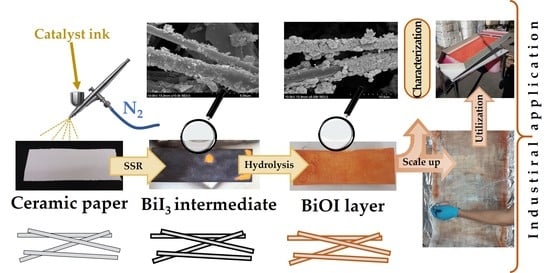
Innovative and Cost-Efficient BiOI Immobilization Technique on Ceramic Paper—Total Coverage and High Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Direct In-Situ Immobilization by Solvothermal Synthesis
2.3. The Spray-Coating Immobilization Technique
- CP_MQ_s.c.: Milli-Q water,
- CP_EtOH_s.c.: Absolute ethanol,
- CP_i-Pr_s.c.: Isopropanol,
- CP_EtOH+MQ_s.c.: Absolute ethanol and water mixture (50–50 w/w%),
- CP_EtOH+EG_s.c.: Absolute ethanol and ethylene glycol mixture (50–50 w/w%).
2.4. Characterization Methods
2.5. Photocatalytic Activity Measurements
3. Results
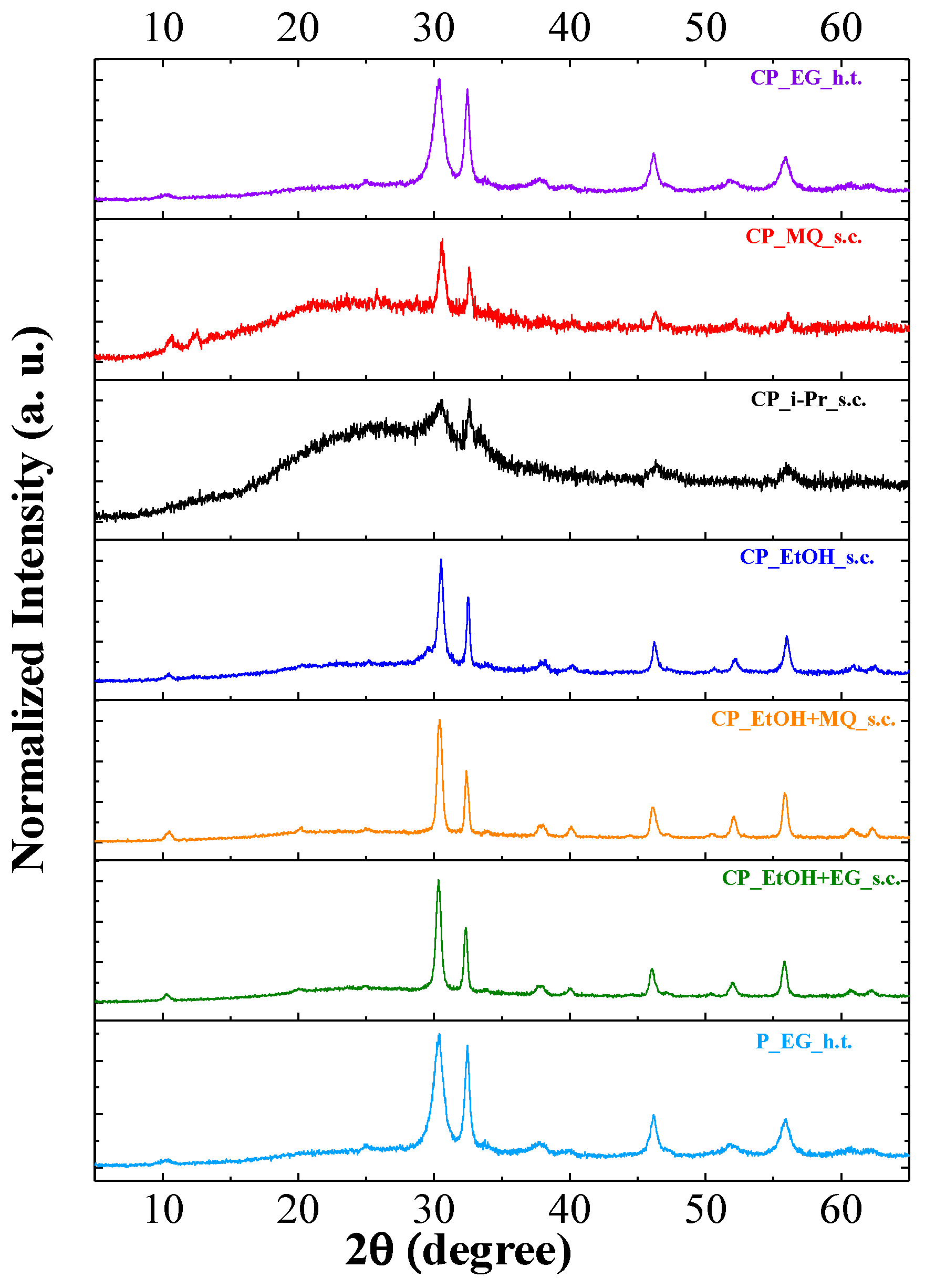
3.1. X-ray Diffraction of Anchored BiOI
3.2. DRS—Diffuse Reflectance Spectroscopy
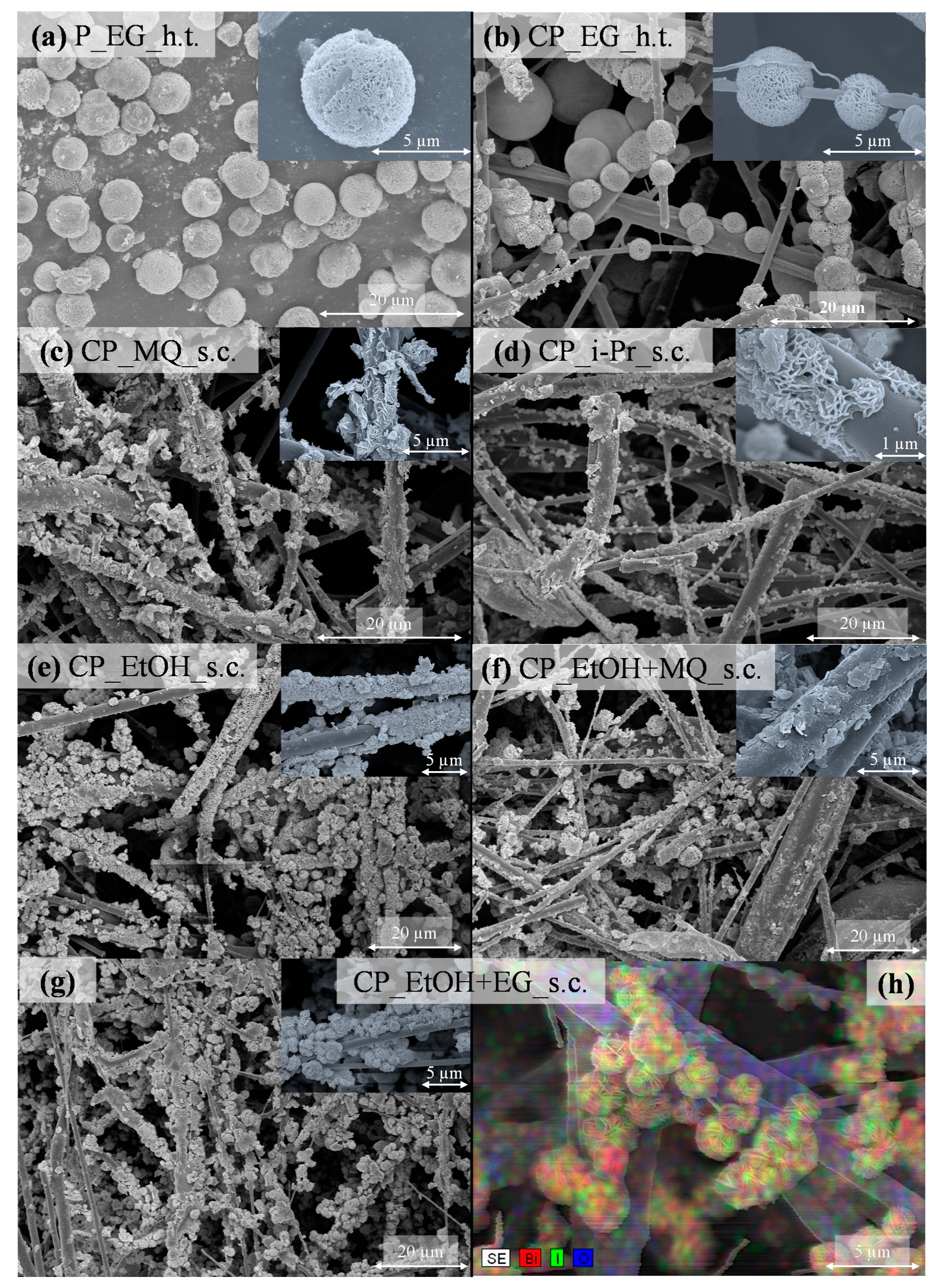
3.3. SEM and Optical Microscope Investigation
3.4. Infrared Measurements
3.5. Raman Measurements
3.6. XPS Measurements
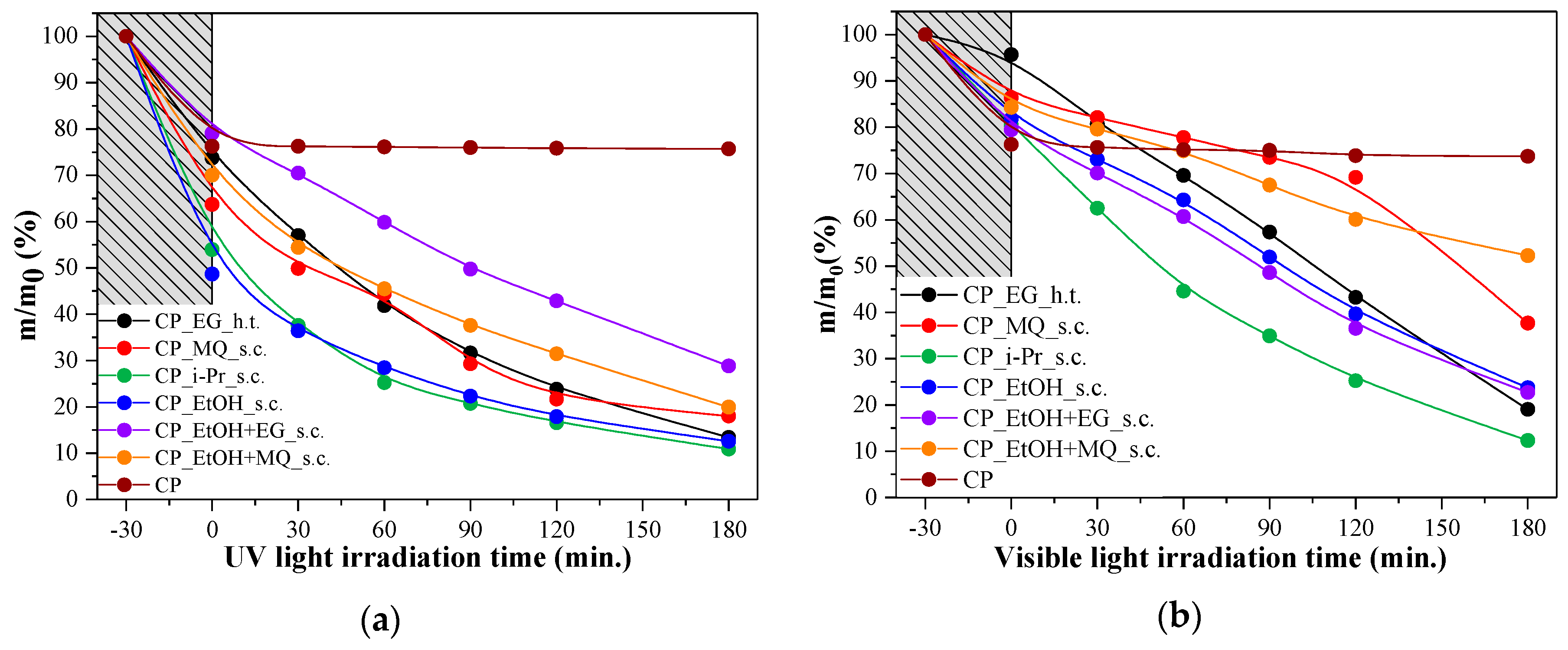
3.7. Photocatalytic Activity Measurements and Different Approaches to the Activity
3.8. Outlook for Scalability
4. Discussion of the BiOI Photocatalysts Uniform Layer Formation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
 , the NTP-NFTÖ scholarship (NTP_NFTÖ-19-B-0016), and the Campus Mundi Programme (EFOP-3.4.2-VEKOP/15-2015-00001). Zs. Pap and K. Magyari acknowledge the financial support for the “Bolyai János” scholarship.
, the NTP-NFTÖ scholarship (NTP_NFTÖ-19-B-0016), and the Campus Mundi Programme (EFOP-3.4.2-VEKOP/15-2015-00001). Zs. Pap and K. Magyari acknowledge the financial support for the “Bolyai János” scholarship.Conflicts of Interest
References
- Blanco–Galvez, J.; Fernández–Ibáñez, P.; Malato-Rodríguez, S. Solar Photocatalytic Detoxification and disinfection of water: Recent overview. J. Sol. Energy Eng. 2007, 129, 4–15. [Google Scholar] [CrossRef]
- Parrino, F.; Bellardita, M.; Garcia-Lopez, E.I.; Marci, G.; Loddo, V.; Palmisano, L. Heterogeneous photocatalysis for selective formation of high-value-added molecules: Some chemical and engineering aspects. ACS Catal. 2018, 8, 11191–11225. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Alarcón, D.C.; Maldonado, M.I.; Fernández–Ibáñez, P.; Gernjak, W. Photocatalytic decontamination and disinfection of water with solar collectors. Catal. Today 2007, 122, 137–149. [Google Scholar] [CrossRef]
- Lin, X.H.; Miao, Y.; Li, S.F.Y. Location of photocatalytic oxidation processes on anatase titanium dioxide. Catal. Sci. Technol. 2017, 7, 441–451. [Google Scholar] [CrossRef]
- Kim, D.S.; Park, Y.S. Photocatalytic decolorization of rhodamine B by immobilized TiO2 onto silicone sealant. Chem. Eng. J. (Lausanne) 2006, 116, 133–137. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Bahnemann, D.W. Photoelectrocatalytic materials for environmental applications. J. Mater. Chem. 2009, 19, 5089–5121. [Google Scholar] [CrossRef]
- Schultz, D.M.; Yoon, T.P. Solar synthesis: Prospects in visible light photocatalysis. Science 2014, 343, 1239176. [Google Scholar] [CrossRef] [Green Version]
- Bahnemann, D. Photocatalytic water treatment: Solar energy applications. Sol. Energy 2004, 77, 445–459. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.-j. Preparation of 2D WO3 Nanomaterials with enhanced catalytic activities: Current status and perspective. ChemBioEng Rev. 2015, 2, 335–350. [Google Scholar] [CrossRef]
- Martin, D.J.; Liu, G.G.; Moniz, S.J.A.; Bi, Y.P.; Beale, A.M.; Ye, J.H.; Tang, J.W. Efficient visible driven photocatalyst, silver phosphate: Performance, understanding and perspective. Chem. Soc. Rev. 2015, 44, 7808–7828. [Google Scholar] [CrossRef]
- Kása, Z.; Baia, L.; Magyari, K.; Hernádi, K.; Pap, Z. Innovative visualization of the effects of crystal morphology on semiconductor photocatalysts. Tuning the Hückel polarity of the shape–tailoring agents: The case of Bi2WO6. CrystEngComm 2019, 21, 1267–1278. [Google Scholar] [CrossRef]
- Kása, Z.; Saszet, K.; Dombi, A.; Hernádi, K.; Baia, L.; Magyari, K.; Pap, Z. Thiourea and Triton X-100 as shape manipulating tools or more for Bi2WO6 photocatalysts? Mater. Sci. Semicond. Process. 2018, 74, 21–30. [Google Scholar] [CrossRef]
- Yi, H.; Qin, L.; Huang, D.; Zeng, G.; Lai, C.; Liu, X.; Li, B.; Wang, H.; Zhou, C.; Huang, F.; et al. Nano-structured bismuth tungstate with controlled morphology: Fabrication, modification, environmental application and mechanism insight. Chem. Eng. J. (Lausanne) 2019, 358, 480–496. [Google Scholar] [CrossRef]
- Adán, C.; Marugán, J.; Obregón, S.; Colón, G. Photocatalytic activity of bismuth vanadates under UV-A and visible light irradiation: Inactivation of Escherichia coli vs oxidation of methanol. Catal. Today 2015, 240, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.J.; Liu, L.Q.; Wang, D.F.; Yang, F.; Ye, J.H. Synthesis of bismuth molybdate photocatalysts for CO2 photo–reduction. J. CO2 Util. 2019, 29, 196–204. [Google Scholar] [CrossRef]
- Dandapat, A.; Horovitz, I.; Gnayem, H.; Sasson, Y.; Avisar, D.; Luxbacher, T.; Mamane, H. Solar photocatalytic degradation of trace organic pollutants in water by Bi(0)-doped bismuth oxyhalide thin films. ACS Omega 2018, 3, 10858–10865. [Google Scholar] [CrossRef]
- Wang, S.-l.; Wang, L.-l.; Ma, W.-h.; Johnson, D.M.; Fang, Y.-f.; Jia, M.-k.; Huang, Y.-p. Moderate valence band of bismuth oxyhalides (BiOXs, X = Cl, Br, I) for the best photocatalytic degradation efficiency of MC-LR. Chem. Eng. J. (Lausanne) 2015, 259, 410–416. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, M.; Huang, D.; Zeng, G.; Xu, P.; Zhou, C.; Lai, C.; Wang, H.; Cheng, M.; Wang, W. Multiply structural optimized strategies for bismuth oxyhalide photocatalysis and their environmental application. Chem. Eng. J. (Lausanne) 2019, 374, 1025–1045. [Google Scholar] [CrossRef]
- Han, Q.; Zhang, K.; Zhang, J.; Gong, S.; Wang, X.; Zhu, J. Effect of the counter ions on composition and morphology of bismuth oxyhalides and their photocatalytic performance. Chem. Eng. J. (Lausanne) 2016, 299, 217–226. [Google Scholar] [CrossRef]
- Bárdos, E.; Király, A.K.; Pap, Z.; Baia, L.; Garg, S.; Hernádi, K. The effect of the synthesis temperature and duration on the morphology and photocatalytic activity of BiOX (X = Cl, Br, I) materials. Appl. Surf. Sci. 2019, 479, 745–756. [Google Scholar] [CrossRef]
- Garg, S.; Yadav, M.; Chandra, A.; Gahlawat, S.; Ingole, P.P.; Pap, Z.; Hernadi, K. Plant leaf extracts as photocatalytic activity tailoring agents for BiOCl towards environmental remediation. Ecotoxicol. Environ. Saf. 2018, 165, 357–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, H.F.; Huang, B.B.; Dai, Y. Engineering BiOX (X = Cl, Br, I) nanostructures for highly efficient photocatalytic applications. Nanoscale 2014, 6, 2009–2026. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, C.; Hu, R.; Zuo, X.; Nan, J.; Li, L.; Wang, L. Oxygen-rich bismuth oxyhalides: Generalized one–pot synthesis, band structures and visible–light photocatalytic properties. J. Mater. Chem. 2012, 22, 22840–22843. [Google Scholar] [CrossRef]
- Garg, S.; Yadav, M.; Chandra, A.; Sapra, S.; Gahlawat, S.; Ingole, P.P.; Pap, Z.; Hernadi, K. Biofabricated BiOI with enhanced photocatalytic activity under visible light irradiation. RSC Adv. 2018, 8, 29022–29030. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.N.; Chen, Z.Z.; Qiu, Y.Y.; Li, M.Y.; Yang, H.; Huang, Y.C.; Chen, J. Preparing BiOI photocatalyst for degradation of methyl blue in wastewater. Inorg. Chem. Commun. 2018, 98, 58–61. [Google Scholar] [CrossRef]
- Long, Y.; Wang, Y.; Zhang, D.; Ju, P.; Sun, Y. Facile synthesis of BiOI in hierarchical nanostructure preparation and its photocatalytic application to organic dye removal and biocidal effect of bacteria. J. Colloid Interface Sci. 2016, 481, 47–56. [Google Scholar] [CrossRef]
- Islam, M.J.; Kim, H.K.; Reddy, D.A.; Kim, Y.; Ma, R.; Baek, H.; Kim, J.; Kim, T.K. Hierarchical BiOI nanostructures supported on a metal organic framework as efficient photocatalysts for degradation of organic pollutants in water. Dalton Trans. 2017, 46, 6013–6023. [Google Scholar] [CrossRef]
- Zanrosso, C.D.; Piazza, D.; Lansarin, M.A. Polymeric hybrid films with photocatalytic activity under visible light. J. Appl. Polym. Sci. 2018, 135, 10. [Google Scholar] [CrossRef]
- Li, L.; Ya, W.; Manhong, H.; Donghui, C. Immobilization of BiOBr/BiOI Hierarchical Microspheres on Fly Ash Cenospheres as Visible Light Photocatalysts. Aust. J. Chem. 2015, 69, 119–125. [Google Scholar]
- Wang, K.; Shao, C.; Li, X.; Miao, F.; Lu, N.; Liu, Y. Room temperature immobilized BiOI nanosheets on flexible electrospun polyacrylonitrile nanofibers with high visible–light photocatalytic activity. J. Sol. Gel Sci. Technol. 2016, 80, 783–792. [Google Scholar] [CrossRef]
- Yadav, M.; Garg, S.; Chandra, A.; Hernadi, K. Immobilization of green BiOX (X = Cl, Br and I) photocatalysts on ceramic fibers for enhanced photocatalytic degradation of recalcitrant organic pollutants and efficient regeneration process. Ceram. Int. 2019, 45, 17715–17722. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, Y.; Wu, S.; Zhu, Y.; Chen, H.; Yu, X.; Zhang, Y. Facile Fabrication of BiOI/BiOCl immobilized films with improved visible light photocatalytic performance. Front. Chem. 2018, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hegedűs, P.; Szabó–Bárdos, E.; Horváth, O.; Szabó, P.; Horváth, K. Investigation of a TiO2 photocatalyst immobilized with poly (vinyl alcohol). Catal. Today 2017, 284, 179–186. [Google Scholar] [CrossRef] [Green Version]
- Simon, G.; Gyulavári, T.; Hernádi, K.; Molnár, M.; Pap, Z.; Veréb, G.; Schrantz, K.; Náfrádi, M.; Alapi, T. Photocatalytic ozonation of monuron over suspended and immobilized TiO2-study of transformation, mineralization and economic feasibility. J. Photochem. Photobiol. A 2018, 356, 512–520. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Song, N.; Zhang, S.; Zou, S.; Zhong, S. Synthesis of sponge-loaded Bi2WO6/ZnFe2O4 magnetic photocatalyst and application in continuous flow photocatalytic reactor. J. Mater. Sci. Mater. Electron. 2017, 28, 8197–8205. [Google Scholar] [CrossRef]
- Mozia, S.; Tomaszewska, M.; Morawski, A.W. Decomposition of nonionic surfactant in a labyrinth flow photoreactor with immobilized TiO2 bed. Appl. Catal. B Environ. 2005, 59, 155–160. [Google Scholar] [CrossRef]
- Cunha, D.L.; Kuznetsov, A.; Achete, C.A.; Machado, A.E.d.H.; Marques, M. Immobilized TiO2 on glass spheres applied to heterogeneous photocatalysis: Photoactivity, leaching and regeneration process. Peer. J. 2018, 6, e4464. [Google Scholar] [CrossRef] [Green Version]
- Bessergenev, V.G.; Mateus, M.C.; Morgado, I.M.; Hantusch, M.; Burkel, E. Photocatalytic reactor, CVD technology of its preparation and water purification from pharmaceutical drugs and agricultural pesticides. Chem. Eng. J. (Lausanne) 2017, 312, 306–316. [Google Scholar] [CrossRef]
- Alalm, M.G.; Samy, M.; Ookawara, S.; Ohno, T. Immobilization of S–TiO2 on reusable aluminum plates by polysiloxane for photocatalytic degradation of 2,4–dichlorophenol in water. J. Water Process Eng. 2018, 26, 329–335. [Google Scholar] [CrossRef]
- Ding, Z.; Hu, X.J.; Yue, P.L.; Lu, G.Q.; Greenfield, P.F. Synthesis of anatase TiO2 supported on porous solids by chemical vapor deposition. Catal. Today 2001, 68, 173–182. [Google Scholar] [CrossRef]
- Yao, S.H.; Li, J.Y.; Shi, Z.L. Immobilization of TiO2 nanoparticles on activated carbon fiber and its photodegradation performance for organic pollutants. Particuology 2010, 8, 272–278. [Google Scholar] [CrossRef]
- De Barros, A.L.; Domingos, A.A.Q.; Fechine, P.B.A.; de Keukeleire, D.; do Nascimento, R.F. PET as a support material for tio2 in advanced oxidation processes. J. Appl. Polym. Sci. 2014, 131, 9. [Google Scholar] [CrossRef]
- Veréb, G.; Ambrus, Z.; Pap, Z.; Mogyorósi, K.; Dombi, A.; Hernádi, K. Immobilization of crystallized photocatalysts on ceramic paper by titanium (IV) ethoxide and photocatalytic decomposition of phenol. React. Kinet. Mech. Cat. 2014, 113, 293–303. [Google Scholar] [CrossRef]
- Han, A.; Sun, J.; Lin, X.; Yuan, C.-H.; Chuah, G.K.; Jaenicke, S. Influence of facets and heterojunctions in photoactive bismuth oxyiodide. RSC Adv. 2015, 5, 88298–88305. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.W.; Lu, C.-S.; Chuang, C.-W.; Chen, Y.-J.; Fu, J.-Y.; Siao, C.-W.; Chen, C.-C. Synthesis of bismuth oxyiodides and their composites: Characterization, photocatalytic activity, and degradation mechanisms. RSC Adv. 2015, 5, 23450–23463. [Google Scholar] [CrossRef]
- Tokoro, C.; Suzuki, S.; Haraguchi, D.; Izawa, S. Silicate Removal in Aluminum Hydroxide co-precipitation process. Materials 2014, 7, 1084–1096. [Google Scholar] [CrossRef]
- Prashanth, P.A.; Raveendra, R.S.; Hari Krishna, R.; Ananda, S.; Bhagya, N.P.; Nagabhushana, B.M.; Lingaraju, K.; Raja Naika, H. Synthesis, characterizations, antibacterial and photoluminescence studies of solution combustion–derived α–Al2O3 nanoparticles. J. Asian Ceram. Soc. 2015, 3, 345–351. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Dong, Y.; Liu, L.; Chang, L.; Zhou, B.; Chi, Q.; Wang, X. High filling alumina/epoxy nanocomposite as coating layer for Fe–based amorphous powder cores with enhanced magnetic performance. J. Mater. Sci. Mater. Electron. 2019, 30, 14869–14877. [Google Scholar] [CrossRef]
- Prajzler, V.; Lyutakov, O.; Hüttel, I.; Špirková, J.; Oswald, J.; Machovič, V.; Jeřábek, V. Properties of epoxy novolak resin layers doped with bismuth for photoluminescence near 1300 nm. J. Appl. Polym. Sci. 2010, 117, 1608–1612. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.; Fu, M.; Wu, Z.; Lee, S.C. Room temperature synthesis and highly enhanced visible light photocatalytic activity of porous BiOI/BiOCl composites nanoplates microflowers. J. Hazard. Mater. 2012, 219, 26–34. [Google Scholar] [CrossRef]
- Putri, A.A.; Kato, S.; Kishi, N.; Soga, T. Relevance of precursor molarity in the prepared bismuth oxyiodide films by successive ionic layer adsorption and reaction for solar cell application. J. Sci. Adv. Mater. Devices 2019, 4, 116–124. [Google Scholar] [CrossRef]
- Shan, L.-w.; He, L.-q.; Suriyaprakash, J.; Yang, L.-x. Photoelectrochemical (PEC) water splitting of BiOI{001} nanosheets synthesized by a simple chemical transformation. J. Alloys Compd. 2016, 665, 158–164. [Google Scholar] [CrossRef]
- Tian, F.; Zhao, H.; Dai, Z.; Cheng, G.; Chen, R. Mediation of Valence Band Maximum of BiOI by Cl Incorporation for Improved Oxidation Power in Photocatalysis. Ind. Eng. Chem. Res. 2016, 55, 4969–4978. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, S.; Wang, X.; Sun, C.; Wang, Y.; Ling, Z. Solvothermal method coupled with thermal decomposition for synthesis of non–stoichiometric BiO1.18I0.64 with excellent photocatalytic activity. RSC Adv. 2016, 6, 2641–2650. [Google Scholar] [CrossRef]
- Liu, A.; Zhu, H.; Park, W.-T.; Kang, S.-J.; Xu, Y.; Kim, M.-G. Room-Temperature Solution-Synthesized p-type copper(i) iodide semiconductors for transparent thin-film transistors and complementary electronics. Adv. Mater. 2018, 30, 1802379. [Google Scholar] [CrossRef] [PubMed]
- Enikő, B.; Viktória, M.; Lucian, B.; Milica, T.; Gábor, K.; Kornélia, B.; Seema, G.; Zsolt, P.; Klara, H. Hydrothermal crystallization of bismuth oxybromide (BiOBr) in the presence of different shape controlling agents. Appl. Surf. Sci. 2020, 518, 146184. [Google Scholar]
- He, R.; Zhang, J.; Yu, J.; Cao, S. Room-temperature synthesis of BiOI with tailorable (001) facets and enhanced photocatalytic activity. J. Colloid Interface Sci. 2016, 478, 201–208. [Google Scholar] [CrossRef]


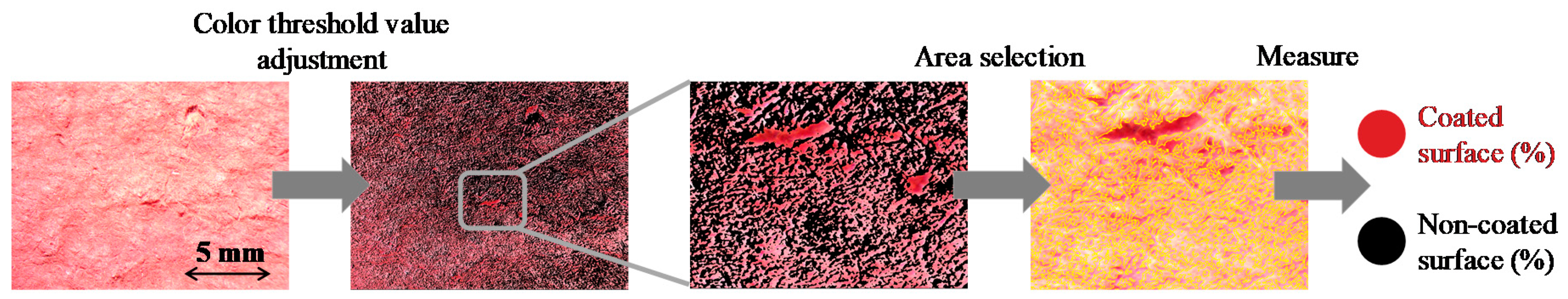
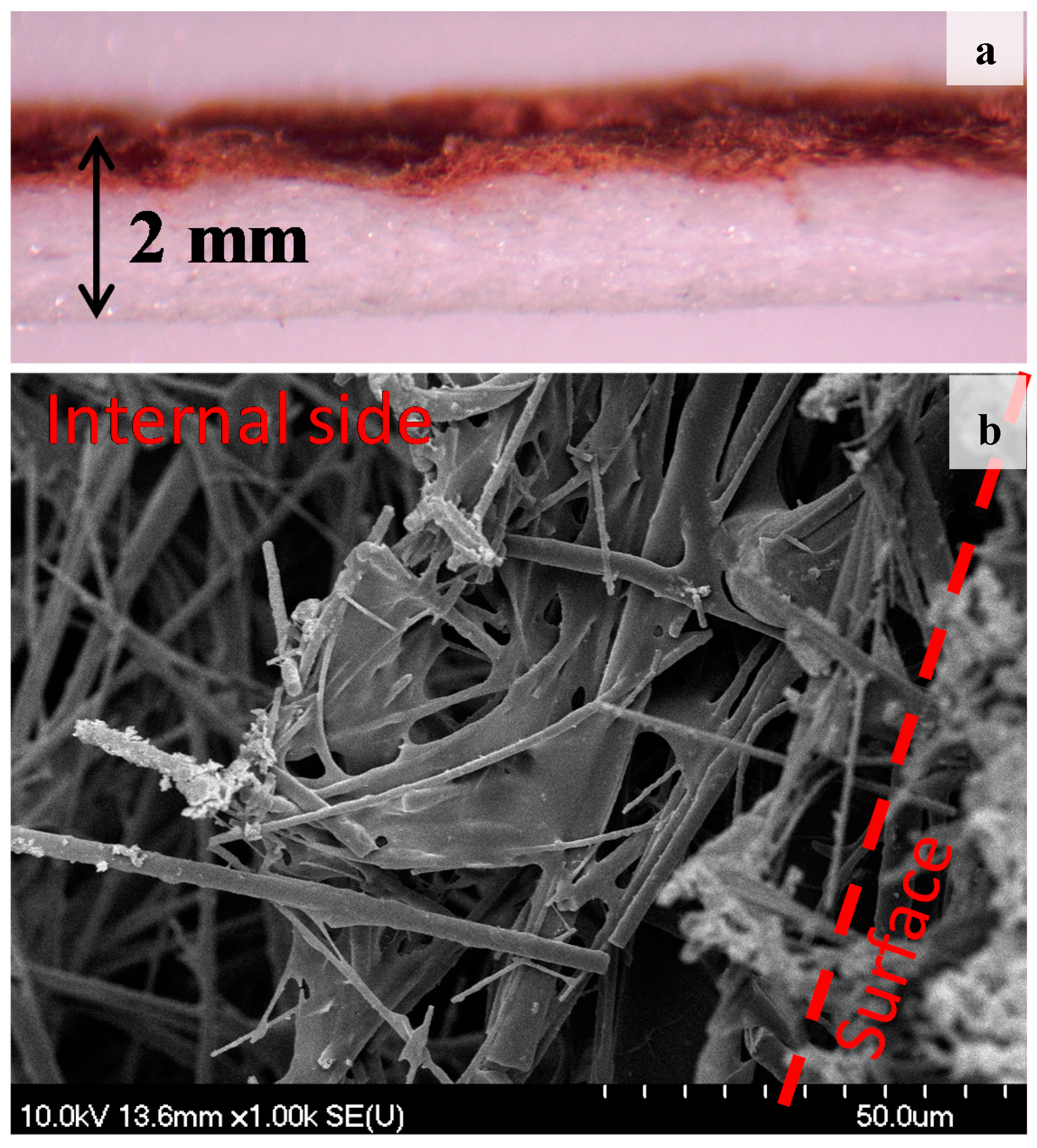

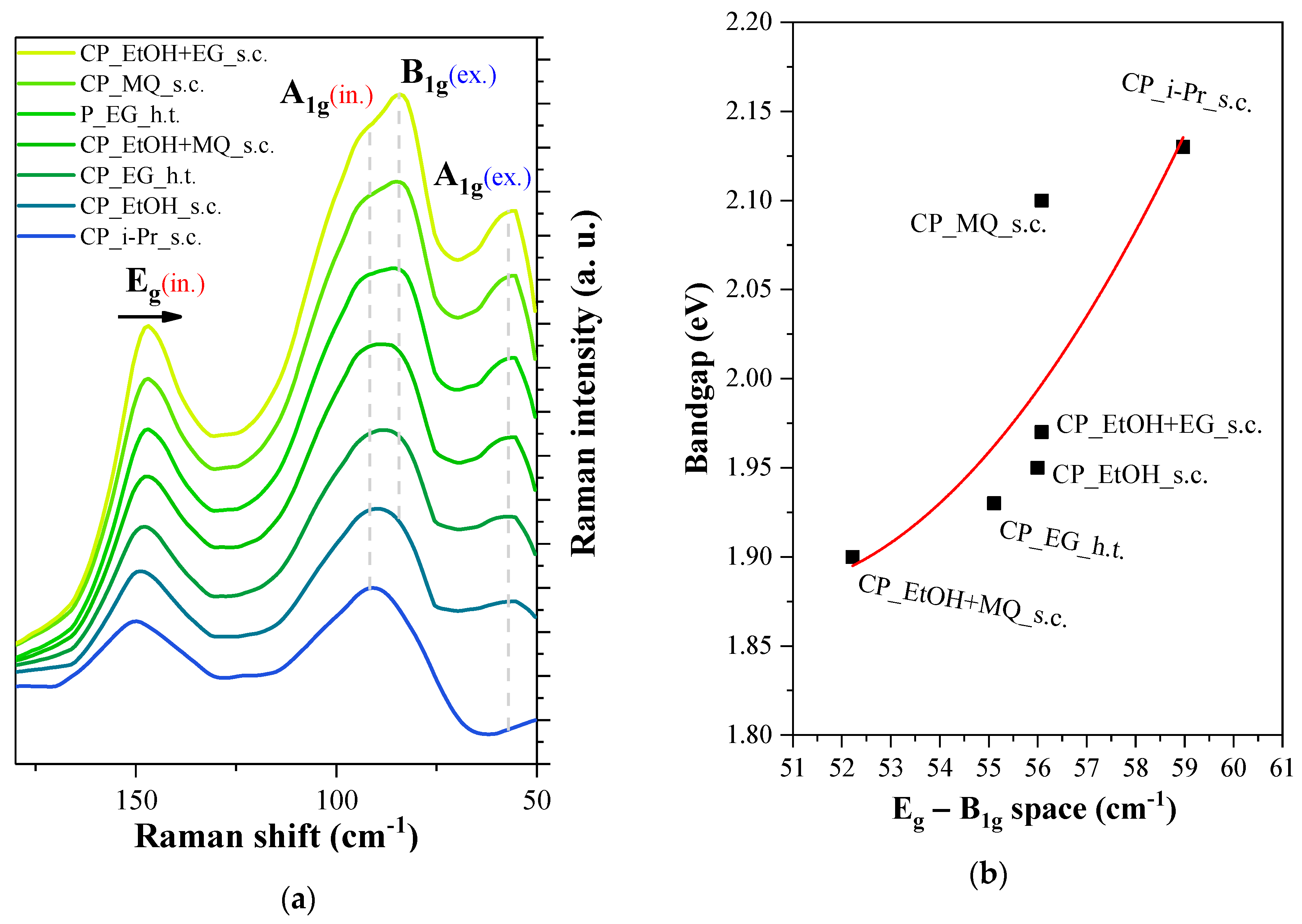
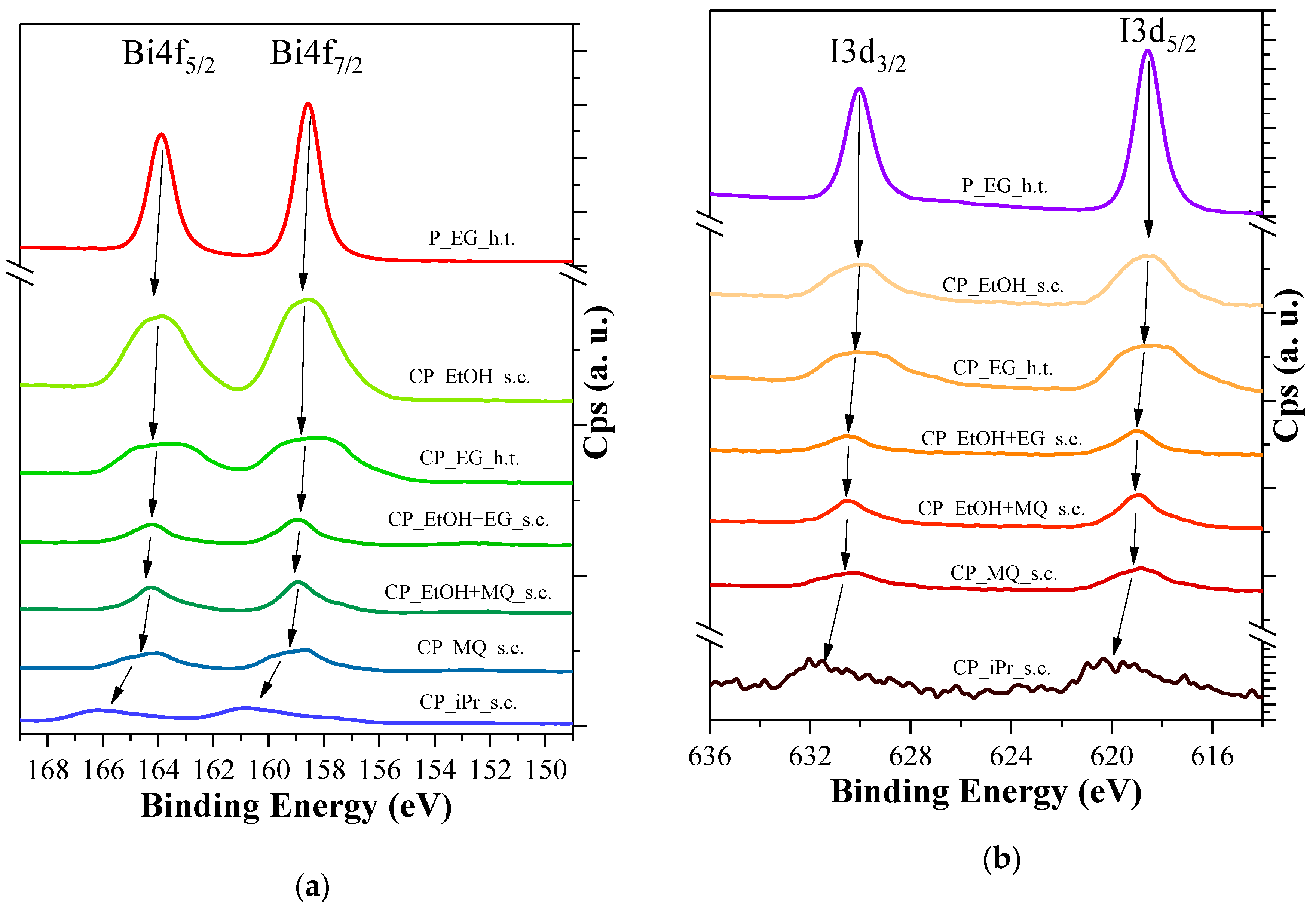
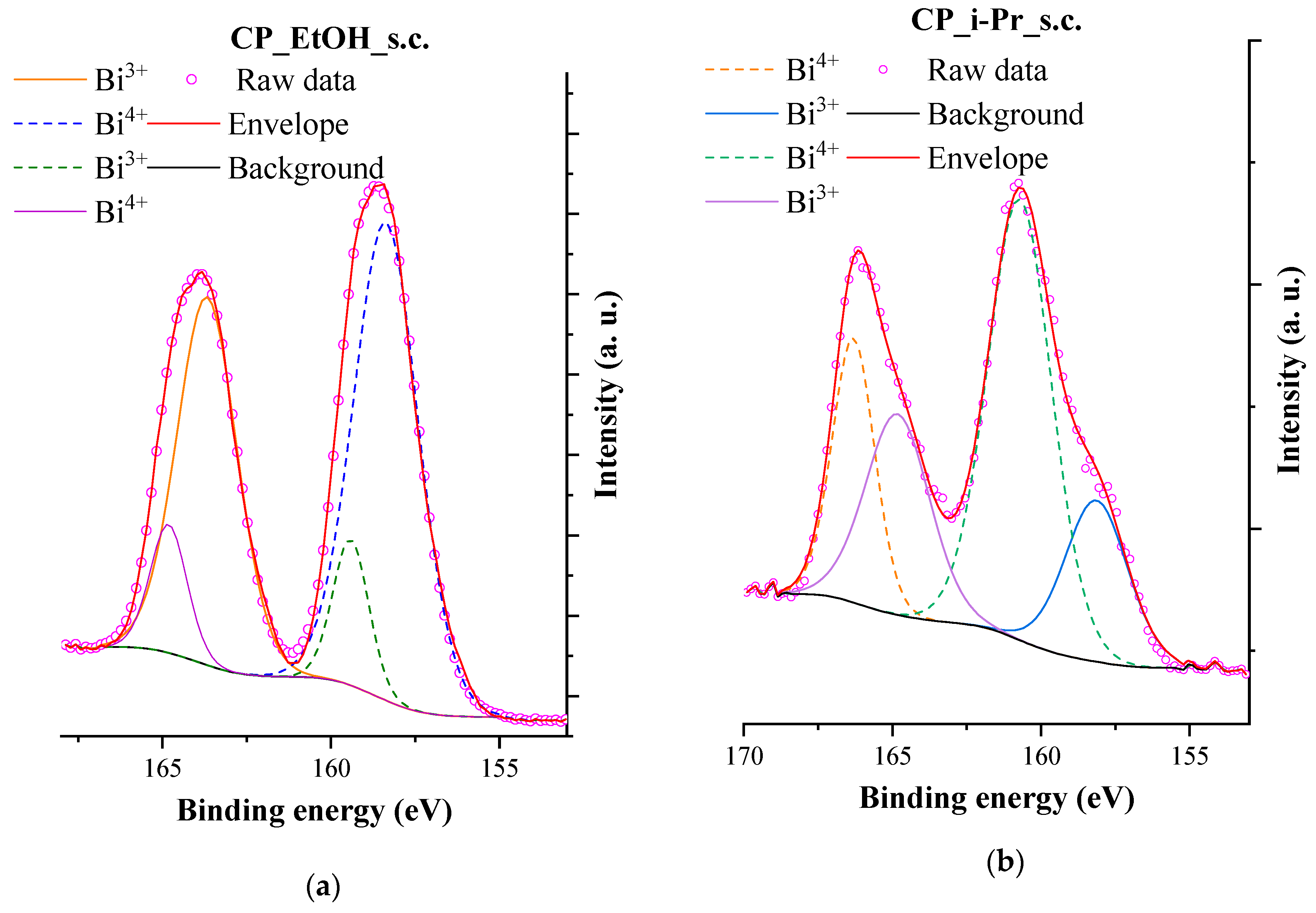
| Sample ID | Particle Size (nm) | Immobilized BiOI (mg) | Surface Coverage (%) |
|---|---|---|---|
| P_EG_h.t. | 11 | × | × |
| CP_EG_h.t. | 12 | 240 | 90.5% |
| CP_MQ_s.c. | 21 | 50 | 82.7% |
| CP_i-Pr_s.c. | 11 | 130 | 70.4% |
| CP_EtOH_s.c. | 23 | 180 | 73.7% |
| CP_EtOH+MQ_s.c. | 21 | 440 | 99.6% |
| CP_EtOH+EG_s.c. | 21 | 80 | 59.3% |
| Sample ID | Iodine (atomic %) | Bismuth (atomic %) |
|---|---|---|
| P_EG_h.t. | 12.50 | 14.49 |
| CP_EG_h.t. | 2.67 | 2.86 |
| CP_MQ_s.c. | 0.78 | 0.84 |
| CP_i-Pr_s.c. | 1.83 | 1.76 |
| CP_EtOH_s.c. | 4.03 | 4.78 |
| CP_EtOH+MQ_s.c. | 3.63 | 4.17 |
| CP_EtOH+EG_s.c. | 3.23 | 3.21 |
| Sample Name | Reaction Rate kUV (min−1) | Reaction Rate kVis (min−1) | UV Degraded RhB/BiOI (µg/mg) | Vis Degraded RhB/BiOI (µg/mg) |
|---|---|---|---|---|
| CP_EG_h.t. | 1.0 × 10−3 | 9.2 × 10−3 | 11 | 17 |
| CP_MQ_s.c. | 7.9 × 10−3 | 4.7 × 10−3 | 34 | 40 |
| CP_i-Pr_s.c. | 9.2 × 10−3 | 10.8 × 10−3 | 13 | 21 |
| CP_EtOH_s.c. | 8.0 × 10−3 | 7.4 × 10−3 | 9 | 13 |
| CP_EtOH+MQ_s.c. | 6.1 × 10−3 | 7.6 × 10−3 | 5 | 6 |
| CP_EtOH+EG_s.c. | 7.2 × 10−3 | 3.2 × 10−3 | 26 | 17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kása, Z.; Orbán, E.; Pap, Z.; Ábrahám, I.; Magyari, K.; Garg, S.; Hernadi, K. Innovative and Cost-Efficient BiOI Immobilization Technique on Ceramic Paper—Total Coverage and High Photocatalytic Activity. Nanomaterials 2020, 10, 1959. https://doi.org/10.3390/nano10101959
Kása Z, Orbán E, Pap Z, Ábrahám I, Magyari K, Garg S, Hernadi K. Innovative and Cost-Efficient BiOI Immobilization Technique on Ceramic Paper—Total Coverage and High Photocatalytic Activity. Nanomaterials. 2020; 10(10):1959. https://doi.org/10.3390/nano10101959
Chicago/Turabian StyleKása, Zsolt, Eszter Orbán, Zsolt Pap, Imre Ábrahám, Klára Magyari, Seema Garg, and Klara Hernadi. 2020. "Innovative and Cost-Efficient BiOI Immobilization Technique on Ceramic Paper—Total Coverage and High Photocatalytic Activity" Nanomaterials 10, no. 10: 1959. https://doi.org/10.3390/nano10101959