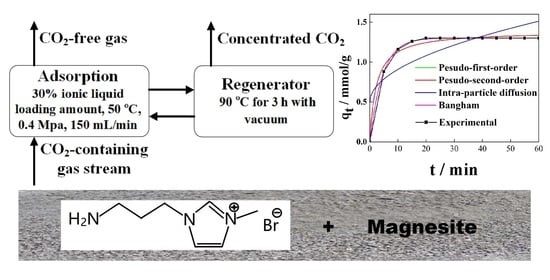
CO2 Adsorption Performance and Kinetics of Ionic Liquid-Modified Calcined Magnesite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Preparation of Adsorbents
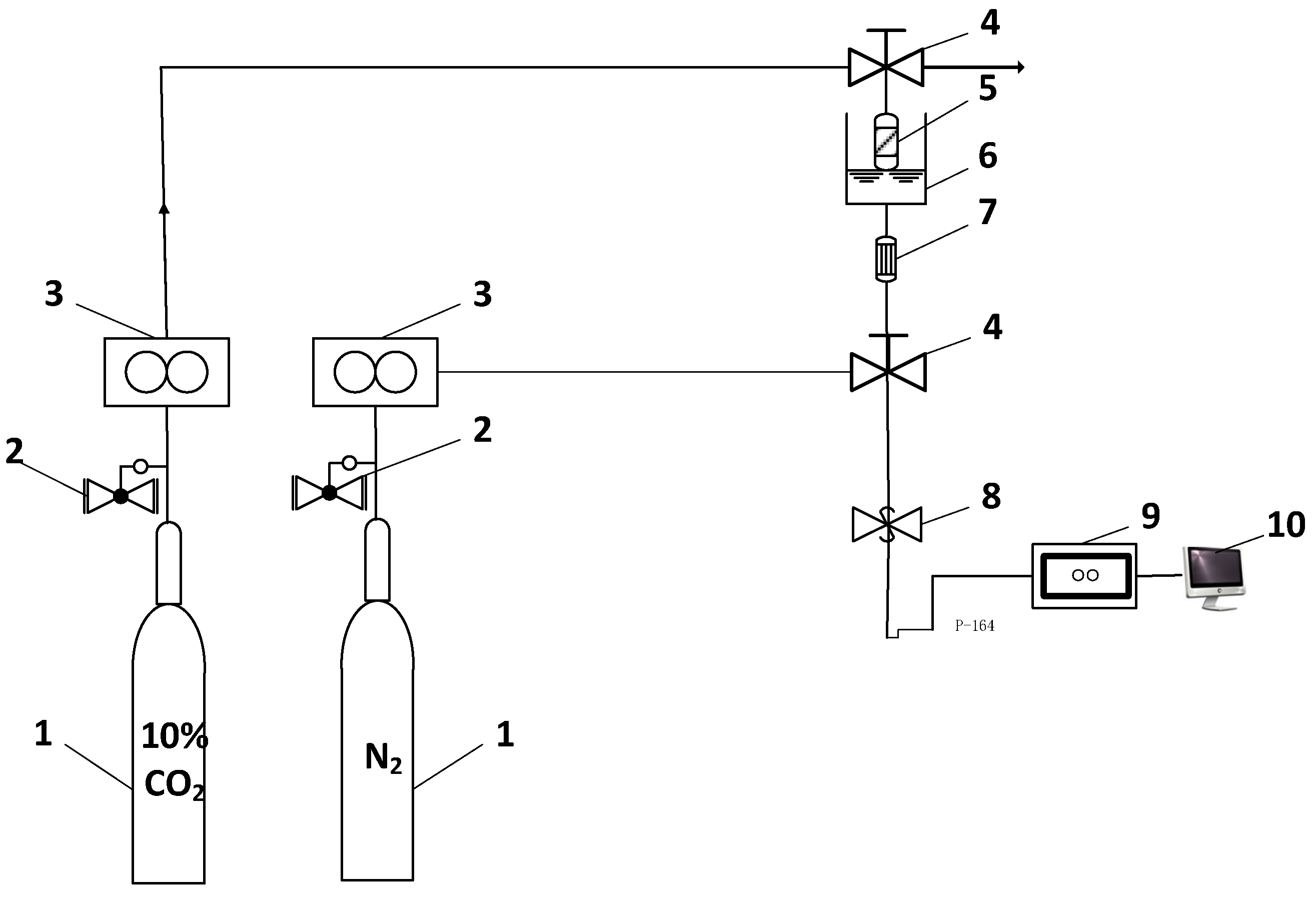
2.3. CO2 Adsorption-Regeneration Experiments
2.4. Characterisation of Adsorbents
3. Results and Discussion
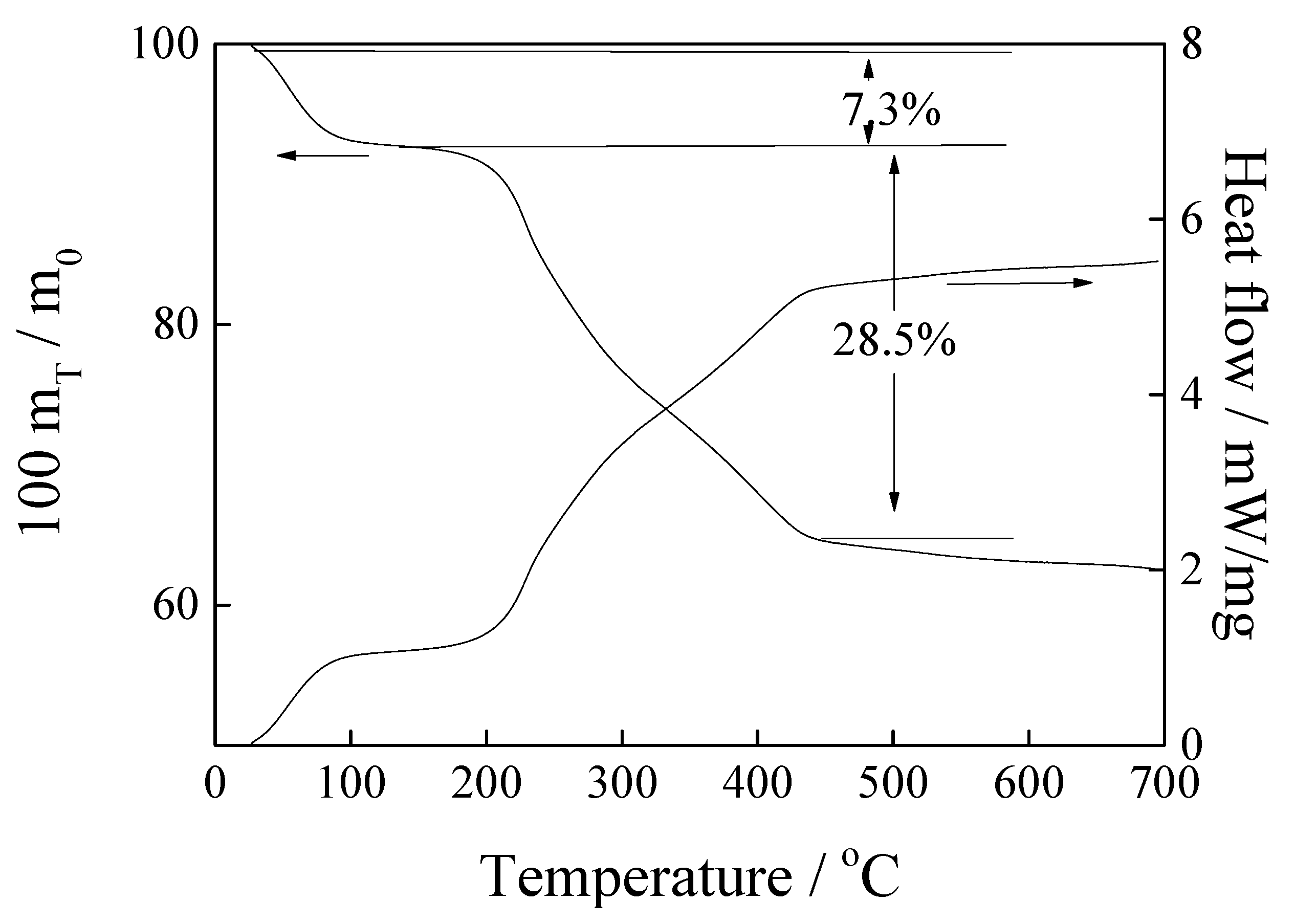
3.1. Properties of the Adsorbents
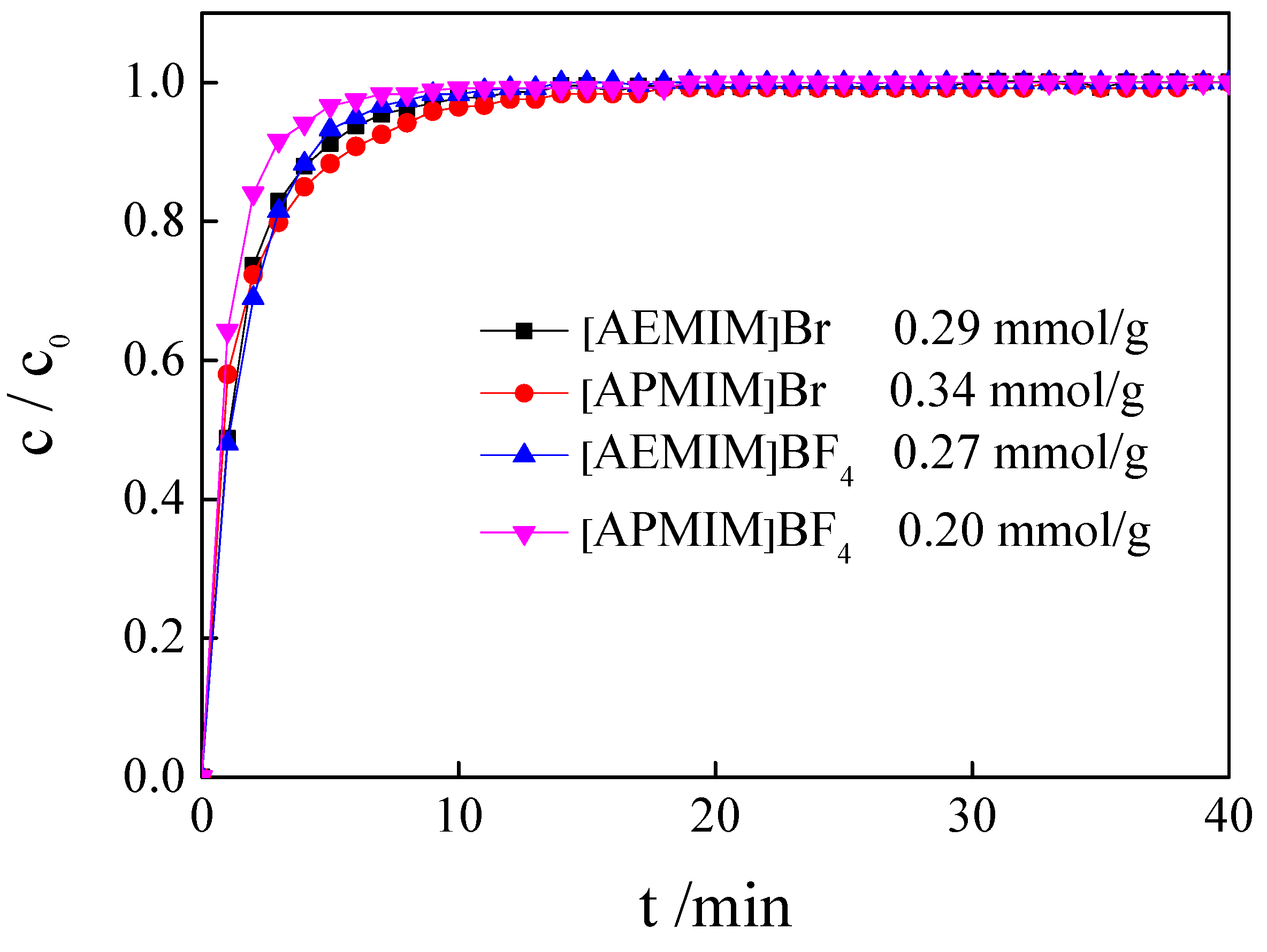
3.2. Influence of Different ILs
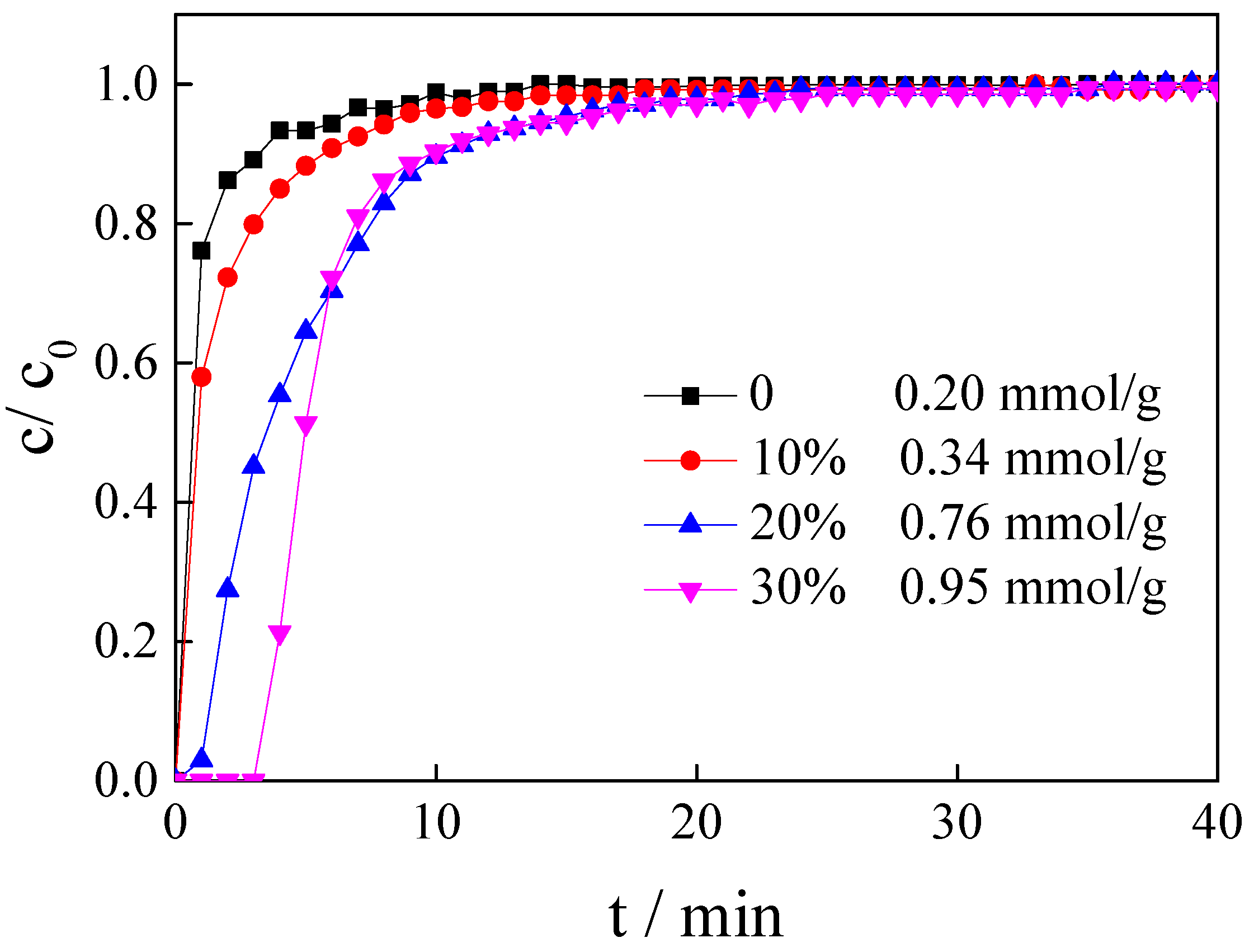
3.3. Influence of IL Loading Amount
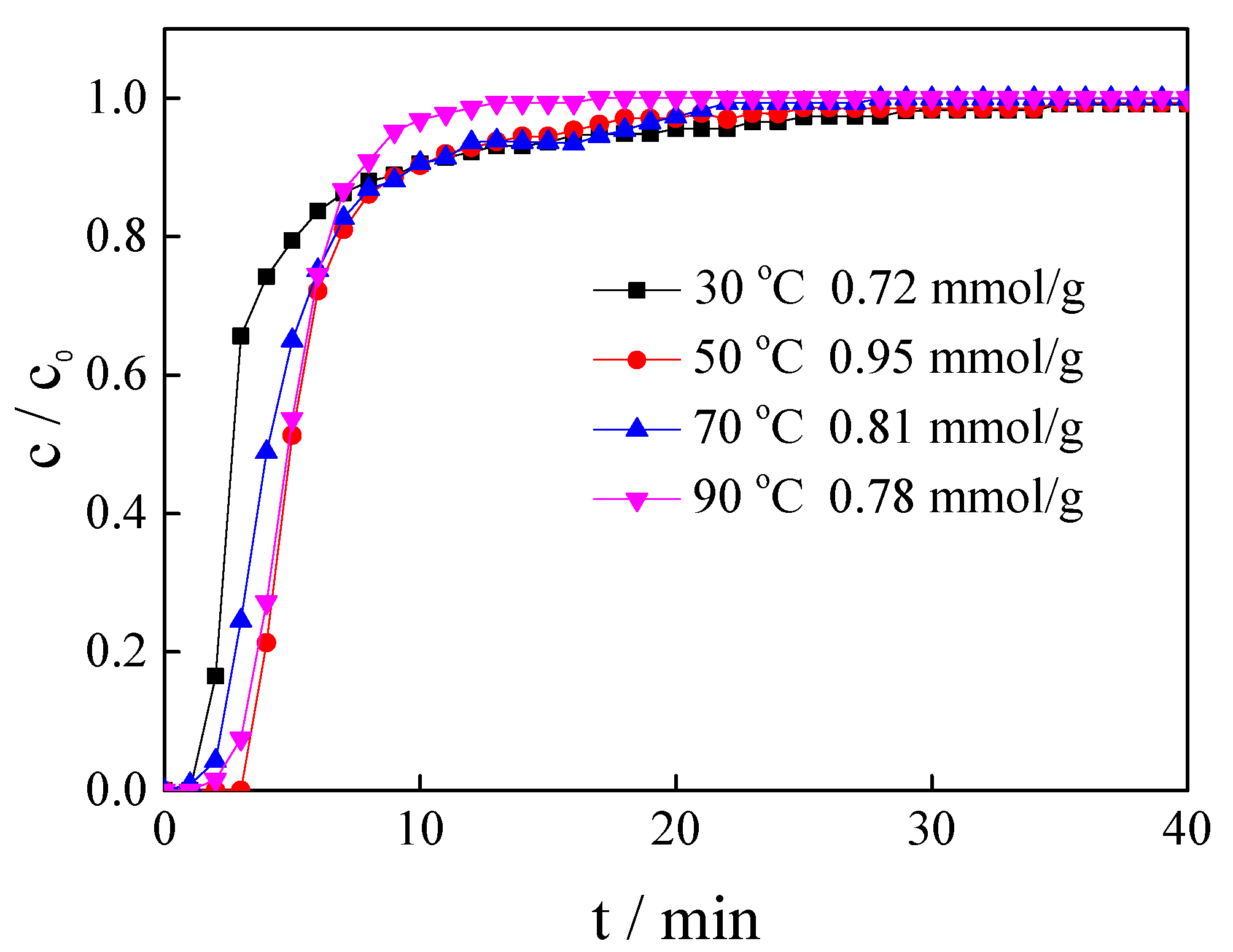
3.4. Influence of Adsorption Temperature
3.5. Influence of Gas Flow Rate
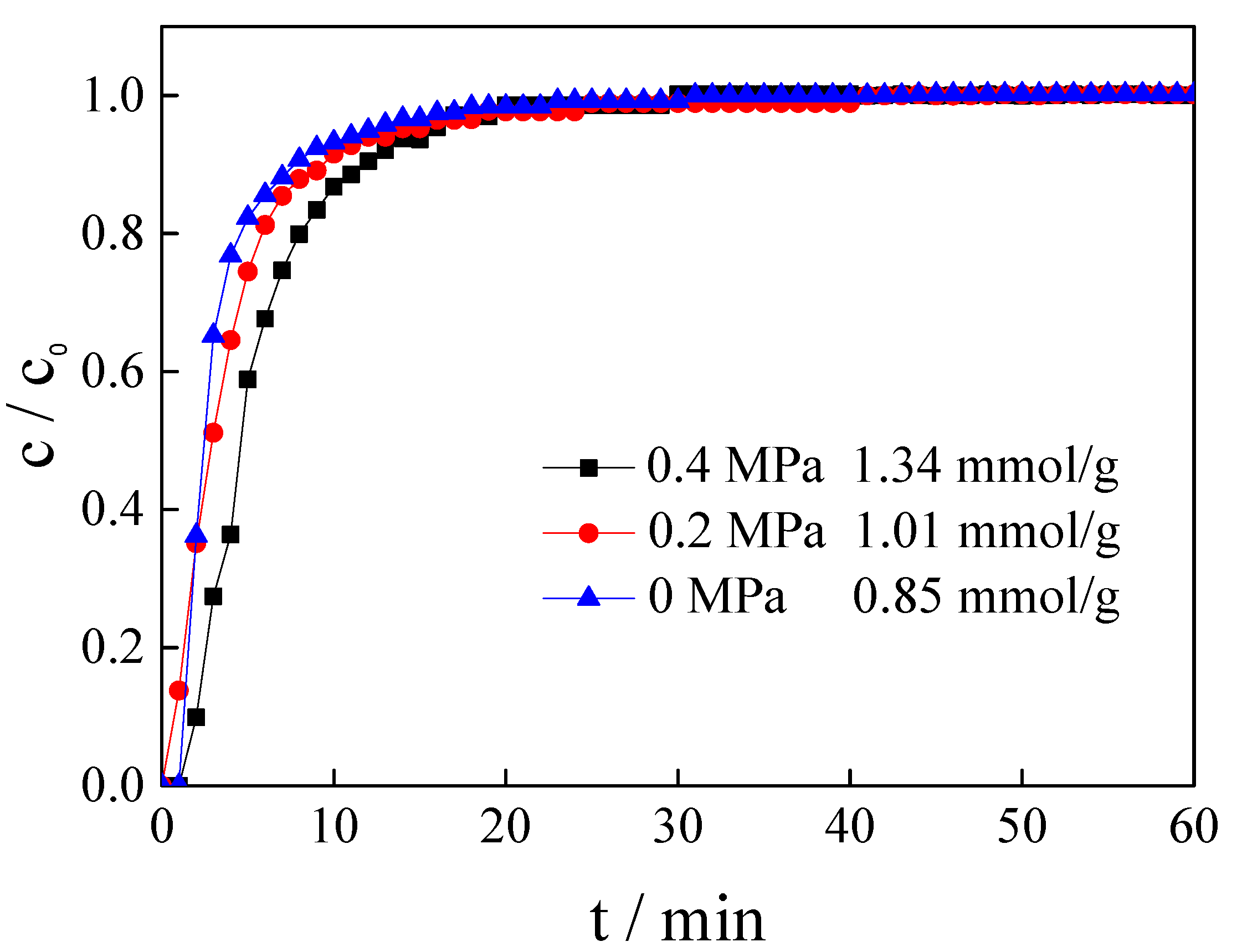
3.6. Influence of Relative Pressure
3.7. Cyclic Regenerative Performance
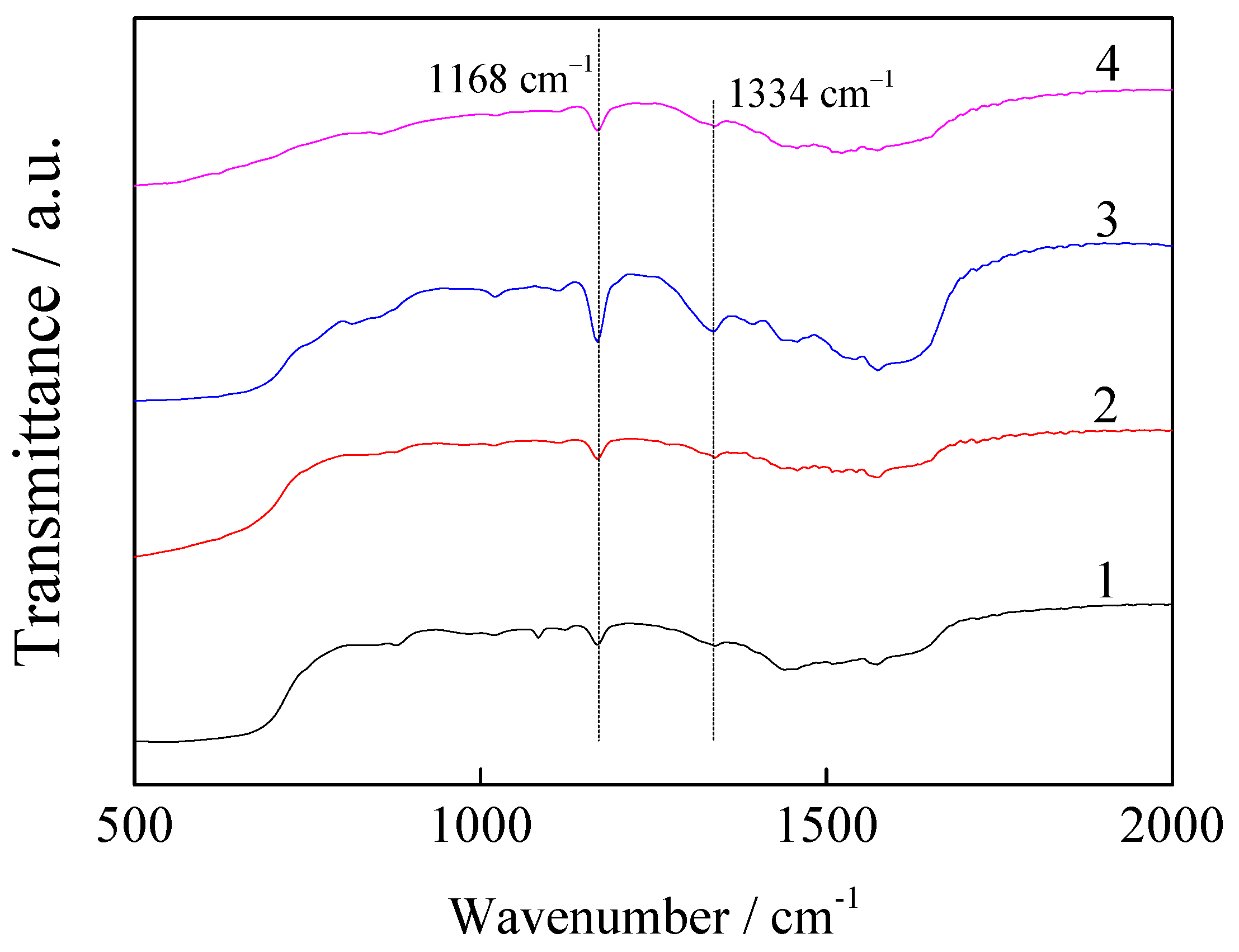
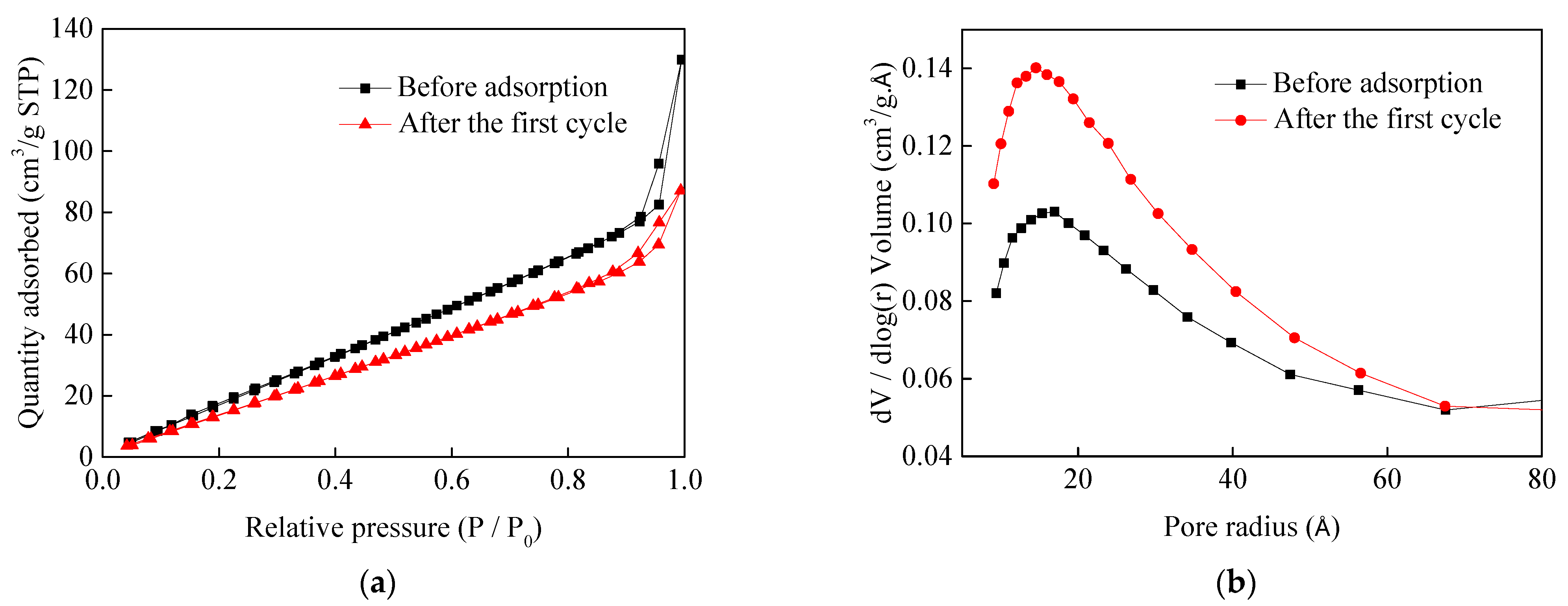
3.8. Characterisation
3.9. Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xian, Z. Carbon capture, utilization and sequestration in China under the carbon neutral target. Ke Chi Xu Fa Zhang Jing Ji Dao Kan 2020, 12, 22–24. [Google Scholar]
- Wang, J.Y.; Huang, L.; Yang, R.; Zhang, Z.; Wu, J.; Gao, Y.; Wang, Q.; O’Hare, D.; Zhong, Z. Recent advances in solid sorbents for CO2 capture and new development trends. Energy Environ. Sci. 2014, 7, 3478–3518. [Google Scholar] [CrossRef]
- Wang, J.; Mei, X.; Huang, L.; Zheng, Q.; Qiao, Y.; Zang, K.; Mao, S.; Yang, R.; Zhang, Z.; Gao, Y.; et al. Synthesis of layered double hydroxides/graphene oxide nanocomposite as a novel high-temperature CO2 adsorbent. J. Energy Chem. 2015, 24, 127–137. [Google Scholar] [CrossRef]
- Wang, Q.; Tay, H.H.; Zhong, Z.; Luo, J.; Borgna, A. Synthesis of high-temperature CO2 adsorbents from organo-layered double hydroxides with markedly improved CO2 capture capacity. Energy Environ. Sci. 2012, 5, 7526–7530. [Google Scholar] [CrossRef]
- Nawar, A.; Ali, M.; Khoja, A.H.; Waqas, A.; Anwar, M.; Mahmood, M. Enhanced CO2 capture using organic acid structure modified waste eggshell derived CaO sorbent. J. Environ. Chem. Eng. 2021, 9, 104871. [Google Scholar] [CrossRef]
- Dashtestani, F.; Nusheh, M.; Siriwongrungson, V.; Hongrapipat, J.; Materic, V.; Pang, S. Effect of H2S and NH3 in biomass gasification producer gas on CO2 capture performance of an innovative CaO and Fe2O3 based sorbent. Fuel 2021, 295, 120586. [Google Scholar] [CrossRef]
- Li, P.; Chen, R.; Lin, Y.N. General approach to facile synthesis of MgO-based porous ultrathin nanosheets enabling high-efficiency CO2 capture. Chem. Eng. J. 2021, 404, 126459. [Google Scholar] [CrossRef]
- Iugai, I.A.; Steksova, Y.P.; Vedyagin, A.A.; Mishakov, I.V.; Bauman, Y.I.; Belyy, V.A.; Danilovich, D.P.; Krivoshapkina, E.F.; Krivoshapkin, P.V. MgO/carbon nanofibers composite coatings on poous ceramic surface for CO2 capture. Surf. Coat. Technol. 2020, 400, 126208. [Google Scholar] [CrossRef]
- Bernabe-Pablo, E.; Duan, Y.; Pfeiffer, H. Developing new alkaline ceramics as possible CO2 chemisorbents at high temperatures: The lithium and sodium yttriates (LiYO2 and NaYO2) cases. Chem. Eng. J. 2020, 396, 125277. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W. Element doped sodium-based sorbents with high attrition resistance and CO2 capture capacity during CO2 sorption / desorption processes. Fuel 2021, 285, 119165. [Google Scholar] [CrossRef]
- Heo, Y.J.; Zhang, Y.; Rhee, K.Y.; Park, S.J. Synthesis of PAN/PVDF nanofiber composites-based carbon adsorbents for CO2 capture. Compos. Part B-Eng. 2019, 156, 95–99. [Google Scholar] [CrossRef]
- Kutorglo, E.M.; Hassouna, F.; Beltzung, A.; Kopecký, D.; Sedlářová, I.; Šoóš, M. Nitrogen-rich hierarchically porous polyaniline-based adsorbents for carbon dioxide (CO2) capture. Chem. Eng. J. 2019, 360, 1199–1212. [Google Scholar] [CrossRef]
- Somkiat, K.; Kingkaew, C.C.; Nuntaporn, K. Synthesis and characterization of NaX-type zeolites prepared by different silica and alumina sources and their CO2 adsorption properties. Micropor. Mesopor. Mater. 2021, 310, 110632. [Google Scholar]
- Zagho, M.M.; Hassan, M.K.; Khraisheh, M.; Al-Maadeed, M.A.; Nazarenko, S. A review on recent advances in CO2 Separation using zeolite and zeolite-like materials as adsorbents and fillers in mixed matrix membranes (MMMs). Chem. Eng. J. Adv. 2021, 6, 100091. [Google Scholar] [CrossRef]
- Zhao, Y.; Ge, H.; Miao, Y.; Chen, J.; Cai, W. CO2 capture ability of Cu-based metal-organic frameworks synergized with amino acid-functionalized layered materials. Catal. Today 2020, 356, 604–612. [Google Scholar] [CrossRef]
- Liao, J.; Jin, B.; Zhao, Y.; Liang, Z. Highly efficient and durable metal-organic framework material derived Ca-based solid sorbents for CO2 capture. Chem. Eng. J. 2019, 372, 1028–1037. [Google Scholar] [CrossRef]
- Abdalazeez, A.; Li, T.; Wang, W.; Abuelgasim, S. A brief review of CO2 utilization for alkali carbonate gasification and biomass/coal cogasification: Reactivity, products and process. J. CO2 Util. 2021, 43, 101370. [Google Scholar] [CrossRef]
- Li, P.; Yang, M.; Chen, D.; Guo, H.; Yan, B. CO fuel and LiAlO2 production through alkali carbonate-assisted CO2 splitting by reusing aluminum wastes. J. CO2 Util. 2020, 39, 101168. [Google Scholar] [CrossRef]
- Cimino, S.; Boccia, F.; Lisi, L. Effect of alkali promoters (Li, Na, K) on the performance of Ru/Al2O3 catalysts for CO2 capture and hydrogenation to methane. J. CO2 Util. 2020, 37, 195–203. [Google Scholar] [CrossRef]
- Jiang, N.; Shen, Y.; Liu, B.; Zhang, D.; Tang, Z.; Li, G.; Fu, B. CO2 capture from dry flue gas by means of VPSA, TSA and TVSA. J. CO2 Util. 2020, 35, 153–168. [Google Scholar] [CrossRef]
- Raganati, F.; Ammendola, P. Sound-assisted fluidization for temperature swing adsorption and calcium looping: A review. Materials 2021, 14, 672. [Google Scholar] [CrossRef]
- Yang, N.; Ning, P.; Li, K.; Wang, J. MgO-based adsorbent achieved from magnesite for CO2 capture in simulate wet flue gas. J. Taiwan Inst. Chem. Eng. 2018, 86, 73–80. [Google Scholar] [CrossRef]
- Yang, N.; Ning, P.; Li, K.; Wang, J. A new method of processing CO2 and magnesite slag simultaneously. J. Serb. Chem. Soc. 2018, 83, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hospital-Benito, D.; Lemus, J.; Moya, C.; Santiago, R.; Ferro, V.R.; Palomar, J. Techno-economic feasibility of ionic liquids-based CO2 chemical capture processes. Chem. Eng. J. 2021, 407, 127196. [Google Scholar] [CrossRef]
- Yang, N.; Wang, R. Molecular sieve-supported ionic liquids as efficient adsorbents for CO2 capture. J. Serb. Chem. Soc. 2015, 80, 265–275. [Google Scholar] [CrossRef]
- Uehara, Y.; Karami, D.; Mahinpey, N. CO2 adsorption using amino acid ionic liquid-impregnated mesoporous silica sorbents with different textural properties. Micropor. Mesopor. Mat. 2019, 278, 378–386. [Google Scholar] [CrossRef]
- Garip, M.; Gizli, N. Ionic liquid containing amin-based silica aerogels for CO2 capture by fixed bed adsorption. J. Mol. Liq. 2020, 300, 113227. [Google Scholar] [CrossRef]
- Orhan, O.Y. Effects of various anions in ionic liquids on CO2 capture. J. Mol. Liq. 2021, 333, 115981. [Google Scholar]
- Huang, Z.; Karami, D.; Mahinpey, N. Study on the efficiency of multiple amino groups in ionic liquids on their sorbents performance for low-temperature CO2 capture. Chem. Eng. Res. Des. 2021, 167, 198–206. [Google Scholar] [CrossRef]
- Nisar, M.; Bernard, F.L.; Duarte, E.; Chaban, V.V.; Einloft, S. New polysulfone microcapsules containing metal oxides and ([BMIM][NTf2]) ionic liquid for CO2 capture. J. Environ. Chem. Eng. 2021, 9, 104781. [Google Scholar] [CrossRef]
- Wei, L.; Guo, R.; Tang, Y. Properties of aqueous amine based protic ionic liquids and its application for CO2 quick capture. Sep. Purif. Technol. 2020, 239, 116531. [Google Scholar] [CrossRef]
- Ramdin, M.; Thijs, J.H.; Theo, W. Solubility of CO2 in the ionic liquids [TBMN][MeSO4] and [TBMP][MeSO4]. J. Chem. Eng. Data 2021, 57, 2275–2280. [Google Scholar] [CrossRef]
- Younas, M.; Rezakazemi, M.; Daud, M.; Wazir, M.B.; Ahmad, S.; Ullah, N.; Ramakrishna, S. Recent progress and remaining challenges in post-combustion CO2 capture using metal-orgnic frameworks(MOFs). Prog. Energy Combust. Sci. 2020, 80, 100849. [Google Scholar] [CrossRef]
- Abuelnoor, N.; AlHajaj, A.; Khaleel, M.; Vega, L.F.; Abu-Zahra, M.R. Acitvated carbon from biomass-based sources for CO2 capture applications. Chemosphere 2021, 283, 131111. [Google Scholar] [CrossRef]
- Kumar, K.V. Linear and non-linear regression analysis for the sorption kinetics of methylene blue on to activated carbon. J. Hazard. Mater. 2006, 137, 1538–1544. [Google Scholar] [CrossRef]
- Bilgili, M.S. Adsorption of 4-chlorophenol from aqueous solutions by xad-4 resin: Isotherm, kinetic and thermodynamic analysis. J. Hazard. Mater. 2006, 137, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Ammendola, P.; Raganati, F.; Chirone, R.; Miccio, F. Fixed bed adsorption as affected by thermodynamics and kinetics: Yellow tuff for CO2 capture. Powder Technol. 2020, 373, 446–458. [Google Scholar] [CrossRef]





| Ionic Liquid | Loading Amount | Adsorption Temperature (°C) | Flow Rate (mL/min) | Adsorption Pressure (MPa) | CO2 Adsorption Capacity (mmol/g) |
|---|---|---|---|---|---|
| [AEMIM]Br | 10% | 50 | 100 | 0 | 0.29 |
| [APMIM]Br | 10% | 50 | 100 | 0 | 0.34 |
| [AEMIM]BF4 | 10% | 50 | 100 | 0 | 0.27 |
| [APMIM]BF4 | 10% | 50 | 100 | 0 | 0.20 |
| [APMIM]Br | 0 | 50 | 100 | 0 | 0.20 |
| [APMIM]Br | 20% | 50 | 100 | 0 | 0.76 |
| [APMIM]Br | 30% | 50 | 100 | 0 | 0.95 |
| [APMIM]Br | 30% | 30 | 100 | 0 | 0.72 |
| [APMIM]Br | 30% | 70 | 100 | 0 | 0.81 |
| [APMIM]Br | 30% | 90 | 100 | 0 | 0.78 |
| [APMIM]Br | 30% | 50 | 150 | 0 | 0.85 |
| [APMIM]Br | 30% | 50 | 200 | 0 | 0.80 |
| [APMIM]Br | 30% | 50 | 150 | 0.2 | 1.01 |
| [APMIM]Br | 30% | 50 | 150 | 0.4 | 1.34 |
| Sample | Adsorption Temperature (°C) | CO2 Adsorption Capacity (mmol/g) | Regeneration Temperature (°C) | Reference |
|---|---|---|---|---|
| TEOS a-APTES b-EMIM(Tf2N) | - | 5.53 | - | [27] |
| [bmim][Ac] c | 30 | 4.49 | - | [28] |
| [AEMIM][Lys] d-immobilized on PMMA e | 30 | 1.50 | 100 | [29] |
| PSF f-[bmim][NTf2]-Fe2O3 | 45 | 1.30 | 70 | [30] |
| [DMAPAH][EOAc] | 30 | 2.44 | 30 | [31] |
| [TBMP][MeSO4] g | 40 | 1.13 | - | [32] |
| [EMIM][Lys]/PMMA | 30 | 1.20 | 100 | [26] |
| [APMIM]Br-modified magnesite | 50 | 1.34 | 90 | This work |
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (Å) |
|---|---|---|---|
| Magnesite modified by [APMIM]Br | 128.5 | 0.2 | 62.5 |
| Magnesite modified by [APMIM]Br after the first cycle | 105.6 | 0.13 | 51.0 |
| Models | Parameter | Value |
|---|---|---|
| Pseudo-first-order | R2 | 0.999 |
| k1 | 0.224 | |
| qe | 1.302 | |
| Pseudo-second-order | R2 | 0.988 |
| k2 | 0.312 | |
| qe | 1.386 | |
| Intraparticle diffusion | R2 | 0.624 |
| ki | 0.131 | |
| C | 0.491 | |
| Bangham | R2 | 0.999 |
| kb | 0.221 | |
| qe | 1.302 | |
| n | 1.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Xue, R.; Huang, G.; Ma, Y.; Wang, J. CO2 Adsorption Performance and Kinetics of Ionic Liquid-Modified Calcined Magnesite. Nanomaterials 2021, 11, 2614. https://doi.org/10.3390/nano11102614
Yang N, Xue R, Huang G, Ma Y, Wang J. CO2 Adsorption Performance and Kinetics of Ionic Liquid-Modified Calcined Magnesite. Nanomaterials. 2021; 11(10):2614. https://doi.org/10.3390/nano11102614
Chicago/Turabian StyleYang, Na, Rong Xue, Guibo Huang, Yunqian Ma, and Junya Wang. 2021. "CO2 Adsorption Performance and Kinetics of Ionic Liquid-Modified Calcined Magnesite" Nanomaterials 11, no. 10: 2614. https://doi.org/10.3390/nano11102614