Synthesis and Characterization of ZnO-TiO2/Carbon Fiber Composite with Enhanced Photocatalytic Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of TiO2-CNFs Composite
2.3. Fabrication of ZnO-TiO2-CNFs Composite
2.4. Characterization
2.5. Phtocatalytic Degradation of MB
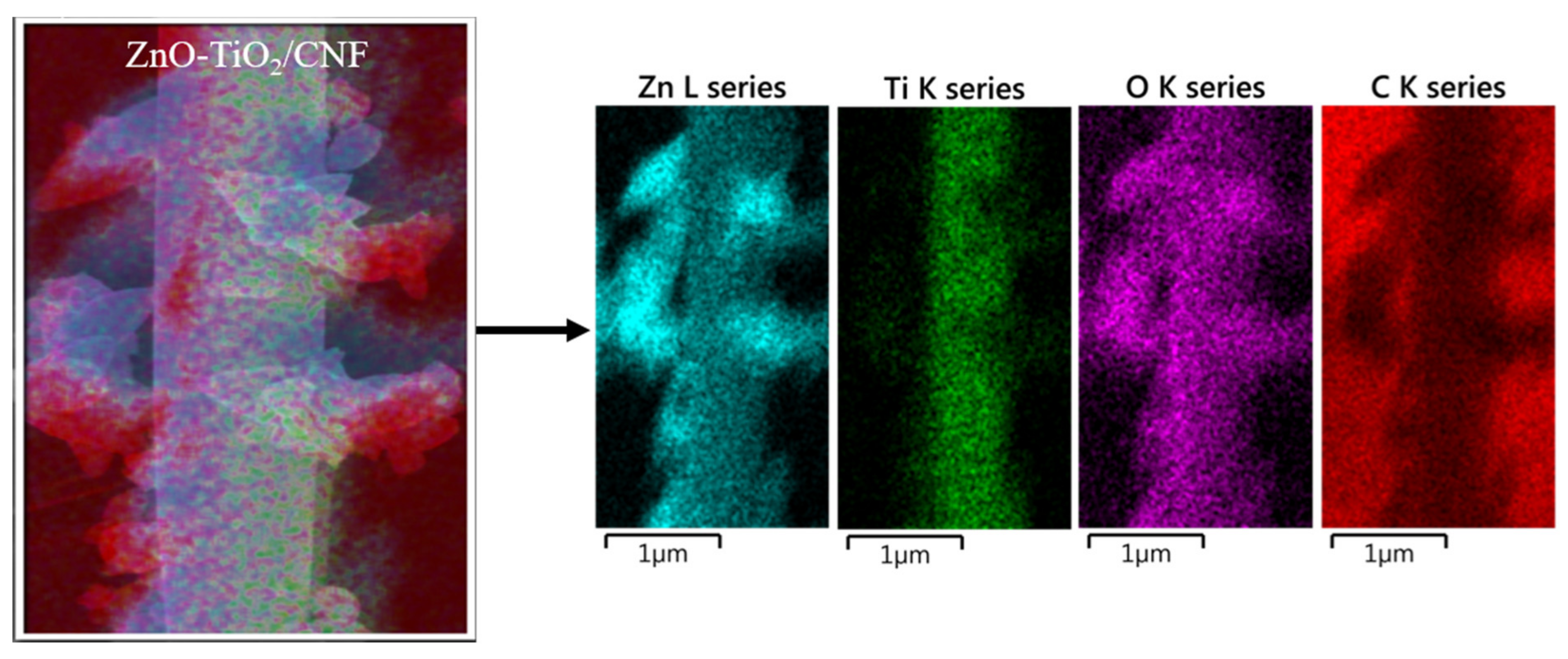
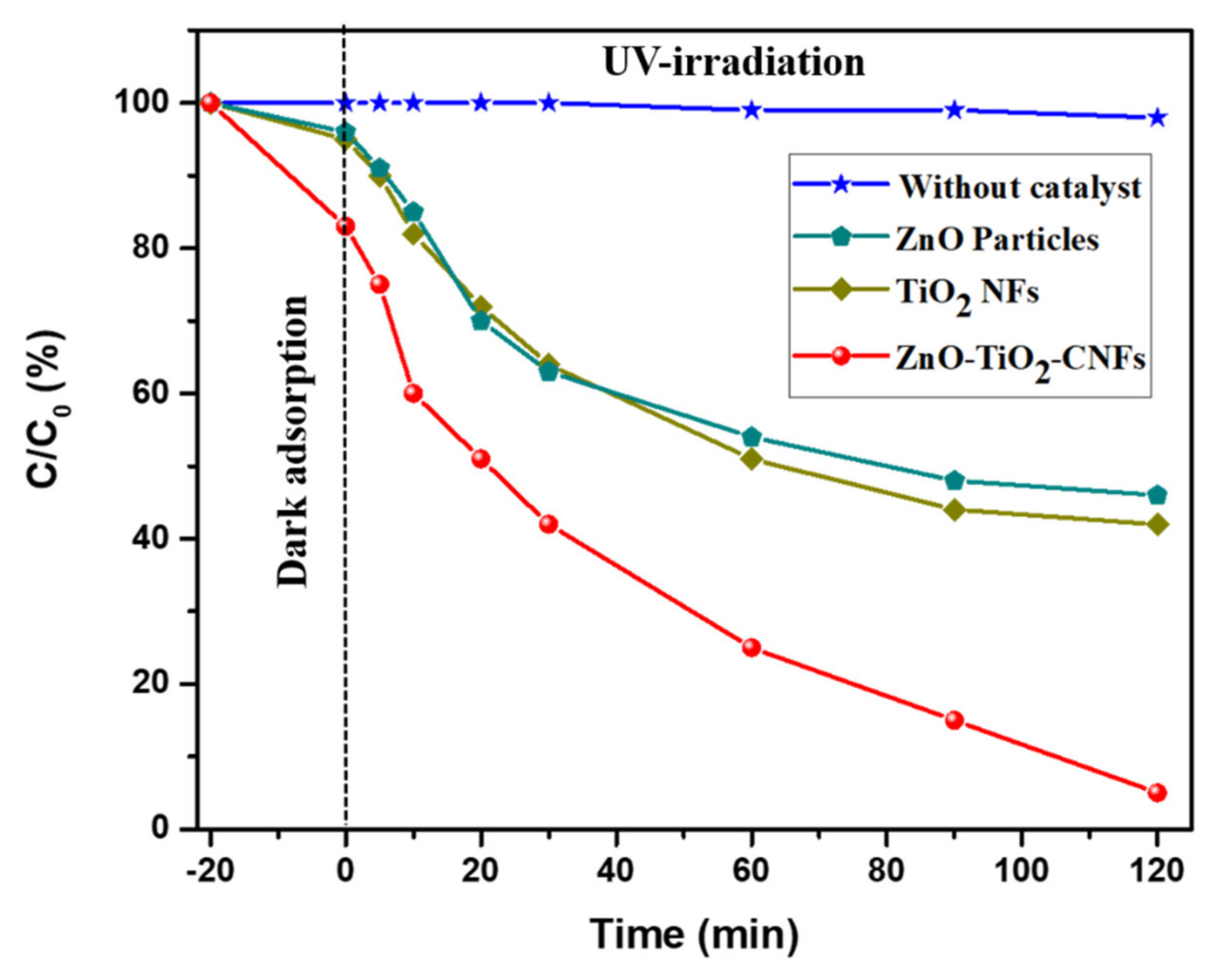
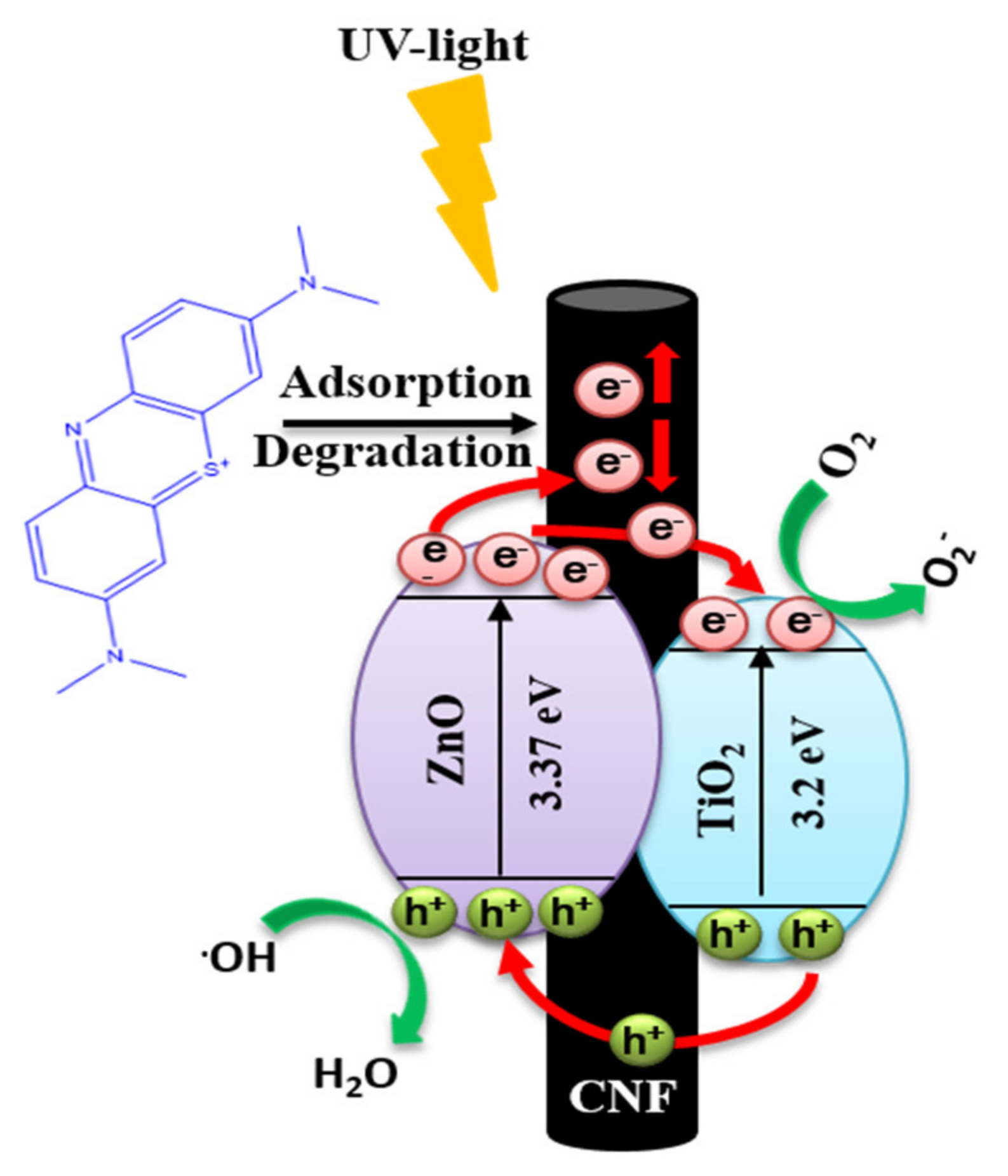
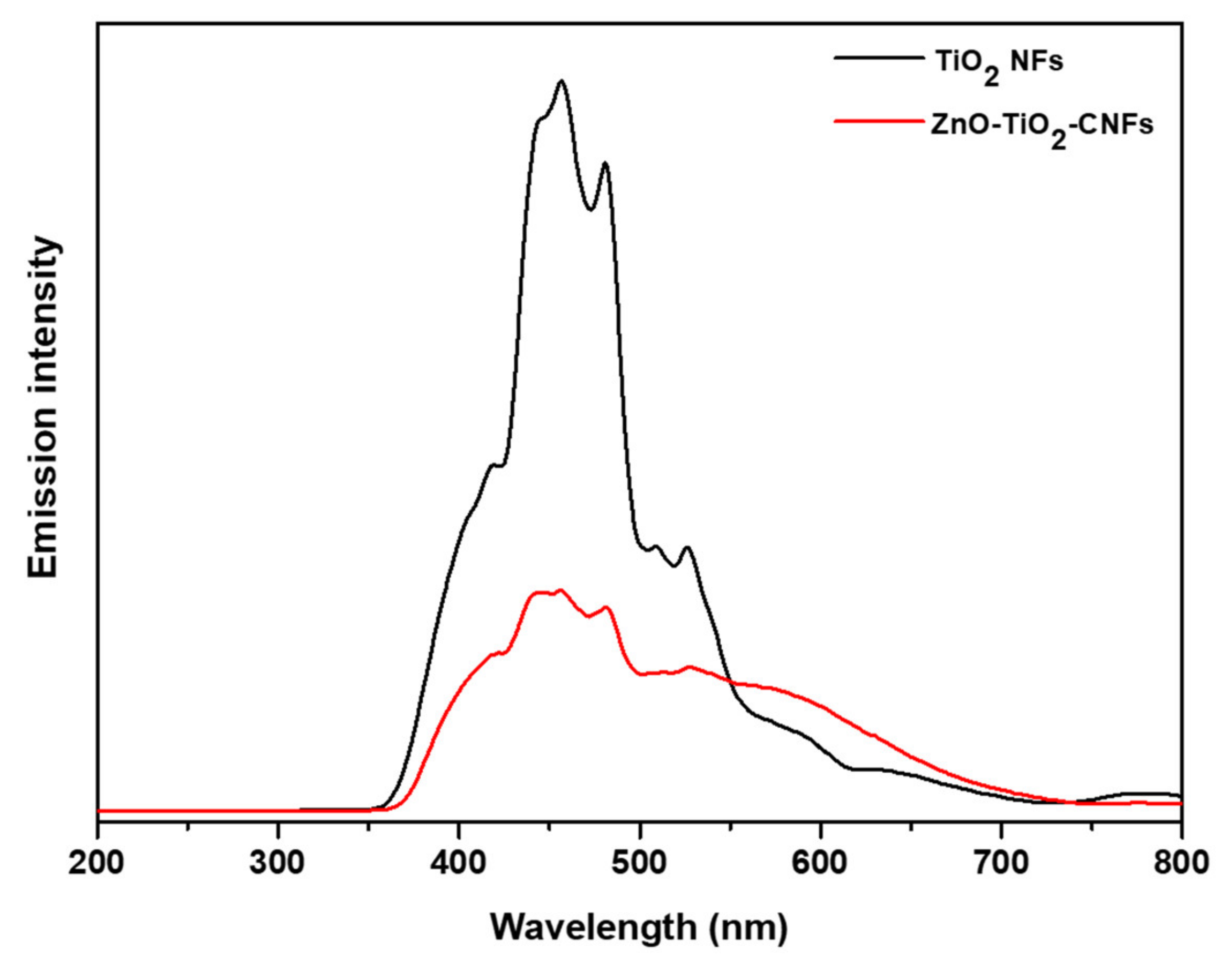
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pant, B.; Park, M.; Park, S.-J. Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef] [Green Version]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Pirilä, M.; Saouabe, M.; Ojala, S.; Rathnayake, B.; Drault, F.; Valtanen, A.; Huuhtanen, M.; Brahmi, R.; Keiski, R.L. Photocatalytic Degradation of Organic Pollutants in Wastewater. Top. Catal. 2015, 58, 1085–1099. [Google Scholar] [CrossRef]
- Zhu, S.; Wang, D. Photocatalysis: Basic Principles, Diverse Forms of Implementations and Emerging Scientific Opportunities. Adv. Energy Mater. 2017, 7, 1700841. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zou, D.; Ma, T.; Liu, Z.; Li, Y. Simultaneous efficient adsorption and accelerated photocatalytic degradation of chlortetracycline hydrochloride over novel Fe-based MOGs under visible light irradiation assisted by hydrogen peroxide. Inorg. Chem. Front. 2019, 6, 1388–1397. [Google Scholar] [CrossRef]
- Pant, B.; Ojha, G.P.; Kim, H.-Y.; Park, M.; Park, S.-J. Fly-ash-incorporated electrospun zinc oxide nanofibers: Potential material for environmental remediation. Environ. Pollut. 2019, 245, 163–172. [Google Scholar] [CrossRef]
- Serpone, N.; Emeline, A.V. Semiconductor Photocatalysis—Past, Present, and Future Outlook. J. Phys. Chem. Lett. 2012, 3, 673–677. [Google Scholar] [CrossRef]
- Mills, A.; Le Hunte, S. An overview of semiconductor photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 108, 1–35. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Xu, D. Recent Progress in Semiconductor-Based Nanocomposite Photocatalysts for Solar-to-Chemical Energy Conversion. Adv. Energy Mater. 2017, 7, 1700529. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Perez, A.; Persson, K.M.; Samuelson, L. Semiconductor Eco-Materials for Water Treatment. In Handbook of Ecomaterials; Martínez, L.M.T., Kharissova, O.V., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–27. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor Composites: Strategies for Enhancing Charge Carrier Separation to Improve Photocatalytic Activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Pant, B.; Pant, H.R.; Barakat, N.A.M.; Park, M.; Jeon, K.; Choi, Y.; Kim, H.-Y. Carbon nanofibers decorated with binary semiconductor (TiO2/ZnO) nanocomposites for the effective removal of organic pollutants and the enhancement of antibacterial activities. Ceram. Int. 2013, 39, 7029–7035. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Kim, H.-Y.; Park, S.-J. Ag-ZnO photocatalyst anchored on carbon nanofibers: Synthesis, characterization, and photocatalytic activities. Synth. Met. 2016, 220, 533–537. [Google Scholar] [CrossRef]
- Liu, L.; Ou, H.; Hong, K.; Wang, L. Evidence of a strong electron–hole separation effect in ZnO@TiO2 core/shell nanowires. J. Alloy. Compd. 2018, 749, 217–220. [Google Scholar] [CrossRef]
- Sakthivel, S.; Neppolian, B.; Shankar, M.V.; Arabindoo, B.; Palanichamy, M.; Murugesan, V. Solar photocatalytic degradation of azo dye: Comparison of photocatalytic efficiency of ZnO and TiO2. Sol. Energy Mater. Sol. Cells 2003, 77, 65–82. [Google Scholar] [CrossRef]
- Dong, H.; Zeng, G.; Tang, L.; Fan, C.; Zhang, C.; He, X.; He, Y. An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res. 2015, 79, 128–146. [Google Scholar] [CrossRef]
- Johar, M.A.; Afzal, R.A.; Alazba, A.A.; Manzoor, U. Photocatalysis and Bandgap Engineering Using ZnO Nanocomposites. Adv. Mater. Sci. Eng. 2015, 2015, 934587. [Google Scholar] [CrossRef] [Green Version]
- Hernández, S.; Hidalgo, D.; Sacco, A.; Chiodoni, A.; Lamberti, A.; Cauda, V.; Tresso, E.; Saracco, G. Comparison of photocatalytic and transport properties of TiO2 and ZnO nanostructures for solar-driven water splitting. Phys. Chem. Chem. Phys. 2015, 17, 7775–7786. [Google Scholar] [CrossRef] [Green Version]
- Pant, B.; Barakat, N.A.M.; Pant, H.R.; Park, M.; Saud, P.S.; Kim, J.-W.; Kim, H.-Y. Synthesis and photocatalytic activities of CdS/TiO2 nanoparticles supported on carbon nanofibers for high efficient adsorption and simultaneous decomposition of organic dyes. J. Colloid Interface Sci. 2014, 434, 159–166. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, X.; Wang, X.; Liu, N.; Liu, X.; Hou, B. Study on the Photocathodic Protection of Q235 Steel by CdIn2S4 Sensitized TiO2 Composite in Splash Zone. Catalysts 2019, 9, 1067. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Yang, J.; Xu, S.; Zhou, Y.; Wang, X.; Peng, H.; Du, J. Preparation of Gd-Doped TiO2 Nanotube Arrays by Anodization Method and Its Photocatalytic Activity for Methyl Orange Degradation. Catalysts 2020, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, M.; Chassagnon, R.; Heintz, O.; Geoffroy, N.; Skompska, M.; Bezverkhyy, I. Improvement of photocatalytic and photoelectrochemical activity of ZnO/TiO2 core/shell system through additional calcination: Insight into the mechanism. Appl. Catal. B Environ. 2017, 204, 200–208. [Google Scholar] [CrossRef]
- Liu, R.; Ye, H.; Xiong, X.; Liu, H. Fabrication of TiO2/ZnO composite nanofibers by electrospinning and their photocatalytic property. Mater. Chem. Phys. 2010, 121, 432–439. [Google Scholar] [CrossRef]
- Pan, L.; Shen, G.-Q.; Zhang, J.-W.; Wei, X.-C.; Wang, L.; Zou, J.-J.; Zhang, X. TiO2–ZnO Composite Sphere Decorated with ZnO Clusters for Effective Charge Isolation in Photocatalysis. Ind. Eng. Chem. Res. 2015, 54, 7226–7232. [Google Scholar] [CrossRef]
- Qin, R.; Meng, F.; Khan, M.W.; Yu, B.; Li, H.; Fan, Z.; Gong, J. Fabrication and enhanced photocatalytic property of TiO2-ZnO composite photocatalysts. Mater. Lett. 2019, 240, 84–87. [Google Scholar] [CrossRef]
- Pei, C.C.; Leung, W.W.-F. Enhanced photocatalytic activity of electrospun TiO2/ZnO nanofibers with optimal anatase/rutile ratio. Catal. Commun. 2013, 37, 100–104. [Google Scholar] [CrossRef]
- Krishna, S.; Sathishkumar, P.; Pugazhenthiran, N.; Guesh, K.; Mangalaraja, R.V.; Kumaran, S.; Gracia-Pinilla, M.A.; Anandan, S. Magnetically recyclable CoFe2O4/ZnO nanocatalysts for the efficient catalytic degradation of Acid Blue 113 under ambient conditions. Rsc Adv. 2020, 10, 16473–16480. [Google Scholar] [CrossRef]
- Jassby, D.; Farner Budarz, J.; Wiesner, M. Impact of Aggregate Size and Structure on the Photocatalytic Properties of TiO2 and ZnO Nanoparticles. Environ. Sci. Technol. 2012, 46, 6934–6941. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. MoS2/CdS/TiO2 ternary composite incorporated into carbon nanofibers for the removal of organic pollutants from water. Inorg. Chem. Commun. 2019, 102, 113–119. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Lee, J.H.; Kim, H.-Y.; Park, S.-J. Novel magnetically separable silver-iron oxide nanoparticles decorated graphitic carbon nitride nano-sheets: A multifunctional photocatalyst via one-step hydrothermal process. J. Colloid Interface Sci. 2017, 496, 343–352. [Google Scholar] [CrossRef]
- Pant, B.; Saud, P.S.; Park, M.; Park, S.-J.; Kim, H.-Y. General one-pot strategy to prepare Ag–TiO2 decorated reduced graphene oxide nanocomposites for chemical and biological disinfectant. J. Alloy. Compd. 2016, 671, 51–59. [Google Scholar] [CrossRef]
- Mu, J.; Shao, C.; Guo, Z.; Zhang, Z.; Zhang, M.; Zhang, P.; Chen, B.; Liu, Y. High Photocatalytic Activity of ZnO−Carbon Nanofiber Heteroarchitectures. ACS Appl. Mater. Interfaces 2011, 3, 590–596. [Google Scholar] [CrossRef]
- Gu, C.; Xiong, S.; Zhong, Z.; Wang, Y.; Xing, W. A promising carbon fiber-based photocatalyst with hierarchical structure for dye degradation. Rsc Adv. 2017, 7, 22234–22242. [Google Scholar] [CrossRef] [Green Version]
- Marks, S.D.; Riascos-Rodriguez, K.; Arrieta-Pérez, R.R.; Yakovenko, A.A.; Exley, J.; Evans, P.G.; Hernández-Maldonado, A.J. Lattice expansion and ligand twist during CO2 adsorption in flexible Cu bipyridine metal–organic frameworks. J. Mater. Chem. A 2020. [Google Scholar] [CrossRef]
- Mondal, K. Recent Advances in the Synthesis of Metal Oxide Nanofibers and Their Environmental Remediation Applications. Inventions 2017, 2, 9. [Google Scholar] [CrossRef]
- Einert, M.; Weller, T.; Leichtweiß, T.; Smarsly, B.M.; Marschall, R. Electrospun CuO Nanofibers: Stable Nanostructures for Solar Water Splitting. ChemPhotoChem 2017, 1, 326–340. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Einert, M.; Ostermann, R.; Weller, T.; Zellmer, S.; Garnweitner, G.; Smarsly, B.M.; Marschall, R. Hollow α-Fe2O3 nanofibres for solar water oxidation: Improving the photoelectrochemical performance by formation of α-Fe2O3/ITO-composite photoanodes. J. Mater. Chem. A 2016, 4, 18444–18456. [Google Scholar] [CrossRef] [Green Version]
- Pant, B.; Park, M.; Park, S.-J. TiO2 NPs Assembled into a Carbon Nanofiber Composite Electrode by a One-Step Electrospinning Process for Supercapacitor Applications. Polymers 2019, 11, 899. [Google Scholar] [CrossRef] [Green Version]
- Nain, R.; Singh, D.; Jassal, M.; Agrawal, A.K. Zinc oxide nanorod assisted rapid single-step process for the conversion of electrospun poly(acrylonitrile) nanofibers to carbon nanofibers with a high graphitic content. Nanoscale 2016, 8, 4360–4372. [Google Scholar] [CrossRef]
- Ranjith, K.S.; Uyar, T. ZnO–TiO2 composites and ternary ZnTiO3 electrospun nanofibers: The influence of annealing on the photocatalytic response and reusable functionality. CrystEngComm 2018, 20, 5801–5813. [Google Scholar] [CrossRef] [Green Version]
- Roongraung, K.; Chuangchote, S.; Laosiripojana, N.; Sagawa, T. Electrospun Ag-TiO2 Nanofibers for Photocatalytic Glucose Conversion to High-Value Chemicals. Acs Omega 2020, 5, 5862–5872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Xia, Y. Fabrication of Titania Nanofibers by Electrospinning. Nano Lett. 2003, 3, 555–560. [Google Scholar] [CrossRef]
- Wang, H.; Huang, X.; Li, W.; Gao, J.; Xue, H.; Li, R.K.Y.; Mai, Y.-W. TiO2 nanoparticle decorated carbon nanofibers for removal of organic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2018, 549, 205–211. [Google Scholar] [CrossRef]
- Chang, T.-H.; Lu, Y.-C.; Yang, M.-J.; Huang, J.-W.; Linda Chang, P.-F.; Hsueh, H.-Y. Multibranched flower-like ZnO particles from eco-friendly hydrothermal synthesis as green antimicrobials in agriculture. J. Clean. Prod. 2020, 262, 121342. [Google Scholar] [CrossRef]
- Teng, M.; Qiao, J.; Li, F.; Bera, P.K. Electrospun mesoporous carbon nanofibers produced from phenolic resin and their use in the adsorption of large dye molecules. Carbon 2012, 50, 2877–2886. [Google Scholar] [CrossRef]
- Reinosa, J.J.; Docio, C.M.Á.; Ramírez, V.Z.; Lozano, J.F.F. Hierarchical nano ZnO-micro TiO2 composites: High UV protection yield lowering photodegradation in sunscreens. Ceram. Int. 2018, 44, 2827–2834. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pant, B.; Ojha, G.P.; Kuk, Y.-S.; Kwon, O.H.; Park, Y.W.; Park, M. Synthesis and Characterization of ZnO-TiO2/Carbon Fiber Composite with Enhanced Photocatalytic Properties. Nanomaterials 2020, 10, 1960. https://doi.org/10.3390/nano10101960
Pant B, Ojha GP, Kuk Y-S, Kwon OH, Park YW, Park M. Synthesis and Characterization of ZnO-TiO2/Carbon Fiber Composite with Enhanced Photocatalytic Properties. Nanomaterials. 2020; 10(10):1960. https://doi.org/10.3390/nano10101960
Chicago/Turabian StylePant, Bishweshwar, Gunendra Prasad Ojha, Yun-Su Kuk, Oh Hoon Kwon, Yong Wan Park, and Mira Park. 2020. "Synthesis and Characterization of ZnO-TiO2/Carbon Fiber Composite with Enhanced Photocatalytic Properties" Nanomaterials 10, no. 10: 1960. https://doi.org/10.3390/nano10101960





