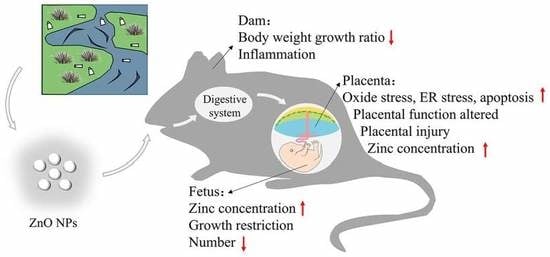
Nano Zinc Oxide Induced Fetal Mice Growth Restriction, Based on Oxide Stress and Endoplasmic Reticulum Stress
Abstract
:1. Introduction
2. Methods
2.1. Characterization of ZnO NPs
2.2. Animals and Treatment
2.3. Inductively Coupled Plasma Atomic Emission Spectrometry (ICP-AES) Analysis of Zn Content
2.4. Histopathological Examination
2.5. Hematological Analysis
2.6. Serum sex Hormone Analysis
2.7. RT-qPCR Analysis
2.8. IHC
2.9. Statistical Analysis
3. Results
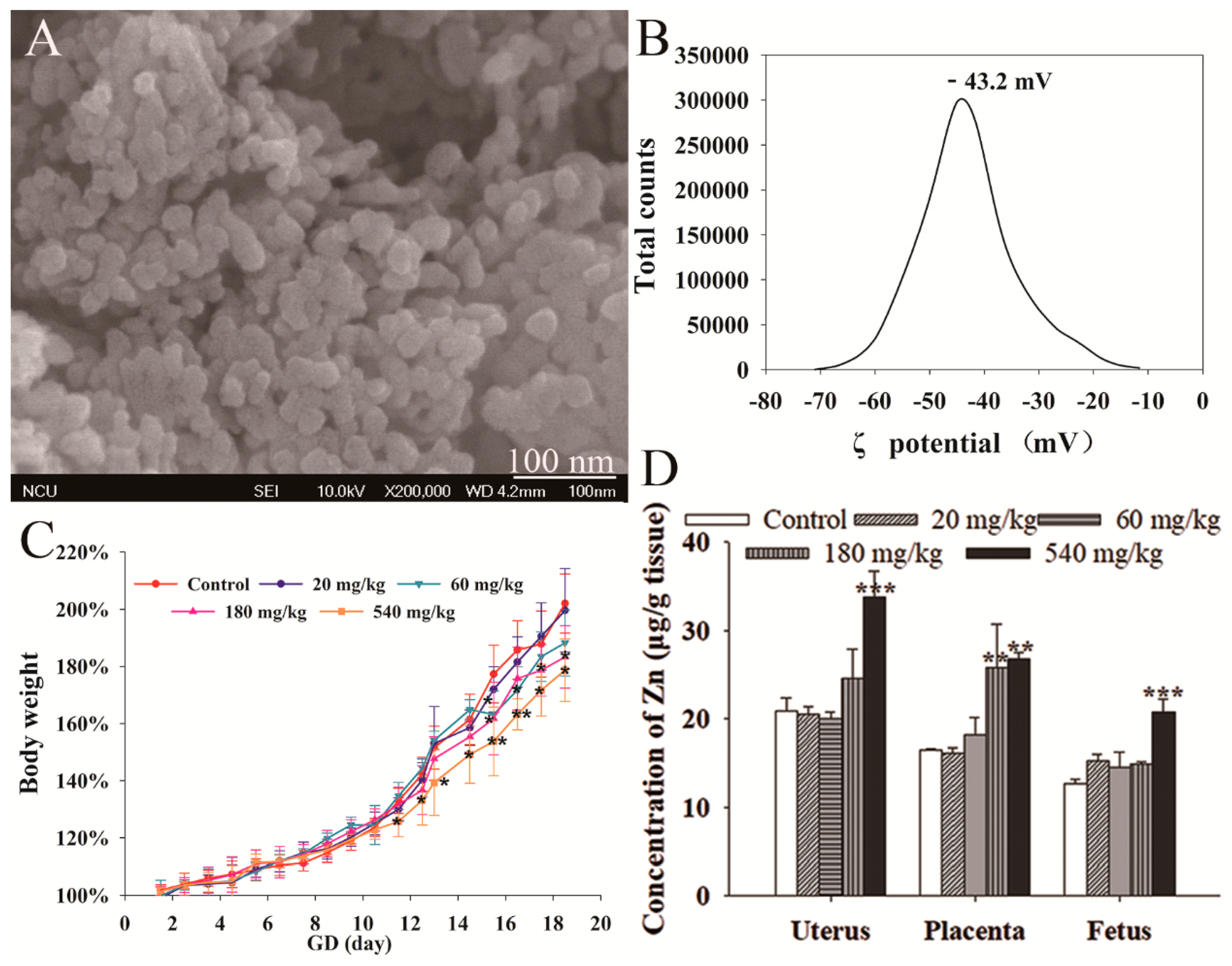
3.1. NPs Characterization
3.2. Maternal Effect of ZnO NPs
3.3. ZnO NPs Distribution
3.4. Fetal Development
3.5. Placental Histological Analysis
3.6. RT-qPCR Analysis
3.7. IHC
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Petchwattana, N.; Covavisaruch, S.; Wibooranawong, S.; Naknaen, P. Antimicrobial food packaging prepared from poly (butylene succinate) and zinc oxide. Measurement 2016, 93, 442–448. [Google Scholar] [CrossRef]
- Keller, A.A.; Vosti, W.; Wang, H.-T.; Lazareva, A. Release of engineered nanomaterials from personal care products throughout their life cycle. J. Nanopart. Res. 2014, 16, 2489. [Google Scholar] [CrossRef]
- Tang, S.; Wang, M.; Germ, K.E.; Du, H.-M.; Sun, W.-J.; Gao, W.-M.; Mayer, G.D. Health implications of engineered nanoparticles in infants and children. World J. Pediat. 2015, 11, 197–206. [Google Scholar] [CrossRef]
- Oliviero, M.; Schiavo, S.; Dumontet, S.; Manzo, S. DNA damages and offspring quality in sea urchin Paracentrotus lividus sperms exposed to ZnO nanoparticles. Sci. Total Environ. 2019, 651, 756–765. [Google Scholar] [CrossRef]
- Kong, T.; Zhang, S.-H.; Zhang, J.-L.; Hao, X.-Q.; Yang, F.; Zhang, C.; Yang, Z.-J.; Zhang, M.-Y.; Wang, J. Acute and cumulative effects of unmodified 50-nm nano-ZnO on mice. Biol. Trace Elem. Res. 2018, 185, 124–134. [Google Scholar] [CrossRef]
- Alimohammadi, S.; Hosseini, M.S.; Behbood, L. Prenatal exposure to zinc oxide nanoparticles can induce depressive-like behaviors in mice offspring. Int. J. Pept. Res. Ther. 2019, 25, 401–409. [Google Scholar] [CrossRef]
- Bai, D.-P.; Zhang, X.-F.; Zhang, G.-L.; Huang, Y.-F.; Gurunathan, S. Zinc oxide nanoparticles induce apoptosis and autophagy in human ovarian cancer cells. Int. J. Nanomed. 2017, 12, 6521. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Leong, W.; Leong, K.W.; Chen, C.-Y.; Zhao, Y.-L. Walking the line: The fate of nanomaterials at biological barriers. Biomaterials 2018, 174, 41–53. [Google Scholar] [CrossRef]
- Vidmar, J.; Loeschner, K.; Correia, M.; Larsen, E.H.; Manser, P.; Wichser, A.; Boodhia, K.; Al-Ahmady, Z.S.; Ruiz, J.; Astruc, D.; et al. Translocation of silver nanoparticles in the ex vivo human placenta perfusion model characterized by single particle ICP-MS. Nanoscale 2018, 10, 11980–11991. [Google Scholar] [CrossRef] [Green Version]
- Hong, J.-S.; Park, M.-K.; Kim, M.-S.; Lim, J.-H.; Park, G.-J.; Maeng, E.-H.; Shin, J.-H.; Kim, Y.-R.; Kim, M.-K.; Lee, J.-K.; et al. Effect of zinc oxide nanoparticles on dams and embryo–fetal development in rats. Int. J. Nanomed. 2014, 9, 145. [Google Scholar]
- Zhao, X.; Ren, X.; Zhu, R.; Luo, Z.; Ren, B. Zinc oxide nanoparticles induce oxidative DNA damage and ROS-triggered mitochondria-mediated apoptosis in zebrafish embryos. Aquat. Toxicol. 2016, 180, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huo, L.; Shi, X.; Bai, R.; Zhang, Z.; Zhao, Y.; Chang, Y.; Chen, C. Endoplasmic reticulum stress induced by zinc oxide nanoparticles is an earlier biomarker for nanotoxicological evaluation. ACS Nano 2014, 8, 2562–2574. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; He, F.; Kuang, L.; Tang, W.; Li, Y.; Che, D. eNOS/iNOS and endoplasmic reticulum stress-induced apoptosis in the placentas of patients with preeclampsia. J. Human Hyperten. 2017, 31, 49. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wang, H.; Xu, Z.M.; Ji, Y.-L.; Chen, Y.-H.; Zhang, Z.-H.; Zhang, C.; Meng, X.-H.; Zhao, M.; Xu, D.-X. Cadmium-induced teratogenicity: Association with ROS-mediated endoplasmic reticulum stress in placenta. Toxicol. Appl. Pharmacol. 2012, 259, 236–247. [Google Scholar] [CrossRef]
- Abraham, T.; Pin, C.L.; Watson, A.J. Embryo collection induces transient activation of XBP1 arm of the ER stress response while embryo vitrification does not. Mol. Human Reprod. 2011, 18, 229–242. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Hong, W.; Zhou, P.; Chen, B.; Xu, H. Nano and bulk ZnO trigger diverse Zn-transport-related gene transcription in distinct regions of the small intestine in mice after oral exposure. Biochem. Biophys. Res. Commun. 2017, 493, 1364–1369. [Google Scholar] [CrossRef]
- Lee, J.; Yu, W.-J.; Song, J.; Sung, C.; Jeong, E.J.; Han, J.-S.; Kim, P.; Jo, E.; Eom, I.; Kim, H.-M.; et al. Developmental toxicity of intravenously injected zinc oxide nanoparticles in rats. Arch. Pharm. Res. 2016, 39, 1682–1692. [Google Scholar] [CrossRef]
- Hao, Y.; Liu, J.; Feng, Y.; Yu, S.; Zhang, W.; Li, L.; Min, L.; Zhang, H.; Shen, W.; Zhao, Y. Molecular evidence of offspring liver dysfunction after maternal exposure to zinc oxide nanoparticles. Toxicol. Appl. Pharm. 2017, 329, 318–325. [Google Scholar] [CrossRef]
- Kinaret, P.; Ilves, M.; Fortino, V.; Rydman, E.; Karisola, P.; a Lähde, A.; Koivisto, J.; Jokiniemi, J.; Wolff, H.; Savolainen, K.; et al. Inhalation and oropharyngeal aspiration exposure to rod-like carbon nanotubes induce similar airway inflammation and biological responses in mouse lungs. ACS Nano 2017, 11, 291–303. [Google Scholar] [CrossRef]
- Paul, E.; Franco-Montoya, M.L.; Paineau, E.; Angeletti, B.; Vibhushan, S.; Ridoux, A.; Tiendrebeogo, A.; Salome, M.; Hesse, B.; Vantelon, D.; et al. Pulmonary exposure to metallic nanomaterials during pregnancy irreversibly impairs lung development of the offspring. Nanotoxicology 2017, 11, 484–495. [Google Scholar] [CrossRef]
- Wang, C.; Lu, J.; Zhou, L.; Li, J.; Xu, J.; Li, W.; Zhang, L.; Zhong, X.; Wang, T. Effects of long-term exposure to zinc oxide nanoparticles on development, zinc metabolism and biodistribution of minerals (Zn, Fe, Cu, Mn) in mice. PLoS ONE 2016, 11, e0164434. [Google Scholar] [CrossRef]
- Perez-Garcia, V.; Fineberg, E.; Wilson, R.; Murray, A.; Mazzeo, C.I.; Tudor, C.; Sienerth, A.; White, J.K.; Tuck, E.; Ryder, E.J.; et al. Placentation defects are highly prevalent in embryonic lethal mouse mutants. Nature 2018, 555, 463. [Google Scholar] [CrossRef]
- Teng, C.; Jia, J.; Wang, Z.; Sharmad, V.K.; Yan, B. Size-dependent maternal-fetal transfer and fetal developmental toxicity of ZnO nanoparticles after oral exposures in pregnant mice. Ecotoxicol. Environ. Saf. 2019, 182, 109439. [Google Scholar] [CrossRef]
- Lin, C.-H.; Yang, M.-H.; Chang, L.-W.; Yang, C.-S.; Chang, H.; Chang, W.-H.; Tsai, M.-H.; Wang, C.-J.; Lin, P. Cd/Se/Te-based quantum dot 705 modulated redox homeostasis with hepatotoxicity in mice. Nanotoxicology 2011, 5, 650–663. [Google Scholar] [CrossRef]
- Sun, Q.; Tan, D.; Zhou, Q.; Liu, X.; Cheng, Z.; Liu, G.; Zhu, M.; Sang, X.; Gui, S.; Cheng, J.; et al. Oxidative damage of lung and its protective mechanism in mice caused by long-term exposure to titanium dioxide nanoparticles. J. Biomed. Mater. Res. A 2012, 100, 2554–2562. [Google Scholar] [CrossRef]
- Nika, J.; Rippel, S.; Hannig, E.M. Biochemical analysis of the eIF2βγ complex reveals a structural function for eIF2α in catalyzed nucleotide exchange. J. Biol. Chem. 2001, 276, 1051–1056. [Google Scholar] [CrossRef] [Green Version]
- Somers, J.; Pöyry, T.; Willis, A.E. A perspective on mammalian upstream open reading frame function. Int. J. Biochem. Cell Biol. 2013, 45, 1690–1700. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Kim, S.B.; Luitel, K.; Shay, J.W. Cholesterol depletion by TASIN-1 induces apoptotic cell death through the ER stress/ROS/JNK signaling in colon cancer cells. Mol. Cancer Ther. 2018, 17, 943–951. [Google Scholar] [CrossRef] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Hong, W.; Yang, P.; Tang, Y.; Zhao, Y.; Aguilar, Z.P.; Xu, H. Nano Zinc Oxide Induced Fetal Mice Growth Restriction, Based on Oxide Stress and Endoplasmic Reticulum Stress. Nanomaterials 2020, 10, 259. https://doi.org/10.3390/nano10020259
Chen B, Hong W, Yang P, Tang Y, Zhao Y, Aguilar ZP, Xu H. Nano Zinc Oxide Induced Fetal Mice Growth Restriction, Based on Oxide Stress and Endoplasmic Reticulum Stress. Nanomaterials. 2020; 10(2):259. https://doi.org/10.3390/nano10020259
Chicago/Turabian StyleChen, Bolu, Wuding Hong, Pengfei Yang, Yizhou Tang, Yu Zhao, Zoraida P. Aguilar, and Hengyi Xu. 2020. "Nano Zinc Oxide Induced Fetal Mice Growth Restriction, Based on Oxide Stress and Endoplasmic Reticulum Stress" Nanomaterials 10, no. 2: 259. https://doi.org/10.3390/nano10020259