.M.; Jung, J.W.; Shim, J.-H.; Oh, J.-H.; Cho, D.-W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication 2014, 6, 24103. [Google Scholar] [CrossRef] [PubMed]
- Phillippi, J.A.; Miller, E.; Weiss, L.; Huard, J.; Waggoner, A.; Campbell, P. Microenvironments Engineered by Inkjet Bioprinting Spatially Direct Adult Stem Cells Toward Muscle- and Bone-Like Subpopulations. Stem Cells 2008, 26, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hockaday, L.A.; Kang, K.H.; Butcher, J.T. 3D bioprinting of heterogeneous aortic valve conduits with alginate/gelatin hydrogels. J. Biomed. Mater. Res. A 2013, 101, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Fedorovich, N.E.; Alblas, J.; de Wijn, J.R.; Hennink, W.E.; Verbout, A.J.; Dhert, W.J.A. Hydrogels as Extracellular Matrices for Skeletal Tissue Engineering: State-of-the-Art and Novel Application in Organ Printing. Tissue Eng. 2007, 13, 1905–1925. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, F.-Y.; Lin, H.-H.; Hsu, S. 3D bioprinting of neural stem cell-laden thermoresponsive biodegradable polyurethane hydrogel and potential in central nervous system repair. Biomaterials 2015, 71, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Suntornnond, R.; An, J.; Chua, C.K. Roles of support materials in 3D bioprinting. Int. J. Bioprint. 2017, 3, 83–89. [Google Scholar] [CrossRef]
- Poldervaart, M.T.; Goversen, B.; de Ruijter, M.; Abbadessa, A.; Melchels, F.P.W.; Öner, F.C.; Dhert, W.J.A.; Vermonden, T.; Alblas, J. 3D bioprinting of methacrylated hyaluronic acid (MeHA) hydrogel with intrinsic osteogenicity. PLoS ONE 2017, 12, e0177628. [Google Scholar] [CrossRef] [PubMed]
- Sa, M.-W.; Nguyen, B.-N.B.; Moriarty, R.A.; Kamalitdinov, T.; Fisher, J.P.; Kim, J.Y. Fabrication and evaluation of 3D printed BCP scaffolds reinforced with ZrO2 for bone tissue applications. Biotechnol. Bioeng. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Pei, P.; Zhu, M.; Du, X.; Xin, C.; Zhao, S.; Li, X.; Zhu, Y. Three dimensional printing of calcium sulfate and mesoporous bioactive glass scaffolds for improving bone regeneration in vitro and in vivo. Sci. Rep. 2017, 7, 42556. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Liu, A.; Shao, H.; Yang, X.; Ma, C.; Yan, S.; Liu, Y.; He, Y.; Gou, Z. Systematical Evaluation of Mechanically Strong 3D Printed Diluted magnesium Doping Wollastonite Scaffolds on Osteogenic Capacity in Rabbit Calvarial Defects. Sci. Rep. 2016, 6, 34029. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, K.; Li, X.; Wei, Q.; Chai, W.; Wang, S.; Che, Y.; Lu, T.; Zhang, B. 3D fabrication and characterization of phosphoric acid scaffold with a HA/β-TCP weight ratio of 60:40 for bone tissue engineering applications. PLoS ONE 2017, 12, e0174870. [Google Scholar] [CrossRef] [PubMed]
- Sandler, N.; Määttänen, A.; Ihalainen, P.; Kronberg, L.; Meierjohann, A.; Viitala, T.; Peltonen, J. Inkjet printing of drug substances and use of porous substrates-towards individualized dosing. J. Pharm. Sci. 2011, 100, 3386–3395. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Scoutaris, N.; Klepetsanis, P.; Chowdhry, B.; Prausnitz, M.R.; Douroumis, D. Inkjet printing of transdermal microneedles for the delivery of anticancer agents. Int. J. Pharm. 2015, 494, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Strobel, L.A.; Rath, S.N.; Maier, A.K.; Beier, J.P.; Arkudas, A.; Greil, P.; Horch, R.E.; Kneser, U. Induction of bone formation in biphasic calcium phosphate scaffolds by bone morphogenetic protein-2 and primary osteoblasts. J. Tissue Eng. Regen. Med. 2014, 8, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Trombetta, R.P.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printed bioceramics for dual antibiotic delivery to treat implant-associated bone infection. Eur. Cells Mater. 2015, 30, 232–247. [Google Scholar] [CrossRef]
- Boehm, R.D.; Miller, P.R.; Daniels, J.; Stafslien, S.; Narayan, R.J. Inkjet printing for pharmaceutical applications. Mater. Today 2014, 17, 247–252. [Google Scholar] [CrossRef]
- Asadi-Eydivand, M.; Solati-Hashjin, M.; Shafiei, S.S.; Mohammadi, S.; Hafezi, M.; Osman, N.A.A. Structure; properties; and in vitro behavior of heat-treated calcium sulfate scaffolds fabricated by 3D printing. PLoS ONE 2016, 11, e0151216. [Google Scholar] [CrossRef] [PubMed]
- Inzana, J.A.; Olvera, D.; Fuller, S.M.; Kelly, J.P.; Graeve, O.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials 2014, 35, 4026–4034. [Google Scholar] [CrossRef] [PubMed]
- Farzadi, A.; Solati-Hashjin, M.; Asadi-Eydivand, M.; Osman, N.A.A. Effect of layer thickness and printing orientation on mechanical properties and dimensional accuracy of 3D printed porous samples for bone tissue engineering. PLoS ONE 2014, 9, e108252. [Google Scholar] [CrossRef] [PubMed]
- Wickström, H.; Hilgert, E.; Nyman, J.; Desai, D.; Şen Karaman, D.; de Beer, T.; Sandler, N.; Rosenholm, J. Inkjet Printing of Drug-Loaded Mesoporous Silica Nanoparticles—A Platform for Drug Development. Molecules 2017, 22, 2020. [Google Scholar] [CrossRef] [PubMed]
- Meess, K.M.; Izzo, R.L.; Dryjski, M.L.; Curl, R.E.; Harris, L.M.; Springer, M.; Siddiqui, A.H.; Rudin, S.; Ionita, C.N. 3D Printed Abdominal Aortic Aneurysm Phantom for Image Guided Surgical Planning with a Patient Specific Fenestrated Endovascular Graft System. Proc. SPIE Int. Soc. Opt. Eng. 2017, 10138, 101380P. [Google Scholar] [PubMed]
- Kuroda, S.; Kobayashi, T.; Ohdan, H. 3D printing model of the intrahepatic vessels for navigation during anatomical resection of hepatocellular carcinoma. Int. J. Surg. Case Rep. 2017, 41, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wei, H.; Zeng, F.; Li, J.; Xia, J.J.; Wang, X. Application of A Novel Three-dimensional Printing Genioplasty Template System and Its Clinical Validation: A Control Study. Sci. Rep. 2017, 7, 5431. [Google Scholar] [CrossRef] [PubMed]
- Gear, J.I.; Cummings, C.; Craig, A.J.; Divoli, A.; Long, C.D.C.; Tapner, M.; Flux, G.D. Abdo-Man: A 3D-printed anthropomorphic phantom for validating quantitative SIRT. EJNMMI Phys. 2016, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Zein, N.N.; Hanouneh, I.A.; Bishop, P.D.; Samaan, M.; Eghtesad, B.; Quintini, C.; Miller, C.; Yerian, L.; Klatte, R. Three-dimensional print of a liver for preoperative planning in living donor liver transplantation. Liver Transplant. 2013, 19, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Waran, V.; Narayanan, V.; Karuppiah, R.; Owen, S.L.F.; Aziz, T. Utility of multimaterial 3D printers in creating models with pathological entities to enhance the training experience of neurosurgeons. J. Neurosurg. 2014, 120, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Mitsouras, D.; Lee, T.C.; Liacouras, P.; Ionita, C.N.; Pietilla, T.; Maier, S.E.; Mulkern, R.V. Three-dimensional printing of MRI-visible phantoms and MR image-guided therapy simulation. Magn. Reson. Med. 2017, 77, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Lan, Q.; Chen, A.; Zhang, T.; Li, G.; Zhu, Q.; Fan, X.; Ma, C.; Xu, T. Development of Three-Dimensional Printed Craniocerebral Models for Simulated Neurosurgery. World Neurosurg. 2016, 91, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Sander, I.; Liepert, T.; Doney, E.; Leevy, W.; Liepert, D. Patient Education for Endoscopic Sinus Surgery: Preliminary Experience Using 3D-Printed Clinical Imaging Data. J. Funct. Biomater. 2017, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, D.K.; Chatzinoff, Y.; Zhang, Y.; Yuan, Q.; Fulkerson, M.; Chopra, R.; Brugarolas, J.; Cadeddu, J.A.; Kapur, P.; Pedrosa, I. Development of a Patient-specific Tumor Mold Using Magnetic Resonance Imaging and 3-Dimensional Printing Technology for Targeted Tissue Procurement and Radiomics Analysis of Renal Masses. Urology 2017, in press. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.-H.T.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Kolesky, D.B.; Truby, R.L.; Gladman, A.S.; Busbee, T.A.; Homan, K.A.; Lewis, J.A. 3D bioprinting of vascularized; heterogeneous cell-laden tissue constructs. Adv. Mater. 2014, 26, 3124–3130. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.J.; Jallerat, Q.; Palchesko, R.N.; Park, J.H.; Grodzicki, M.S.; Shue, H.-J.; Ramadan, M.H.; Hudson, A.R.; Feinberg, A.W. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Sci. Adv. 2015, 1, e1500758. [Google Scholar] [CrossRef] [PubMed]
- Hassana, B.O.; Guessasma, S.; Belhabib, S.; Nouri, H. Explaining the Difference between Real Part and Virtual Design of 3D Printed Porous Polymer at the Microstructural Level. Macromol. Mater. Eng. 2016, 301, 566–576. [Google Scholar] [CrossRef]
- Ventola, C.L. Medical Applications for 3D Printing: Current and Projected Uses. Pharm. Ther. 2014, 39, 704–711. [Google Scholar] [PubMed]
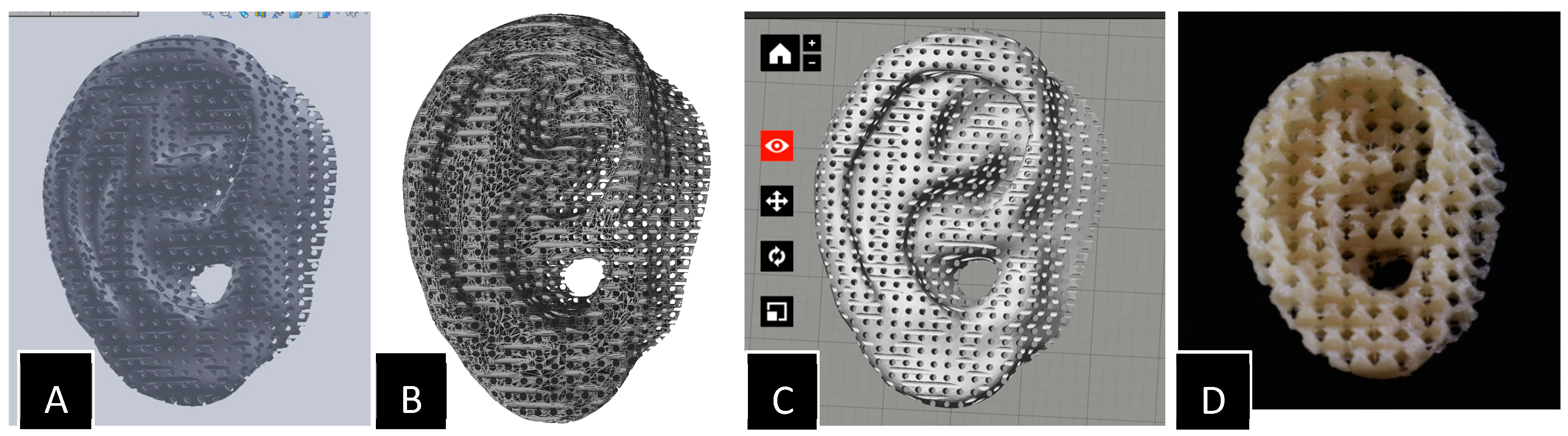
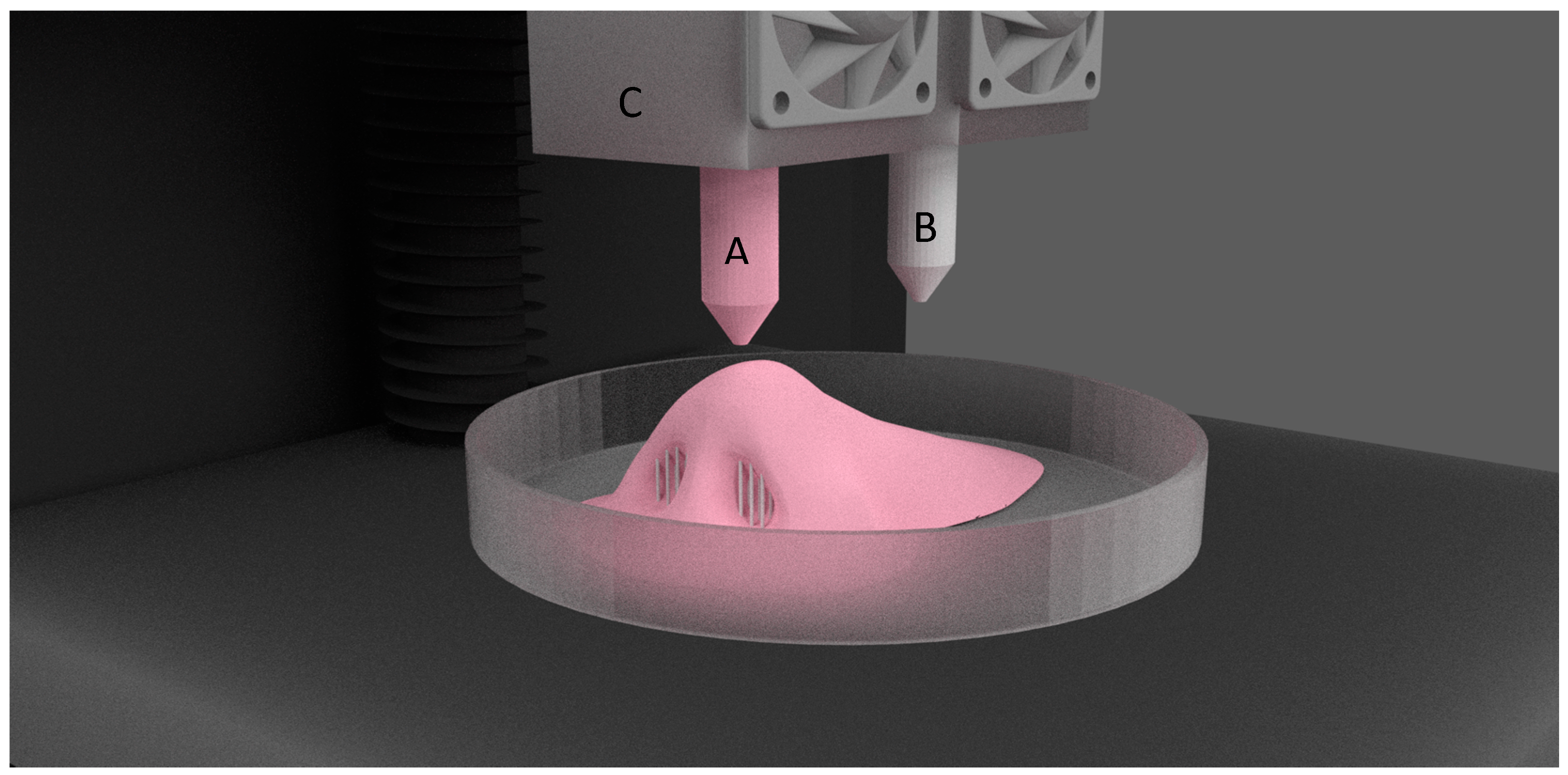
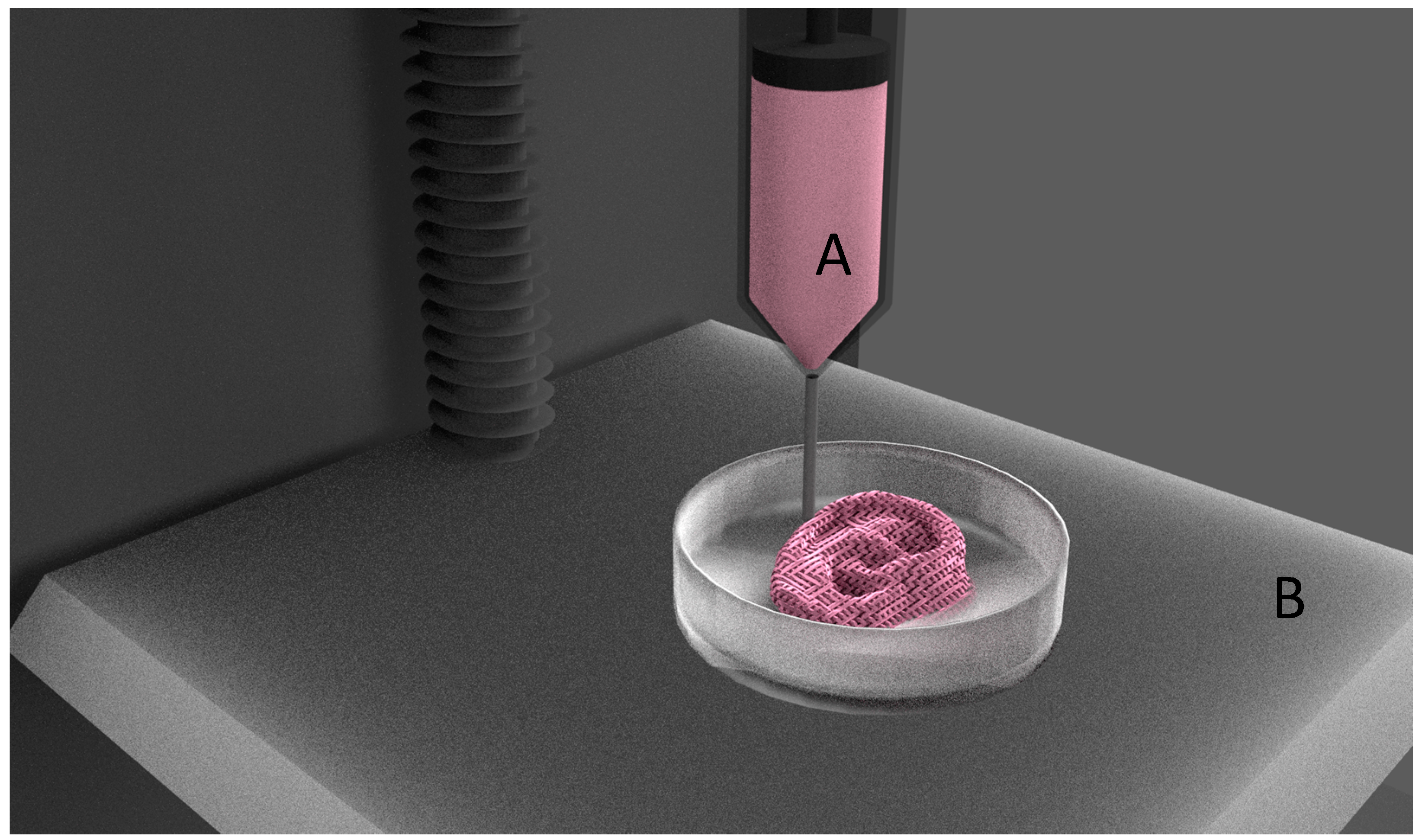
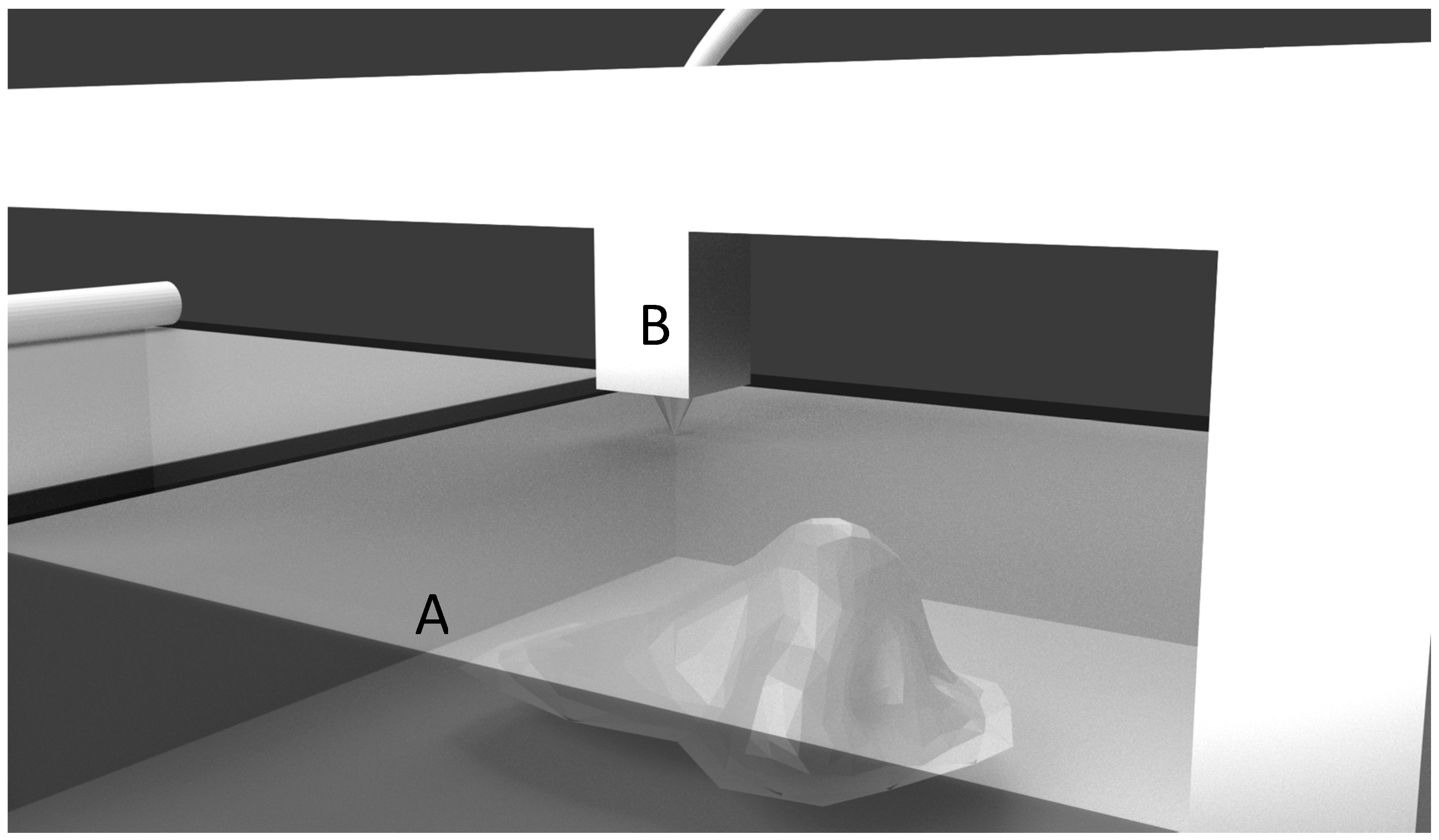
| Process | Principle |
|---|---|
| Extrusion Printing | |
| Fused Deposition Modeling (FDM) [1] | A thermoplastic material is melted and laid on to the build platform in layer-by-layer fashion, until the object is formed. |
| Materials: acrylonitrile butadiene styrene (ABS), poly-lactic acid (PLA), nylon. | |
| Bioprinting [2] | Biological materials are extruded through a nozzle under pressure to lay down materials in sequential layers till the scaffold is built. |
| Materials: alginate, chitosan, gelatin, collagen, fibrin. | |
| Material Sintering | |
| Selective Laser Sintering (SLS) [3] | A high-power laser beam fuses the powdered materials in layer-by-layer pattern to form an object. |
| Materials: nylon, polyamide. | |
| Electron Beam Manufacturing (EBM) | EBM is similar to SLS, except for high power electron beam is used to fuse the powdered particles. |
| Materials: titanium, cobalt−chrome alloy. | |
| Stereolithography (SLA) [4] | A UV laser beam selectively hardens the photo-polymer resin in layers. |
| Each layer is solidified and built on top of next until the object is formed. | |
| Materials: photopolymers. | |
| Continuous Liquid Interface Production (CLIP) [3] | CLIP is similar to SLA, except for UV beam is passed through a transparent window at the bottom of the resin and build platform raises upwards holding the 3D printed object. |
| Materials: photopolymers. | |
| Material Binding | |
| Binder Jetting/Inkjet [5] | A liquid binding material is selectively dropped into the powder bed in alternative layers of powder–binding liquid–powder, until the final object is formed. |
| Materials: starch or gypsum (powder bed) and water (binding agent) | |
| Polyjet | Polyjet printing is similar to inkjet, but instead of binding agents, photopolymer liquid is sprayed in layers onto the build platform and is instantaneously cured using UV light. |
| Materials: polypropylene, polystyrene, polycarbonate. | |
| Lamination | |
| Laminated Object Manufacturing (LOM) | Layers of adhesive coated material are successively glued together and cut in required shapes using a laser. |
| Materials: thin sheets of paper, polyvinyl caprolactam (PVC) plastic, or metal laminates | |
| Sector | Applications |
|---|---|
| Industry | Jigs, fixtures, and end-use parts for aeronautical industry |
| Prototypes and spare parts for automotive industry | |
| Medical | Surgical models for perioperative surgical preparations |
| Dental fixtures, bridges, and crowns | |
| Customized patient specific implants and prostheses | |
| Living tissue scaffolds for tissue engineering and regenerative medicine | |
| Pharmaceutical | Customized implants for drug delivery |
| Tablets, capsules, and other patient specific dosages | |
| Food | Designing and 3D printing complex shaped cakes, cookies, candies, pizzas, and other desserts |
| Fashion | Jewelry, clothes, shoes, and other accessories |
| Household | Plates, cups, spoons, holders, and other common household objects |
| Miscellaneous | Space: building prototypes and parts in space |
| Chemical industry: fabricating complex molecules and compounds | |
| Construction: scale models with intricate architectures |
| Type | Advantages | Disadvantages | Applications |
|---|---|---|---|
| Metals and metal alloys | * High material strength | * Corrosive | * Orthopedic implants, screws, pins, and plates |
| E.g.,: gold, platinum, titanium, steel, chromium, cobalt | * Easy to fabricate and sterilize | * Aseptic loosening | |
| * Excessive elastic modulus | |||
| Ceramics and carbon compounds | * High material strength | * Difficult to mold | * Bioactive orthopedic implants |
| E.g.,: calcium phosphate salts (HA), glass, oxides of aluminum and titanium | * Biocompatibility | * Excessive elastic modulus | * Dental implants |
| * Corrosion resistance | * Artificial hearing aids | ||
| Polymers | * Biodegradable | * Leachable in body fluids | * Orthopedic and dental implants |
| * Biocompatible | * Hard to sterilize | * Prostheses | |
| * Easily moldable and readily available | * Tissue engineering scaffolds | ||
| E.g.,: PMMA*, Polycaprolactone(PCL), PLA, polycarbonates, polyurethanes | * Suitable mechanical strength | * Drug delivery systems | |
| Composites | * Excellent mechanical properties | * Expensive | * Porous orthopedic implants |
| E.g.,: Dental filling composites, carbon fiber reinforced methyl methacrylate bone cement + ultra-high molecular weight polyethylene | * Corrosive resistant | * Laborious manufacturing methods | * Dental fillings |
| * Rubber catheters and gloves |
| Materials | Fabrication Process | In Vivo/In Vitro Model | Key Findings | Ref. |
|---|---|---|---|---|
| Scaffolds for tissue engineering and regeneration | ||||
| PCL + Chitosan | Porous PCL scaffolds were 3D printed at 130 °C, print head speed of 1–3 mm/s and 1.5–3.0 bar pressure. Thermosensitive chitosan hydrogel was filled inside the pores of PCL scaffold. | Rabbit bone marrow mesenchymal stem cells (BMMSCs) | 3D printed scaffolds showed greater cell retention and proliferation of BMMSCs. Stronger osteogenesis and higher bone matrix formation shows their applications in bone tissue engineering | [8] |
| PCL + β-TCP | PCL melted at 110 °C and β-TCP powder is added. Membranes were 3D printed at 110 °C and at 500 kPa. | Alveolar bone defects on beagles | The 3D printed PCL/β-TCP membranes showed enhanced bone regeneration capabilities than PCL or collagen membranes alone | [9] |
| PLA + biodegradable calcium phosphate glass | Printing pressure 40–80 psi, 3 mm/s motor speed, print head temperature 40 ± 5 °C, Cross-linked with 8% (w/v) NaOH in 70% ethanol. | Human monocytes | PLA based scaffolds increased the production of IL-6, IL-12/23 and IL-10 | [19] |
| Drug Delivery | ||||
| PCL | Extruded PCL filaments with female sex hormones (E1, E2, E3 and progesterone) at 90 °C and 3D printed at 110 °C in the shape of commonly used implants including discs, pessaries, subdermal rods, intrauterine devices (IUDs) and surgical mesh. | Estrogen receptor luciferase reporter cells (T47D) | FDM can be used to fabricate patient specific personalized medicine for drug delivery. The 3D printed hormonal constructs showed biocompatibility and bioactive retention | [12] |
| PLA | PLA pellets coated with gentamicin and methotrexate were extruded as filaments at 170 °C and 3D printed as beads and catheters using Makerbot 3D printer (FDM based) at 220 °C | Osteosarcoma cells (for chemotherapeutics) and E. coli (for antibiotics) | 3D printed PLA constructs successfully retained the bioactivity. Clear demarcating zones of inhibition was seen for gentamicin constructs and decrease in cell viability of osteosarcoma cells proved the cytostatic effect of methotrexate constructs. | [11] |
| Olea-gum-resins (benzoin, myrrha and olibanum) doped with metal oxide nanoparticles (TiO2, P25, Cu2O, and MoO3) | Natural gum resins added with 10% metal oxides were extruded as filaments at 70–85 °C and 3D printed into discs (10 mm × 5 mm) at 80 °C while maintaining the build platform temperature at 60 °C and at a print head speed of 10 mm/min. | Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Candida albicans. | Naturally occurring polymers can be successfully 3D printed. Discs with just the resins prevented only surface associated microbial growth. Additionally, metal oxide nanoparticles increased the bacteriostatic effects of the natural polymers | [13] |
| PVA | PVA filament was milled and powdered. Paracetamol and caffeine were added and extruded as filaments at 180 °C. These filaments were 3D printing into tablets and capsules at 200 °C with print head speed of 150 mm/s | Novel oral dosage forms were successfully fabricated. Capsules with alternating layers of caffeine and paracetamol were 3D printed. | [16] | |
| Surgical guides and implants | ||||
| ABS | CAD models were developed using CT files of patient and 3D printed. FDM fabricated models were scanned again for comparison | Perioperative surgical simulation of conjoined twin separation surgery | The 3D printed models resembled the CT data of the patients and had an overall mean deviation of less than 2 mm. | [17] |
| TPU * | Pharmaceutical grade TPU powder was extruded into filaments and 3D printed into fistula stents, which were modelled from patient’s 3D reconstructed fistulography and CT scan images | A 45-year-old man was implanted with this tailor-made fistula implant | The 3D printed implant was effective in treating the enterocutaneous fistula | [18] |
| Materials | Process | In Vivo/In Vitro Model | Key Findings | Ref. |
|---|---|---|---|---|
| Gelatin (partially crosslinked) | The partially polymerized gel in the print head was extruded at 30 °C through a 100 µm diameter nozzle on to a cooled platform (10 °C). These were later crosslinked with chemicals EDC/NHS * for thermal and mechanical stability. Sterilization was done by overnight incubation in 70% ethanol and one hour of UV exposure. | CD-1 strain (Harlan) female mice | 3D printed implant restored ovarian function in the sterilized mice. Additionally, these mice successfully bore offspring. | [21] |
| Nano-fibrillated cellulose (NFC) + alginate | Using regenHU bioprinter, scaffolds (4.8 mm × 4.8 mm × 1 mm) were printed at printing pressure 40 kPa and 5 mm/s printing speed. Crosslinked using CaCl2 for 10 min, followed by rinsing with culture medium. | Human nasoseptal chondrocytes | Successfully 3D printed constructs resembling human organs (ear). The cytotoxicity and cell viability analysis proved the biocompatibility of this novel hydrogel (bioink) formulation. | [22] |
| NFC + alginate; NFC + HA | RegenHu bioprinter was used to 3D print the constructs of 7 mm × 7 mm × 1.2 mm dimensions with the two bioinks loaded with iPSCs. Printing speed was maintained at 10–20 mm/s at 20–30 kPa printing pressure. NFC-alginate constructs were crosslinked with CaCl2 for 5 min and NFC–HA constructs were crosslinked for 5 min using H2O2. | Human derived induced pluripotent stem cells (iPSCs) | The iPSCs in NFC-alginate constructs were pluripotent for at least 5 weeks, and then formed into hyaline like cartilage expressing type II collagen. NFC-hyaluronic acid constructs have shown lower proliferation rate. | [23] |
| Methacrylated hyaluronic acid (MeHA) | MeHA was dissolved in culture medium along with photoinitiator Irgacure 2959. Porous cubic scaffolds were bioprinted using Bioscaffolder dispensing system 3D bioprinter and scaffolds were UV crosslinked at 1800 mJ/cm2. | Mesenchymal stromal cells | Bioprinted scaffolds maintained good cell viability for more than 3 weeks. Increased concentrations of MeHA promoted osteogenic differentiation. | [31] |
| PVA and phytagel (1:1) | Printing was done at room temperature with a print speed of 5 mm/s and flow rate of 6 mL/h on to a cold build plate (−78.5 °C). The scaffolds were stored at −25 °C for 15 h. Constructs were later coated with collagen, poly-l-lysine or gelatin | Human dermal fibroblast cells | PVA/phytagel hydrogel was successfully 3D printed cryogenically and have mechanical properties similar to soft tissue. Additionally, coating with natural polymers (chitosan or gelatin) increased the cell attachment of the fibroblasts | [24] |
| Biphasic calcium phosphate (HA/β-TCP = 60:40) + HPMC + Polyethylenimine + ZrO2 | Extruded at pressure of 600 kPa and at printing speed of 100 mm/min. Samples were sintered at 1100 °C | Tested on osteoblast like sarcoma cells for cytotoxicity and hMSCs for differentiation potential of the scaffolds | Improved mechanical properties of scaffolds at 10% (w/w) of ZrO2 was reported along with improved BMP-2 expression | [32] |
| Calcium sulfate hydrate + mesoporous bioglass + PCL | Extruded under pressure of 2.2–3.6 bar and speed of 4.5–8.2 mm/s | In vitro evaluation on hBMSc cells and in vivo evaluation on rat model | Addition of bioglass promoted bone formation significantly in the animal model | [33] |
| Calcium silicate + Magnesium + PVA | Extruded using a 450 µm nozzle and printed at speed of 6 mm/s. Scaffolds were sintered at 1150 °C | In vitro testing on MC3T3 cells an in vivo evaluation on rabbit skull defects | Mechanical strength was significantly improved along with degradation rate and new bone formation | [34] |
| Materials | Process | In Vivo/In Vitro Model | Key Findings | Ref. |
|---|---|---|---|---|
| Powders: hydroxyapatite + β-TCP); Binding liquid: (0.6 wt % PVA + 0.25 wt % Tween 80) and (8.75 wt % phosphoric acid + 0.25 wt % Tween 80) | Microporous cylindrical scaffolds (3 mm × 10 mm) were 3D printed using ZPrinter 250 printer at 0.1 mm powder thickness and 0.3 L/m3 binder spray velocity. Scaffolds were set to dry at 50 °C for 2 h. | Rabbit bone marrow stromal cells (BMSCs) | Constructs printed with phosphoric acid showed better fabrication accuracy and mechanical properties than constructs printed with PVA. Both binding liquids showed good cellular affinity with BMSCs. | [35] |
| Substrate: paper and polyethylene terephthalate (PET); Binding liquid: concentrated solution of paracetamol, theophylline, and caffeine | Concentrated drug solutions were selectively placed on the substrates at 30 °C, and at 10 µm dropping distance using dimatix materials printer (DMP) 2800 inkjet printer. | Active pharmaceutical ingredients were successfully 3D printed using inkjet technology. The accurate deposition and crystallization of the drugs can be highly controlled. Precise and personalized dosing of the drug substances is possible with this technology. | [36] | |
| Powders: β-TCP + hydroxyapatite + dextrin; Binding liquid: water + glycerol | Powder bed thickness was maintained 100 µm at 0.006 m/s print head speed. Constructs were gradually heated up to 350 °C and sintered at 1200 °C for 4 h. Fibrin and BMP-2 were coated. Osteoblasts were seeded on the scaffolds. | Male Lewis rats | 3D printed constructs with BMP-2 and osteoblast cells showed enhanced ectopic bone formation. | [38] |
| Powder: α-TCP; Binding liquid: 8.75 wt % phosphoric acid + 0.25 wt % Tween 80 | Powder layer thickness 89 µm and binder liquid to powder ratio 0.46. Vancomycin and rifampin were added to the powder bed. Polylactic-co-glucolic acid (PLGA) was coated in some groups. | Female BALB/cJ mice | Unlike PMMA, co-delivery of drugs vancomycin and rifampin was possible with 3D printed constructs. Thus, significantly improving implant-associated osteomyelitis. Additional PLGA coating further prolonged the antibiotic release. | [39] |
| Binding liquid: Soluplus (co-polymer of PVC-PVA-PEG); Substrate: stainless steel microneedles | Drugs curcumin, 5-fluorouracil, cis-platin were added to the polymer and jetted as fine droplets (300 pL) on the needles at 1–5 m/s. Multiple coatings were given to acquire desired drug concentration. | Dermatomed porcine skin | Inkjet printing technology was proved effective in coating metallic microneedles for transdermal drug delivery. | [37] |
| Binding liquid: miconazole; Substrate: Gantrez AN 169 BF (poly (methyl vinyl ether-co-maleic anhydride)) microneedles | Miconazole in dimethyl sulfoxide was sprayed at a rate of 10 pL/droplet of solution. Drop spacing of 30 µm and 32.0 V jet voltage was used. | Candida albicans | Antifungal agents were successfully incorporated using inkjet printing technology and clear zone of inhibition was demonstrated. Fabricated constructs can be effectively used for transdermal treatment of cutaneous fungal infections. | [40] |
| Binding liquid: 2-pyrolidinone; Substrate: calcium sulfate hemihydrate | 89 µm layer height | Osteoblast like sarcoma cells | Binder solution toxicity was assayed by sintering specimens at temperature ranging from 300–1100 °C. High temperature sintered samples were compatible | [41] |
| Binding liquid: 8.75% phosphoric acid + 0.25% Tween80 + 1%–2% collagen; Substrate: hydroxyapatite and α-TCP | 89 µm layer height and binding liquid to powder ratio was 0.46 was used | In vitro cytocompatibility was tested on C3H/10T1/2 cells and in vivo evaluation was done on critical size femoral defects on female BLAB/cJ | Macroporosity up to 0.5 mm was achieved. Incorporation of collagen favored better cellular response and improved mechanical properties. | [42] |
| Binding liquid: aqueous solution of 2-pyrrolidone (zb63); Substrate: calcium sulfate (plaster), vinyl polymer and carbohydrate | Pore sizes of 0.4, 0.6, and 0.8 mm were designed and printed at binder to powder ratio of 0.24 (shell) and 0.12 (core) | Effect of layer thickness and orientation of printing were evaluated by measuring physical and mechanical properties | Layer thickness of 0.1125 mm and printing along X direction resulted in specimens with best mechanical strength and dimensional accuracy | [43] |
| Binder liquid: mesoporous silica nanoparticles, polyethyleneimine, furosemide, and propylene glycol; Substrate: hydroxypropyl methyl cellulose (HPMC), and polyester transparency films | Print speed at 200 mm/s, resolution of 150 and 500 dpi, and wet thickness of 500 µm | Drug release from inks, rheological properties, dynamic viscosity and other important properties were evaluated | Successfully demonstrated the feasibility of printing drug loaded nano particle suspension for poorly water-soluble drugs | [44] |
| Materials | Process | Test Model | Key Findings | Ref. |
|---|---|---|---|---|
| Elastic photopolymer (FullCure 930 TangoPlus) by Stratasys | 3D printed live size aortic aneurysum phantom from patients CT files using a Stratasys Eden 260 polyjet printer. The phantom cost was $254.49 and took 13 hours to 3D print. | Mock surgical procedure was performed under live fluoroscope using the 3D printed phantom | Pre-surgical planning & simulation was possible with patient-specific abdominal aortic aneurysm phantom. Simulation was effective in planning surgical challenges & complications than standard procedures (2D image diagnostics). | [45] |
| Rigid acrylic resin (AR-M2) for Agilista-3200 3D printer, Japan | 3D printed patient-specific intrahepatic vessel models | Preoperative planning in hepatocellular carcinoma resection procedure | The use of 3D printed intrahepatic vessel models from patient’s data (CT files) has greatly improved the surgical quality of the hepatocellular carcinoma procedure. | [46] |
| Photopolymer resin | 3D printed customized surgical aids (cutting and repositioning guides) for genioplasty. CAD/CAM models were created from the patients CT images and patient specific surgical guides were fabricated using SLA based 3D printer (3D systems). | Genioplasty performed on 88 patients with dentofacial deformities | 3D printed genioplasty templates provided greater accuracy in the surgical procedures than traditional intraoperative measurements. | [47] |
| Multiple photopolymer resins on Connex 3 polyjet | Printed at 16 µm layer height | 3D printed anatomical phantoms of liver and microspheres from patient’s CT data | These phantoms offered a method to quantify radiation dose form Y-90 microspheres for treatment of liver cancer | [48] |
| Multiple photopolymer resins printed using Connex 350 | Printed anatomical liver with different materials for vasculature and biliary structures | Used as preoperative surgical guidance model for 3 cases of liver transplant | 6 patient specific liver models were 3D printed (3 living donor and 3 recipients). Significantly improved surgery and minimized intraoperative complications. | [49] |
| Multiple photopolymer resins printed using Objet 500 Connex | Printed anatomical model of head with different materials for skin, bone and tissues | Used these models as a training tool for neuro surgery | Significantly improved the training experience of surgeons by improving navigation and planning | [50] |
| Photopolymer RGD525 and Connex 500 | Printed with polymers that are visible under MRI scanners | Spine model containing C6–C8 vertebrae including tumors in them. | Anatomically accurate phantoms that can be imaged under CT and MRI were developed. Improving preoperative planning for MR guided minimally invasive surgeries. | [51] |
| Multiple photopolymers and Objet 350 Connex | Materials with different rigidity were used to mimic native tissue’s mechanical properties. | Different models such as hollow aneurysm, craniocerebral aneurysm, and craniocerebral tumors | Aneurysm clippings and tumor resection planning were efficiently planned with these models | [52] |
| Multiple photopolymers and Objet studio | Materials with different flexibilities were used | 50 patients were randomly chosen to explain medical procedure using 3D printed model | 3D printed model of nasal sinus anatomy was used as educational tool to enable patients to make informed decision. Results suggest improved patient comfort levels and outcomes. | [53] |
| Projet 3512 HD | Rigid material was used to create molds for nephrology sectioning. | 5 patient specific slicing guides were 3D printed for partial nephrectomy | Enabled accurate sectioning of tumors for colocalization analysis for radiomic and radiogenomic analyses | [54] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tappa, K.; Jammalamadaka, U. Novel Biomaterials Used in Medical 3D Printing Techniques. J. Funct. Biomater. 2018, 9, 17. https://doi.org/10.3390/jfb9010017
Tappa K, Jammalamadaka U. Novel Biomaterials Used in Medical 3D Printing Techniques. Journal of Functional Biomaterials. 2018; 9(1):17. https://doi.org/10.3390/jfb9010017
Chicago/Turabian StyleTappa, Karthik, and Udayabhanu Jammalamadaka. 2018. "Novel Biomaterials Used in Medical 3D Printing Techniques" Journal of Functional Biomaterials 9, no. 1: 17. https://doi.org/10.3390/jfb9010017





