Toxicity of TiO2, ZnO, and SiO2 Nanoparticles in Human Lung Cells: Safe-by-Design Development of Construction Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanoparticles and Their Characterization
- Aerosil® SiO2 A200 (hereinafter referred to as SiO2), which is a nanopowder with a surface area of 220 m2 g−1 and particle size of 12 nm (both specifications are that provided by the manufacturer).
- Aerosil® R9200 (hereinafter referred to as SiO2–methyl), which is a nanopowder of SiO2 with a methylated surface, its surface area and particle size were 150–190 m2 g−1 and 12 nm (as stated by the manufacturer), respectively.
- Aerosil® R805 (hereinafter referred to as SiO2–octyl), which is a nanopowder of SiO2 with an octylated surface, its surface area and particle size was 125–175 m2 g−1 and 12 nm (as stated by the manufacturer), respectively.
- Aeroxide® TiO2 P25 (hereinafter referred to as TiO2), which is a photocatalyst that is widely used, owing to its high activity in many photocatalytic reactions. It contains more than 70% of the anatase phase with a minor proportion of rutile (about 20%) and a small percentage of the amorphous phase [17]. It exhibits a specific surface area in the range of 35–65 m2 g−1 and a particle size of 25 nm (as stated by the manufacturer).
- NanoZnO (hereinafter referred to as ZnO), which is a photocatalyst with a surface area and particle size of 90–110 m2 g−1 and 17 nm (as stated by the manufacturer), respectively.
2.2. Preparation of the Nanoparticle Dispersions for Cytotoxicity Testing
2.3. Cell Cultivation and Exposure to the Nanoparticles
2.4. Cytotoxicity Testing
2.5. Data Analysis and Statistics
2.6. Nanoparticle Uptake by the Cells Determined by Transmission Electron Microscopy
2.7. Preparation of the Nanoparticle-Modified Consolidants
2.8. Evaluation of the Consolidation Effect
3. Results and Discussion
3.1. Physico-Chemical Properties of the Nanoparticles
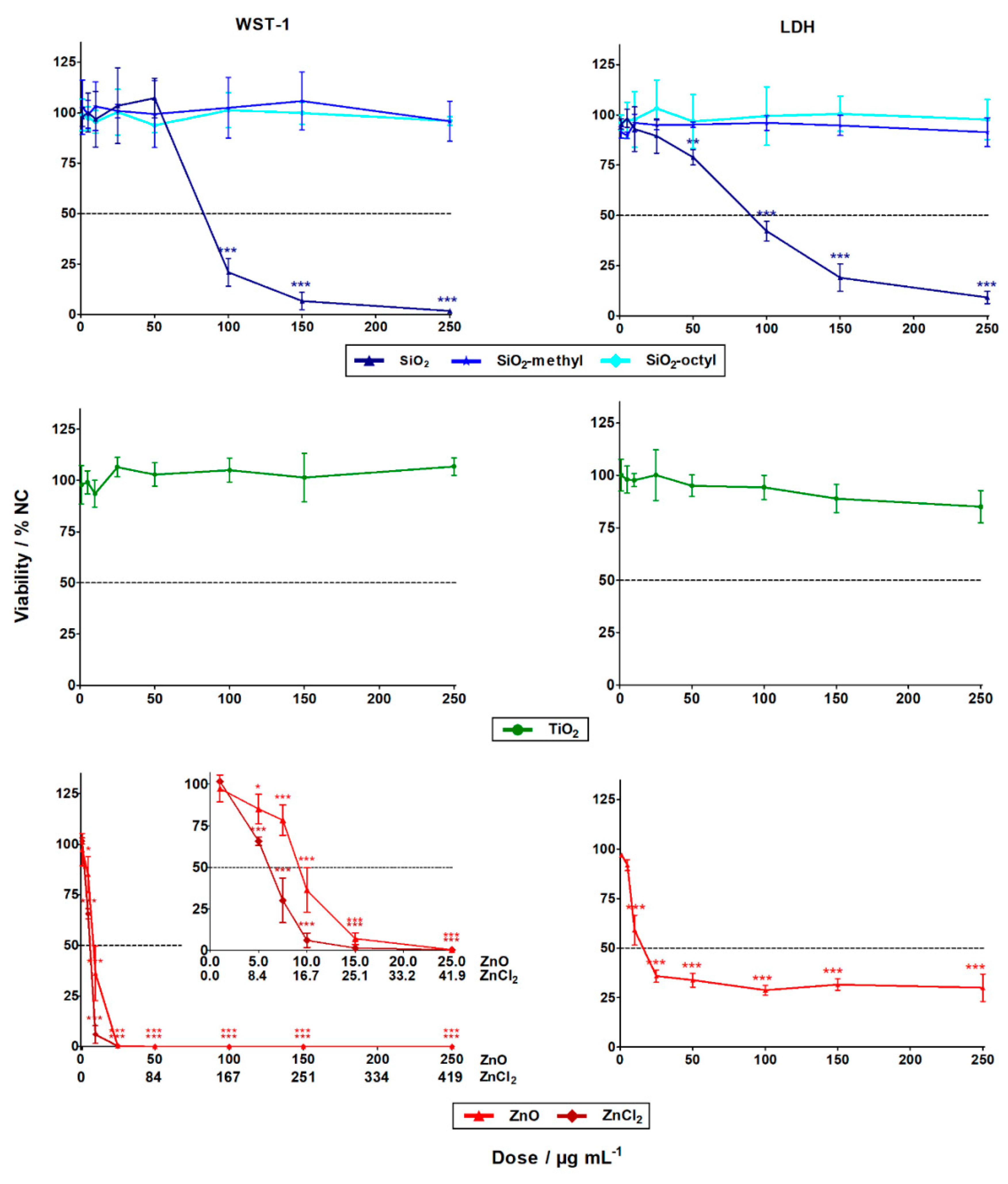
3.2. Evaluation of the Nanoparticles Cytotoxicity
3.3. Nanoparticles Uptake by The Lung Cells
3.4. Improved Strength of Sandstone by Nanoparticle-Modified Consolidants
3.5. Study Significance and Limitations
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hornyak, G.; Tibbals, H.; Dutta, J.; Moore, J. Introduction to Nanoscience and Nanotechnology, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 847–848. ISBN 9781420047806. [Google Scholar]
- Miliani, C.; Velo-Simpson, M.L.; Scherer, G.W. Particle-modified consolidants: A study on the effect of particles on sol-gel properties and consolidation effectiveness. J. Cult. Herit. 2007, 8, 1–6. [Google Scholar] [CrossRef]
- Kislov, N.; Lahiri, J.; Verma, H.; Goswami, D.Y.; Stefanakos, E.; Batzill, M. Photocatalytic degradation of methyl orange over single crystalline ZnO: Orientation dependence of photoactivity and photostability of ZnO. Langmuir 2009, 25, 3310–3315. [Google Scholar] [CrossRef] [PubMed]
- Guérin, V.-M.; Zouzelka, R.; Bibova-Lipsova, H.; Jirkovsky, J.; Rathousky, J.; Pauporté, T. Experimental and DFT study of the degradation of 4-chlorophenol on hierarchical micro-/nanostructured oxide films. Appl. Catal. B Environ. 2015, 168–169, 132–140. [Google Scholar] [CrossRef]
- Zouzelka, R.; Kusumawati, Y.; Remzova, M.; Rathousky, J.; Pauporte, T. Photocatalytic activity of porous multiwalled carbon nanotube-TiO2 composite layers for pollutant degradation. J. Hazard. Mater. 2016, 317, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Zouzelka, R.; Rathousky, J. Photocatalytic abatement of NOx pollutants in the air using commercial functional coating with porous morphology. Appl. Catal. B Environ. 2017, 217, 466–476. [Google Scholar] [CrossRef]
- Zouzelka, R.; Remzova, M.; Brabec, L.; Rathousky, J. Photocatalytic performance of porous TiO2 layers prepared by quantitative electrophoretic deposition from organic solvents. Appl. Catal. B Environ. 2018, 227, 70–78. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Sun, Z.; Duan, J.; Tian, L.; Ho, K.; Zhou, X.; Liu, X.; Yu, Y. Silica nanoparticles induce autophagy and autophagic cell death in HepG2 cells triggered by reactive oxygen species. J. Hazard. Mater. 2014, 270, 176–186. [Google Scholar]
- Xu, Y.; Wang, N.; Yu, Y.; Li, Y.; Li, Y.B.; Yu, Y.B.; Zhou, X.Q.; Sun, Z.W. Exposure to Silica Nanoparticles Causes Reversible Damage of the Spermatogenic Process in Mice. PLoS ONE 2014, 9, 1–11. [Google Scholar] [CrossRef]
- Nemmar, A.; Beegam, S.; Yuvaraju, P.; Yasin, J.; Attoub, S.; Ali, B.; Albarwani, S. Amorphous silica nanoparticles impair vascular homeostasis and induce systemic inflammation. Int. J. Nanomedicine 2014, 9, 2779. [Google Scholar] [CrossRef]
- Stark, W.J. Nanoparticles in biological systems. Angew. Chemie—Int. Ed. 2011, 50, 1242–1258. [Google Scholar] [CrossRef]
- Krug, H.F.; Wick, P. Nanotoxicology: An interdisciplinary challenge. Angew. Chemie—Int. Ed. 2011, 50, 1260–1278. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Wu, C.; Jiang, H.; Li, Q.; Wang, X.; Chen, B. Synergistic cytotoxic effect of different sized ZnO nanoparticles and daunorubicin against leukemia cancer cells under UV irradiation. J. Photochem. Photobiol. B Biol. 2008, 93, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ekstrand-Hammarström, B.; Akfur, C.M.; Andersson, P.O.; Lejon, C.; Österlund, L.; Bucht, A. Human primary bronchial epithelial cells respond differently to titanium dioxide nanoparticles than the lung epithelial cell lines A549 and BEAS-2B. Nanotoxicology 2012, 6, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Clemente, Z.; Castro, V.L.S.S.; Moura, M.A.M.; Jonsson, C.M.; Fraceto, L.F. Toxicity assessment of TiO2 nanoparticles in zebrafish embryos under different exposure conditions. Aquat. Toxicol. 2014, 147, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Zouzelka, R.; Cihakova, P.; Rihova Ambrozova, J.; Rathousky, J. Combined biocidal action of silver nanoparticles and ions against Chlorococcales (Scenedesmus quadricauda, Chlorella vulgaris) and filamentous algae (Klebsormidium sp.). Environ. Sci. Pollut. Res. 2016, 23, 8317–8326. [Google Scholar] [CrossRef] [PubMed]
- Ohtani, B.; Li, D.; Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef]
- Ritz, C.; Streibig, J.C. Bioassay Analysis using R. J. Stat. Softw. 2015, 12, e0146021. [Google Scholar]
- Li, L.; Yan, J.; Wang, T.; Zhao, Z.-J.; Zhang, J.; Gong, J.; Guan, N. Sub-10 nm rutile titanium dioxide nanoparticles for efficient visible-light-driven photocatalytic hydrogen production. Nat. Commun. 2015, 6, 5881. [Google Scholar] [CrossRef]
- Arrouvel, C.; Digne, M.; Breysse, M.; Toulhoat, H.; Raybaud, P. Effects of morphology on surface hydroxyl concentration: A DFT comparison of anatase-TiO2 and γ-alumina catalytic supports. J. Catal. 2004, 222, 152–166. [Google Scholar] [CrossRef]
- Tedesco, E.; Mičetić, I.; Micheletti, C.; Ciappellano, S.G.; Benetti, F.; Venturini, M. Cytotoxicity and antibacterial activity of a new generation of nanoparticle-based consolidants for restoration and contribution to the safe-by-design implementation. Toxicol. Vitr. 2015, 29, 1736–1744. [Google Scholar] [CrossRef]
- Lison, D.; Martens, J.A.; Hoet, P.H.; Napierska, D.; Thomassen, L.C. The nanosilica hazard: another variable entity. Part. Fibre Toxicol. 2010, 7, 39. [Google Scholar]
- Fruijtier-Pölloth, C. The toxicological mode of action and the safety of synthetic amorphous silica-A nanostructured material. Toxicology 2012, 294, 61–79. [Google Scholar] [CrossRef] [PubMed]
- Oh, W.; Kim, S.; Choi, M.; Kim, C.; Jeong, Y.S.; Cho, B.; Hahn, J.; Jang, J. Cellular uptake, cytotoxicity, and innate immune response of silica− titania hollow nanoparticles based on size and surface functionality. ACS Nano 2010, 4, 5301–5313. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.S.; Duffin, R.; Howie, S.E.M.; Scotton, C.J.; Wallace, W.A.H.; MacNee, W.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Amara, S.; Slama, I.B.; Mrad, I.; Rihane, N.; Khemissi, W.; El Mir, L.; Rhouma, K.B.; Abdelmelek, H.; Sakly, M. Effects of zinc oxide nanoparticles and/or zinc chloride on biochemical parameters and mineral levels in rat liver and kidney. Hum. Exp. Toxicol. 2014, 33, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Wätjen, W.; Haase, H.; Biagioli, M.; Beyersmann, D. Induction of apoptosis in mammalian cells by cadmium and zinc. Environ. Health Perspect. 2002, 110, 865–867. [Google Scholar]
- Velintine, V.A.; Wee, B.S.; Chin, S.F.; Kok, K.Y. Transformation of zinc oxide nanoparticles under environmentally relevant conditions: influence of pH and ionic strength. Trans. Sci. Technol. 2017, 4, 123–136. [Google Scholar]
- Remzova, M.; Sasek, P.; Frankeova, D.; Slizkova, Z.; Rathousky, J. Effect of modified ethylsilicate consolidants on the mechanical properties of sandstone. Constr. Build. Mater. 2016, 112, 674–681. [Google Scholar] [CrossRef]




| Consolidant | Catalyst |
|---|---|
| KSE OH (commercial reference) | dibutyltin dilaurate |
| SiGel SiO2 | n-octylamine |
| SiGel SiO2-methyl | n-octylamine |
| SiGel SiO2-octyl | n-octylamine |
| SiGel TiO2 | n-octylamine |
| SiGel ZnO | n-octylamine + dibutyltin dilaurate |
| Nanoparticles | Surface Modification | SBET */m2 g−1 | C* | d */nm |
|---|---|---|---|---|
| SiO2 | - | 204 | 79 | 13 |
| SiO2–methyl | –CH3 | 220 | 31 | 12 |
| SiO2–octyl | –(CH2)7–CH3 | 164 | 27 | 16 |
| TiO2 | - | 51 | 93 | 30 |
| ZnO | - | 46 | 139 | 23 |
| Sample | WST-1 Assay/µg mL−1 | LDH Assay/µg mL−1 |
|---|---|---|
| SiO2 | 89.4 ± 1.2 | 92.0 ± 12.4 |
| SiO2–methyl | no toxic * | no toxic |
| SiO2–octyl | no toxic | not toxic |
| TiO2 | no toxic | not toxic |
| ZnO | 9.6 ± 0.2 | 9.5 ± 0.6 |
| ZnO as Zn2+ | 7.8 ± 0.2 | 7.7 ± 0.5 |
| ZnCl2 | 10.1 ± 0.6 | NA |
| ZnCl2 as Zn2+ | 4.8 ± 0.3 | NA |
| Consolidant | Drilling resistance force/N |
|---|---|
| Reference sandstone | 2.4 ± 0.7 |
| KSE OH | 7.4 ± 1.2 |
| SiGel SiO2 | 22.3 ± 4.4 |
| SiGel SiO2-methyl | 16.9 ± 3.3 |
| SiGel SiO2-octyl | 17.3 ± 2.7 |
| SiGel TiO2 | 25.9 ± 6.8 |
| SiGel ZnO | 14.4 ± 2.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remzova, M.; Zouzelka, R.; Brzicova, T.; Vrbova, K.; Pinkas, D.; Rőssner, P.; Topinka, J.; Rathousky, J. Toxicity of TiO2, ZnO, and SiO2 Nanoparticles in Human Lung Cells: Safe-by-Design Development of Construction Materials. Nanomaterials 2019, 9, 968. https://doi.org/10.3390/nano9070968
Remzova M, Zouzelka R, Brzicova T, Vrbova K, Pinkas D, Rőssner P, Topinka J, Rathousky J. Toxicity of TiO2, ZnO, and SiO2 Nanoparticles in Human Lung Cells: Safe-by-Design Development of Construction Materials. Nanomaterials. 2019; 9(7):968. https://doi.org/10.3390/nano9070968
Chicago/Turabian StyleRemzova, Monika, Radek Zouzelka, Tana Brzicova, Kristyna Vrbova, Dominik Pinkas, Pavel Rőssner, Jan Topinka, and Jiri Rathousky. 2019. "Toxicity of TiO2, ZnO, and SiO2 Nanoparticles in Human Lung Cells: Safe-by-Design Development of Construction Materials" Nanomaterials 9, no. 7: 968. https://doi.org/10.3390/nano9070968
APA StyleRemzova, M., Zouzelka, R., Brzicova, T., Vrbova, K., Pinkas, D., Rőssner, P., Topinka, J., & Rathousky, J. (2019). Toxicity of TiO2, ZnO, and SiO2 Nanoparticles in Human Lung Cells: Safe-by-Design Development of Construction Materials. Nanomaterials, 9(7), 968. https://doi.org/10.3390/nano9070968