Nanoporous TiN/TiO2/Alumina Membrane for Photoelectrochemical Hydrogen Production from Sewage Water
Abstract
:1. Introduction
2. Experimental Methods
2.1. Al2O3 Template Synthesis
2.2. Synthesis of TiN/TiO2 and Au/TiN/TiO2
2.3. Samples Characterization
2.4. Water-Splitting Test
3. Results and Discussion
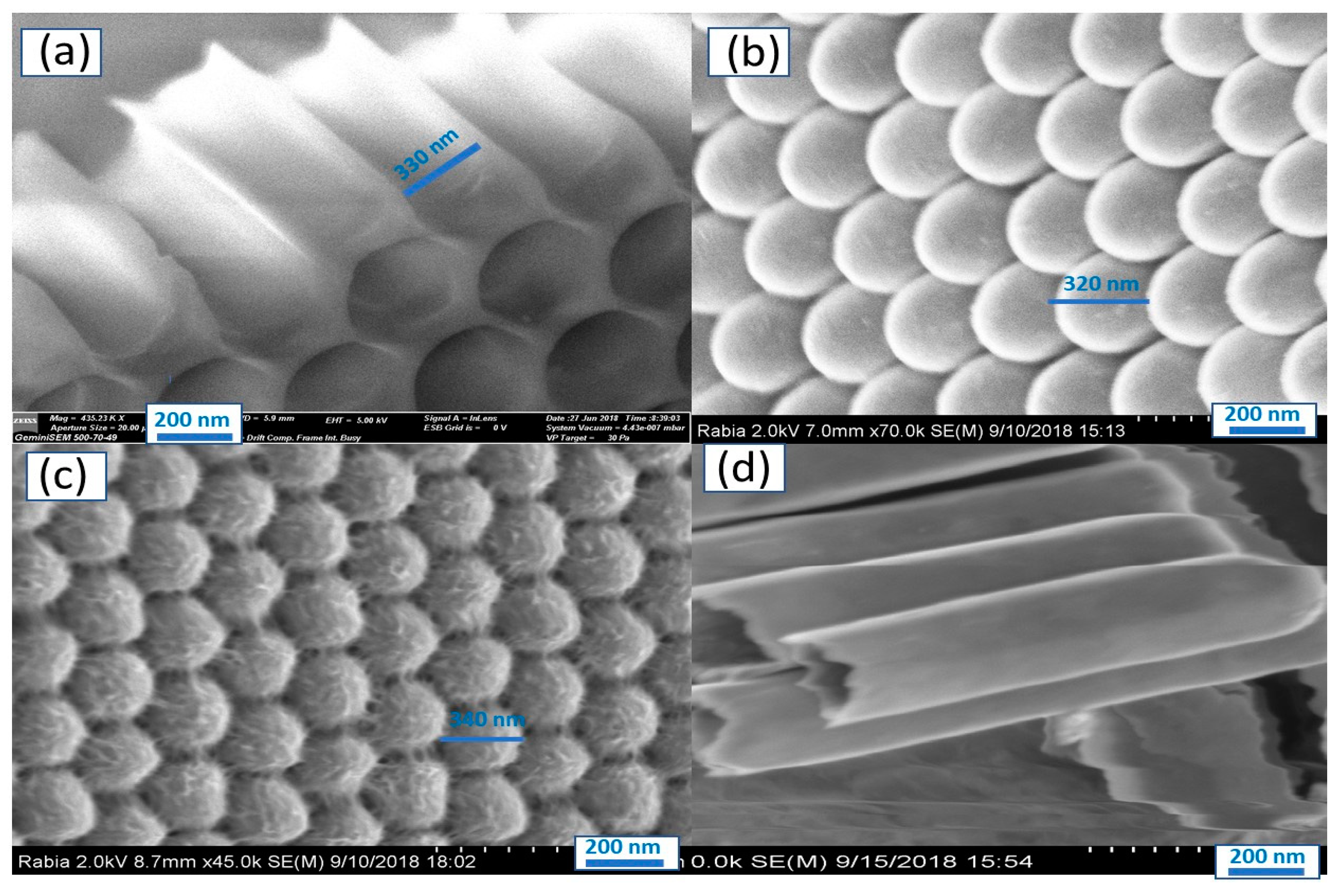
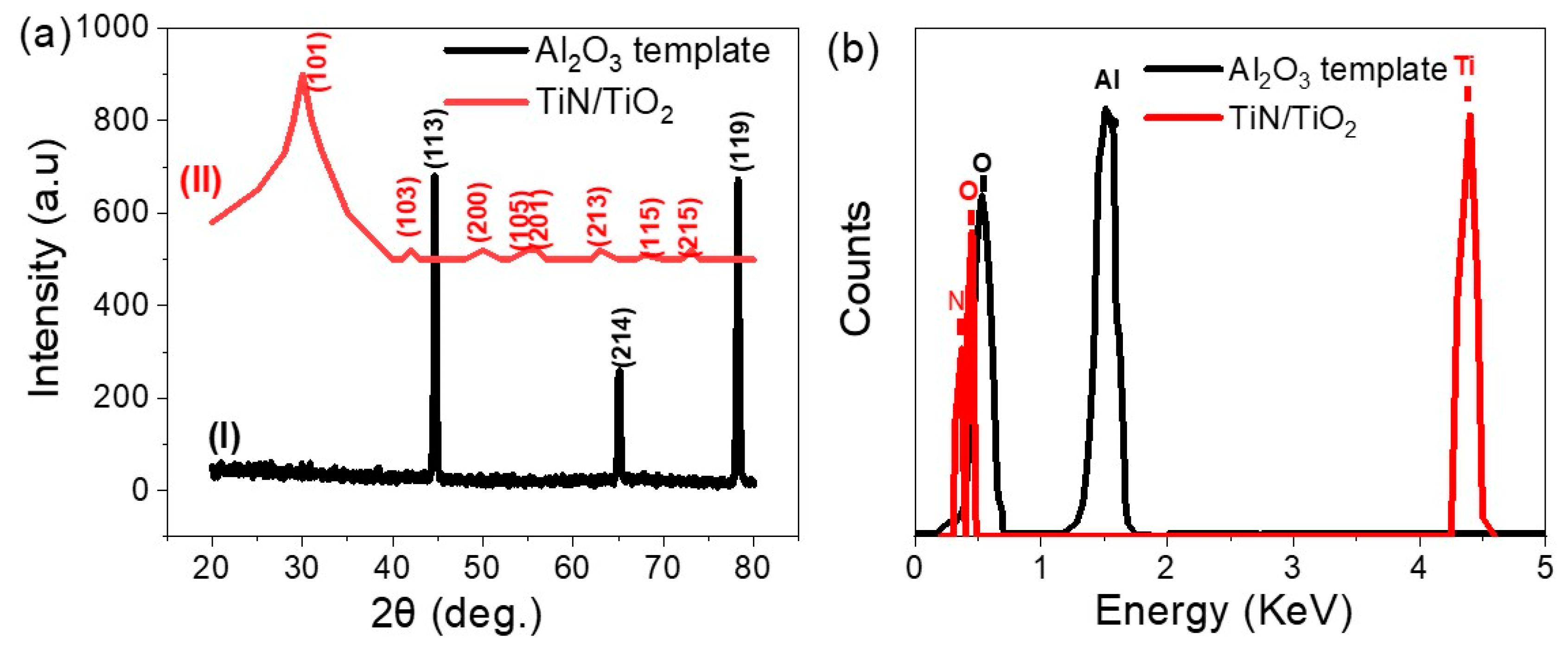
3.1. SEM and XRD Studies
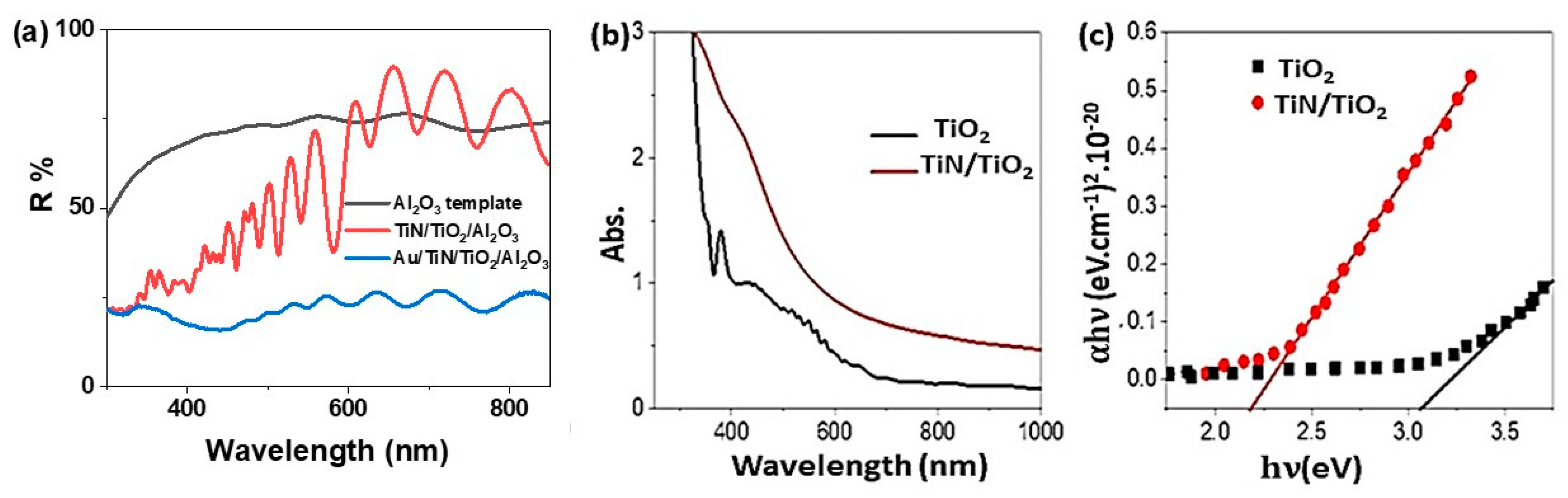
3.2. Optical Properties of TiN/TiO2/Al2O3
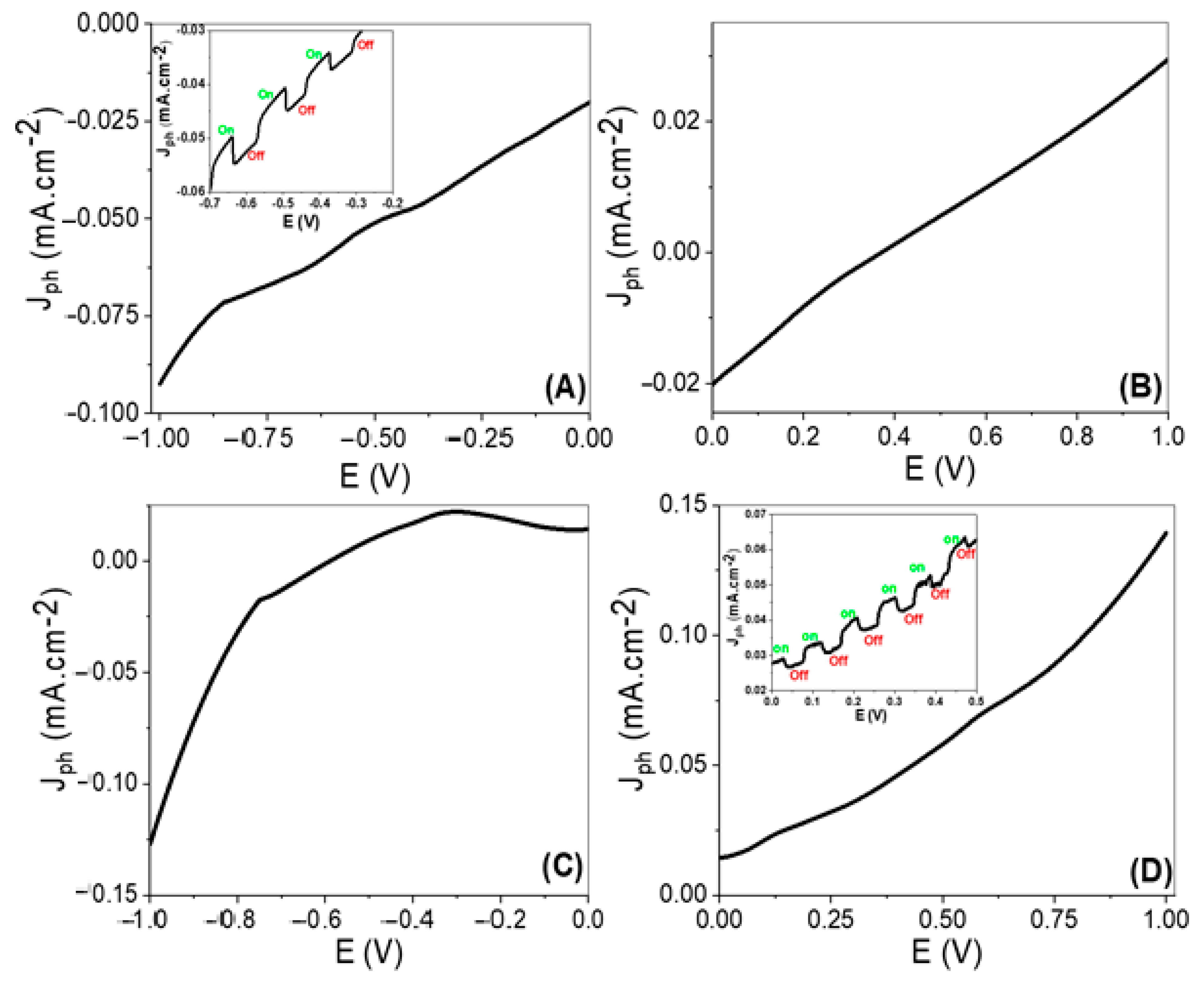
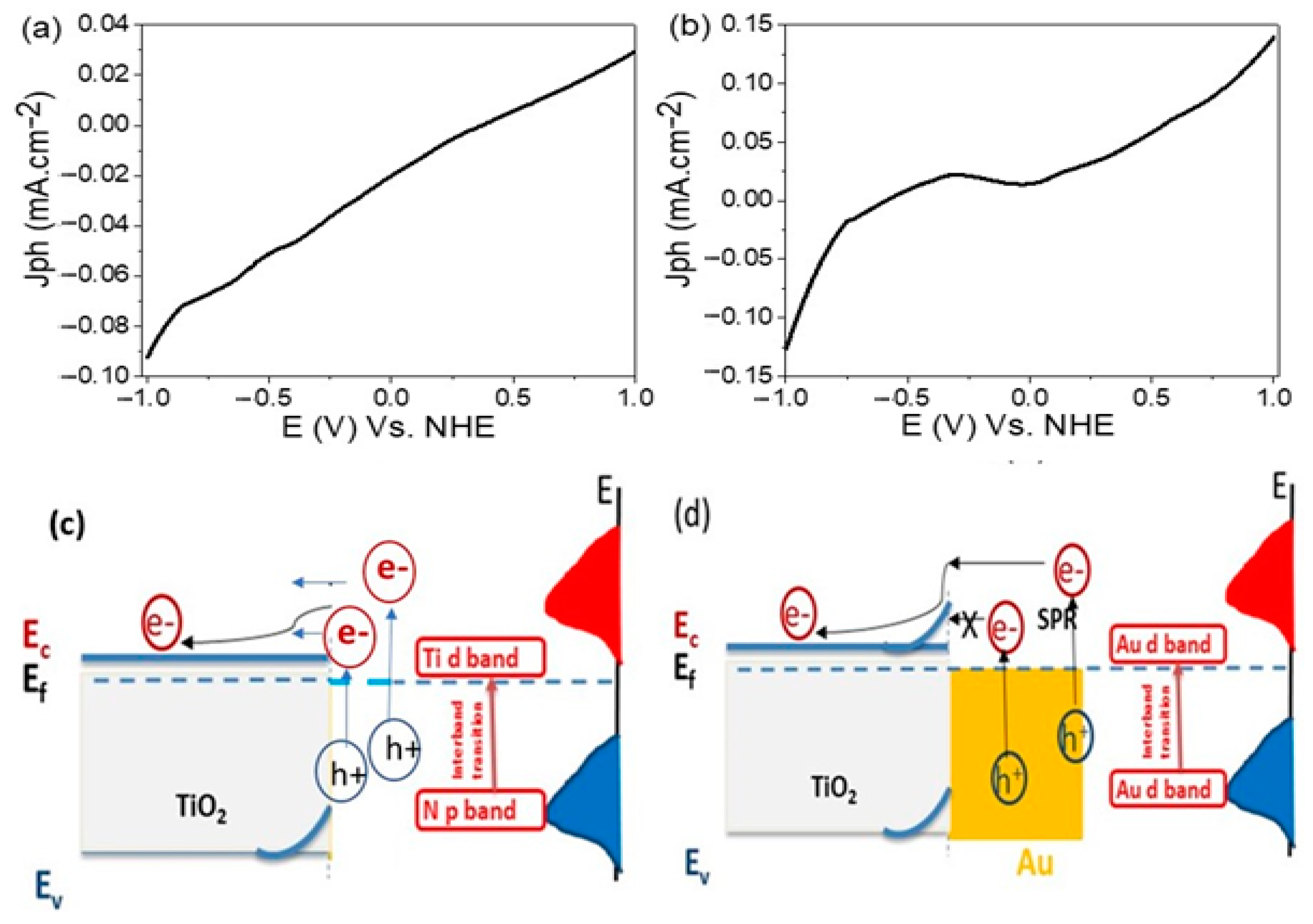
3.3. The Photoelectrochemical Performance
3.4. Effect of Temperature and Thermodynamic Parameters
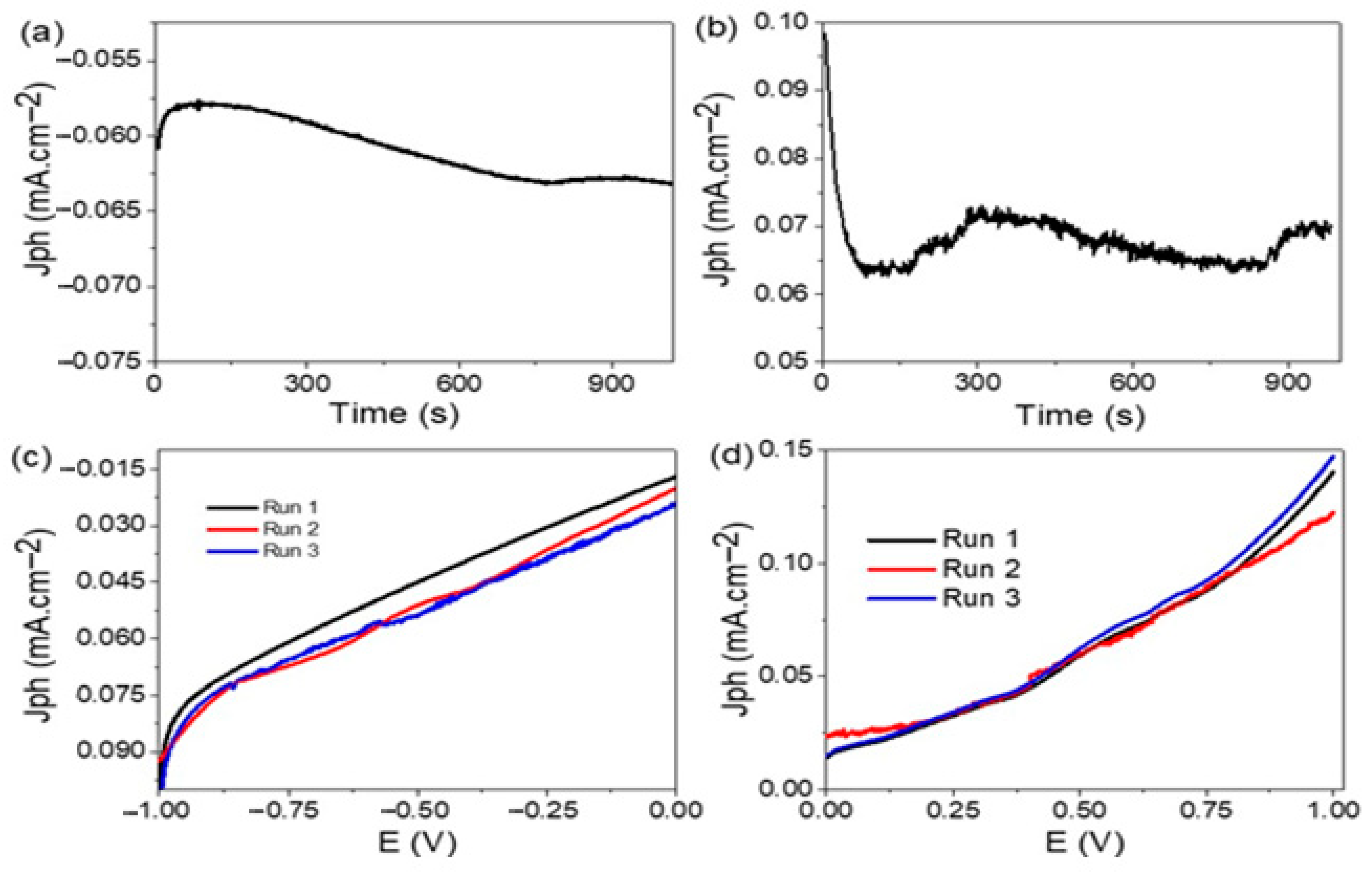
3.5. Stability of the Photoelectrode
3.6. Mechanism of Electron Transition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kunwar, S.; Pandit, S.; Jeong, J.H.; Lee, J. Improved Photoresponse of UV Photodetectors by the Incorporation of Plasmonic Nanoparticles on GaN Through the Resonant Coupling of Localized Surface Plasmon Resonance. Nano-Micro Lett. 2020, 12, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Liu, K.; Hu, L.; Al-Ghamdi, A.A.; Fang, X. New concept ultraviolet photodetectors. Mater. Today 2015, 18, 493–502. [Google Scholar] [CrossRef]
- Tan, M.; Hu, C.; Lan, Y.; Khan, J.; Deng, H.; Yang, X.; Wang, P.; Yu, X.; Lai, J.; Song, H. 2D Lead Dihalides for High-Performance Ultraviolet Photodetectors and their Detection Mechanism Investigation. Small 2017, 13, 1702024. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qian, Y.; Kong, X. Photon Counting Based on Solar-Blind Ultraviolet Intensified Complementary Metal-Oxide-Semiconductor (ICMOS) for Corona Detection. IEEE Photonics J. 2018, 10. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; El-Sayed, A.M.A.; Ahmed, A.; Sayed, S. Photocatalytic properties of PbS/graphene oxide/polyaniline electrode for hydrogen generation. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaban, M.; Benghanem, M.; Almohammedi, A.; Rabia, M. Optimization of the Active Layer P3HT:PCBM for Organic Solar Cell. Coatings 2021, 11, 863. [Google Scholar] [CrossRef]
- Kang, Z.; Cheng, Y.; Zheng, Z.; Cheng, F.; Chen, Z.; Li, L.; Tan, X.; Xiong, L.; Zhai, T.; Gao, Y. MoS2-Based Photodetectors Powered by Asymmetric Contact Structure with Large Work Function Difference. Nano-Micro Lett. 2019, 11, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Lee, W.W.; Yang, D.W.; Chang, W.J.; Kwon, S.S.; Park, W.I. Anomalous Photovoltaic Response of Graphene-on-GaN Schottky Photodiodes. ACS Appl. Mater. Interfaces 2018, 10, 14170–14174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Yang, W.; Leng, B.; Niu, P.; Jiang, X.; Liu, B. High-Performance Flexible Ultraviolet Photodetectors Based on AZO/ZnO/PVK/PEDOT:PSS Heterostructures Integrated on Human Hair. ACS Appl. Mater. Interfaces 2019, 11, 24459–24467. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.C.; Kim, K.H.; Hwang, M.S.; Park, J.S.; Lee, J.M.; So, J.P.; Choi, J.H.; Kwon, S.H.; Barrelet, C.J.; et al. Photon-triggered nanowire transistors. Nat. Nanotechnol. 2017, 12, 963–968. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Liu, B.; Yang, W.; Li, J.; Niu, P.; Jiang, X. Giant UV photoresponse of a GaN nanowire photodetector through effective Pt nanoparticle coupling. J. Mater. Chem. C 2017, 5, 4319–4326. [Google Scholar] [CrossRef]
- Rabia, M.; Mohamed, H.S.H.; Shaban, M.; Taha, S. Preparation of polyaniline/PbS core-shell nano/microcomposite and its application for photocatalytic H2 electrogeneration from H2O. Sci. Rep. 2018, 8, 1107. [Google Scholar] [CrossRef] [Green Version]
- Rabia, M.; Shaban, M.; Adel, A.; Abdel-Khaliek, A.A. Effect of plasmonic au nanoparticles on the photoactivity of polyaniline/indium tin oxide electrodes for water splitting. Environ. Prog. Sustain. Energy 2019, 38, 13171. [Google Scholar] [CrossRef]
- Shi, X.F.; Xia, X.Y.; Cui, G.W.; Deng, N.; Zhao, Y.Q.; Zhuo, L.H.; Tang, B. Multiple exciton generation application of PbS quantum dots in ZnO@PbS/graphene oxide for enhanced photocatalytic activity. Appl. Catal. B Environ. 2015, 163, 123–128. [Google Scholar] [CrossRef]
- Li, M.; Yu, M.; Su, D.; Zhang, J.; Jiang, S.; Wu, J.; Wang, Q.; Liu, S. Ultrahigh Responsivity UV Photodetector Based on Cu Nanostructure/ZnO QD Hybrid Architectures. Small 2019, 15, 1901606. [Google Scholar] [CrossRef]
- Surre, F.; Bernal, O.D.; Seat, H.C.; Sharp, J.H. Estimation of Transition Metal Nitride Surface Plasmon Refractometer Sensitivity. In Proceedings of the IEEE Sensors, Montorel, QC, Canada, 27–30 October 2019. [Google Scholar]
- Liu, J.; Guler, U.; Lagutchev, A.; Kildishev, A.; Malis, O.; Boltasseva, A.; Shalaev, V.M. Quasi-coherent thermal emitter based on refractory plasmonic materials. Opt. Mater. Express 2015, 5, 2721. [Google Scholar] [CrossRef] [Green Version]
- Cao, Y.Q.; Zhao, X.R.; Chen, J.; Zhang, W.; Li, M.; Zhu, L.; Zhang, X.J.; Wu, D.; Li, A.D. TiOxNy Modified TiO2 Powders Prepared by Plasma Enhanced Atomic Layer Deposition for Highly Visible Light Photocatalysis. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Yang, W.; Li, Q. TiO2-based photocatalysts prepared by oxidation of TiN nanoparticles and their photocatalytic activities under visible light illumination. J. Mater. Sci. Technol. 2018, 34, 969–975. [Google Scholar] [CrossRef]
- Yuan, Y.; Wang, J.; Adimi, S.; Shen, H.; Thomas, T.; Ma, R.; Attfield, J.P.; Yang, M. Zirconium nitride catalysts surpass platinum for oxygen reduction. Nat. Mater. 2019, 19, 282–286. [Google Scholar] [CrossRef]
- Awad, M.A.; Aly, A.H. Experimental and theoretical studies of hybrid multifunctional TiO2/TiN/TiO2. Ceram. Int. 2019, 45, 19036–19043. [Google Scholar] [CrossRef]
- Li, G.; Zhang, P.; Bian, Z.; Zhu, J.; Wu, L.; Li, H. Mesoporous TiN microspheres with hierarchical chambers and enhanced visible light-driven hydrogen evolution. ChemSusChem 2013, 6, 1461–1466. [Google Scholar] [CrossRef]
- Kumar, R.; Pasupathi, S.; Pollet, B.G.; Scott, K. Nafion-stabilised platinum nanoparticles supported on titaniumnitride: An efficient and durable electrocatalyst for phosphoric acidbased polymer electrolyte fuel cells. Electrochim. Acta 2013, 109, 365–369. [Google Scholar] [CrossRef] [Green Version]
- Rabia, M.; Mohamed, S.H.; Zhao, H.; Shaban, M.; Lei, Y.; Ahmed, A.M. TiO2/TiOxNY hollow mushrooms-like nanocomposite photoanode for hydrogen electrogeneration. J. Porous Mater. 2020, 27, 133–139. [Google Scholar] [CrossRef]
- Noothongkaew, S.; Thumthan, O.; An, K.S. Minimal layer graphene/TiO2 nanotube membranes used for enhancement of UV photodetectors. Mater. Lett. 2018, 218, 274–279. [Google Scholar] [CrossRef]
- Wang, L.; Yang, W.; Chong, H.; Wang, L.; Gao, F.; Tian, L.; Yang, Z. Efficient ultraviolet photodetectors based on TiO2 nanotube arrays with tailored structures. RSC Adv. 2015, 5, 52388–52394. [Google Scholar] [CrossRef]
- Lee, Y.B.; Han, J.K.; Noothongkaew, S.; Kim, S.K.; Song, W.; Myung, S.; Lee, S.S.; Lim, J.; Bu, S.D.; An, K.-S. Toward Arbitrary-Direction Energy Harvesting through Flexible Piezoelectric Nanogenerators Using Perovskite PbTiO3 Nanotube Arrays. Adv. Mater. 2017, 29, 1604500. [Google Scholar] [CrossRef]
- Liu, G.; Karuturi, S.K.; Chen, H.; Wang, D.; Ager, J.W.; Simonov, A.N.; Tricoli, A. Enhancement of the photoelectrochemical water splitting by perovskite BiFeO3 via interfacial engineering. Sol. Energy 2020, 202, 198–203. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Rabia, M.; Shaban, M.; Verpoort, F. Heulandite/polyaniline hybrid composite for efficient removal of acidic dye from water; kinetic, equilibrium studies and statistical optimization. Adv. Powder Technol. 2018, 29, 2501–2511. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Rabia, M.; Shaban, M. The structure and photoelectrochemical activity of Cr-doped PbS thin films grown by chemical bath deposition. RSC Adv. 2020, 10, 14458–14470. [Google Scholar] [CrossRef]
- Sherman, B.D.; Ashford, D.L.; Lapides, A.M.; Sheridan, M.V.; Wee, K.R.; Meyer, T.J. Light-Driven Water Splitting with a Molecular Electroassembly-Based Core/Shell Photoanode. J. Phys. Chem. Lett. 2015, 6, 3213–3217. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Shaban, M. Nanoporous chromium thin film for active detection of toxic heavy metals traces using surface-enhanced Raman spectroscopy. Mater. Res. Express 2020, 7, 1–8. [Google Scholar] [CrossRef]
- Mohamed, S.H.; Zhao, H.; Romanus, H.; El-Hossary, F.M.; Abo EL-Kassem, M.; Awad, M.A.; Rabia, M.; Lei, Y. Optical, water splitting and wettability of titanium nitride/titanium oxynitride bilayer films for hydrogen generation and solar cells applications. Mater. Sci. Semicond. Process. 2020, 105, 104704. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A sensor of m-toluidine/m-cresol polymer film for detection of lead ions by potentiometric methods. Sens. Lett. 2016, 14, 522–529. [Google Scholar] [CrossRef]
- Sayyah, E.-S.M.; Shaban, M.; Rabia, M. A sensor of m-cresol nanopolymer/Pt-electrode film for detection of lead ions by potentiometric methods. Adv. Polym. Technol. 2018, 37, 1296–1304. [Google Scholar] [CrossRef]
- Mohamed, F.; Rabia, M.; Shaban, M. Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J. Mater. Res. Technol. 2020, 9, 4255–4271. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, D.; Khare, N. Plasmonic Ag nanoparticles decorated Bi2S3 nanorods and nanoflowers: Their comparative assessment for photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 3538–3552. [Google Scholar] [CrossRef]
- Zayed, M.; Ahmed, A.M.; Shaban, M. Synthesis and characterization of nanoporous ZnO and Pt/ZnO thin films for dye degradation and water splitting applications. Int. J. Hydrogen Energy 2019, 44, 17630–17648. [Google Scholar] [CrossRef]
- Yang, L.; Liu, Z. Study on light intensity in the process of photocatalytic degradation of indoor gaseous formaldehyde for saving energy. Energy Convers. Manag. 2007, 48, 882–889. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. Electropolymerization of m-Toluidin on Platinum Electrode from Aqueous Acidic Solution and Character of the Obtained Polymer. Adv. Polym. Technol. 2018, 37, 126–136. [Google Scholar] [CrossRef]
- Baniasadi, E.; Dincer, I.; Naterer, G.F. Measured effects of light intensity and catalyst concentration on photocatalytic hydrogen and oxygen production with zinc sulfide suspensions. Int. J. Hydrogen Energy 2013, 38, 9158–9168. [Google Scholar] [CrossRef]
- Sayyah, S.M.; Shaban, M.; Rabia, M. A High-Sensitivity Potentiometric Mercuric Ion Sensor Based on m-Toluidine Films. IEEE Sens. J. 2016, 16, 1541–1548. [Google Scholar] [CrossRef]
- Bell, S.; Will, G.; Bell, J. Light intensity effects on photocatalytic water splitting with a titania catalyst. Int. J. Hydrogen Energy 2013, 38, 6938–6947. [Google Scholar] [CrossRef]
- Shaban, M.; Ahmed, A.M.; Abdel-Rahman, E.; Hamdy, H. Tunability and Sensing Properties of Plasmonic/1D Photonic Crystal. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Razek, S.A.; Popeil, M.R.; Wangoh, L.; Rana, J.; Suwandaratne, N.; Andrews, J.L.; Watson, D.F.; Banerjee, S.; Piper, L.F.J. Designing catalysts for water splitting based on electronic structure considerations. Electron. Struct. 2020, 2, 023001. [Google Scholar] [CrossRef]
- Freeman, E.; Kumar, S.; Thomas, S.R.; Pickering, H.; Fermin, D.J.; Eslava, S. PrFeO3 Photocathodes Prepared Through Spray Pyrolysis. ChemElectroChem 2020, 7, 1365–1372. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, D.; Wen, L.; Xu, R.; Obergfell, M.; Mi, Y.; Zhan, Z.; Nasori, N.; Demsar, J.; Lei, Y. Manipulation of charge transfer and transport in plasmonic-ferroelectric hybrids for photoelectrochemical applications. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Wang, D.; Huang, Q.; Du, J.; Sun, L.; Meyer, T.J. Stabilization of a molecular water oxidation catalyst on a dye−sensitized photoanode by a pyridyl anchor. Nat. Commun. 2020, 11, 4610. [Google Scholar] [CrossRef]
- Xiao, X.; Engelbrekt, C.; Zhang, M.; Li, Z.; Ulstrup, J.; Zhang, J.; Si, P. A straight forward approach to electrodeposit tungsten disulfide/poly(3,4-ethylenedioxythiophene) composites onto nanoporous gold for the hydrogen evolution reaction. Appl. Surf. Sci. 2017, 410, 308–314. [Google Scholar] [CrossRef]
- Modibane, K.D.; Waleng, N.J.; Ramohlola, K.E.; Maponya, T.C.; Monama, G.R.; Makgopa, K.; Hato, M.J. Poly(3-aminobenzoic acid) Decorated with Cobalt Zeolitic Benzimidazolate Framework for Electrochemical Production of Clean Hydrogen. Polymers 2020, 12, 1581. [Google Scholar] [CrossRef]
- Elsayed, A.M.; Rabia, M.; Shaban, M.; Aly, A.H.; Ahmed, A.M. Preparation of hexagonal nanoporous Al2O3/TiO2/TiN as a novel photodetector with high efficiency. Sci. Rep. 2021, 11, 17572. [Google Scholar] [CrossRef]
- Shaban, M.; Ali, S.; Rabia, M. Design and application of nanoporous graphene oxide film for CO2, H2, and C2H2 gases sensing. J. Mater. Res. Technol. 2019, 5, 4510–4520. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Dissociationswärme und den Einfluss der Temperatur auf den Dissociationsgrad der Elektrolyte. Z. Phys. Chem. 1889, 4U, 96–116. [Google Scholar] [CrossRef] [Green Version]
- Helmy, A.; Rabia, M.; Shaban, M.; Ashraf, A.M.; Ahmed, S.; Ahmed, A.M. Graphite/rolled graphene oxide/carbon nanotube photoelectrode for water splitting of exhaust car solution. Int. J. Energy Res. 2020, 44, 7687–7697. [Google Scholar] [CrossRef]
- Shaban, N.; Abukhadra, M.R.; Rabia, M.; Elkader, Y.; Abd El-Halim, M.R. Investigation the adsorption properties of graphene oxide and polyaniline nano/micro structures for efficient removal of toxic Cr(VI) contaminants from aqueous solutions; kinetic and equilibrium studies. Rendiconti Lincei. Scienze Fisiche e Naturali 2018, 29, 154. [Google Scholar] [CrossRef]
- Shaban, M.; Rabia, M.; Eldakrory, M.G.; Maree, R.M.; Ahmed, A.M. Efficient photoselectrochemical hydrogen production utilizing of APbI3 (A = Na, Cs, and Li) perovskites nanorods. Int. J. Energy Res. 2020, er.6326. [Google Scholar] [CrossRef]
- Zaki, S.E.; Basyooni, M.A.; Shaban, M.; Rabia, M.; Eker, Y.R.; Attia, G.F.; Yilmaz, M.; Ahmed, A.M. Role of oxygen vacancies vanadium oxide and oxygen functional groups in graphene oxide for room temperature CO2 gas sensors. Sens. Actuator A Phys. 2019, 294, 17–24. [Google Scholar] [CrossRef]
- Rabia, M.; Shaban, M.; Jibali, B.M.; Abdelkhaliek, A.A. Effect of Annealing Temperature on the Photoactivity of ITO/VO2 (M)/Au Film Electrodes for Water Splitting. J. Nanosci. Nanotechnol. 2020, 20, 4120–4130. [Google Scholar] [CrossRef]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 63–71. [Google Scholar] [CrossRef]
- Thompson, M.C.; Barad, B.A.; Wolff, A.M. Temperature-jump solution X-ray scattering reveals distinct motions in a dynamic enzyme. Nat. Chem. 2019, 11, 1058–1066. [Google Scholar] [CrossRef]
- Mohamed, H.S.H.; Rabia, M.; Zhou, X.G.; Qin, X.S.; Khabiri, G.; Shaban, M.; Younus, H.A.; Taha, S.; Hu, Z.Y.; Liu, J.; et al. Phase-junction Ag/TiO2 nanocomposite as photocathode for H2 generation. J. Mater. Sci. Technol. 2021, 83, 179–187. [Google Scholar] [CrossRef]
- Cannelli, Q.; Bacellar, C.; Ingle, R.A.; Bohinc, R.; Kinschel, D.; Bauer, B.; Ferreira, D.S.; Grolimund, D.; Mancini, G.F.; Cherqui, M. Toward time-resolved laser T-jump/X-ray probe spectroscopy in aqueous solutions. Struct. Dyn. 2019, 6, 064303. [Google Scholar] [CrossRef]
- Mohamed, H.S.H.; Rabia, M.; Shaban, M.; Taha, S. Controlled synthesis of CdS nanoflowers thin films for H2 electro-generation. Mater. Sci. Semicond. Process. 2020, 120. [Google Scholar] [CrossRef]
- Naldoni, A.; Guler, U.; Wang, Z.; Marelli, M.; Malara, F.; Meng, X.; Besteiro, L.V.; Govorov, A.O.; Kildishev, A.V.; Boltasseva, A.; et al. Broadband Hot-Electron Collection for Solar Water Splitting with Plasmonic Titanium Nitride. Adv. Opt. Mater. 2017, 5, 1601031. [Google Scholar] [CrossRef] [Green Version]
- Hao, Q.; Wang, C.; Huang, H.; Li, W.; Du, D.; Han, D.; Qiu, T.; Chu, P.K. Aluminum plasmonic photocatalysis. Sci. Rep. 2015, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Jiang, L.; Zhou, G.; Mi, J.; Wu, Z. Fabrication of visible-light-driven one-dimensional anatase TiO2/Ag heterojunction plasmonic photocatalyst. Catal. Commun. 2012, 24, 48–51. [Google Scholar] [CrossRef]
- Sellappan, R.; Nielsen, M.G.; González-Posada, F.; Vesborg, P.C.K.; Chorkendorff, I.; Chakarov, D. Effects of plasmon excitation on photocatalytic activity of Ag/TiO2 and Au/TiO2 nanocomposites. J. Catal. 2013, 307, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Langhammer, C.; Schwind, M.; Kasemo, B.; Zorić, I. Localized Surface Plasmon Resonances in Aluminum Nanodisks. Nano Lett. 2008, 8, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Long, R.; Prezhdo, O.V. Instantaneous Generation of Charge-Separated State on TiO2 Surface Sensitized with Plasmonic Nanoparticles. J. Am. Chem. Soc. 2014, 136, 4343–4354. [Google Scholar] [CrossRef]
- Christopher, P.; Ingram, D.B.; Linic, S. Enhancing photochemical activity of semiconductor nanoparticles with optically active Ag nanostructures: Photochemistry mediated by Ag surface plasmons. J. Phys. Chem. C 2010, 114, 9173–9177. [Google Scholar] [CrossRef]
- Ingram, D.B.; Linic, S. Water splitting on composite plasmonic-metal/semiconductor photoelectrodes: Evidence for selective plasmon-induced formation of charge carriers near the semiconductor surface. J. Am. Chem. Soc. 2011, 133, 5202–5205. [Google Scholar] [CrossRef]
- Guler, U.; Shalaev, V.M.; Boltasseva, A. Nanoparticle plasmonics: Going practical with transition metal nitrides. Mater. Today 2015, 18, 227–237. [Google Scholar] [CrossRef]
- Yick, S.; Murdock, A.T.; Martin, P.J.; Kennedy, D.F.; Maschmeyer, T.; Bendavid, A. Tuning the plasmonic response of TiN nanoparticles synthesised by the transferred arc plasma technique. Nanoscale 2018, 10, 7566–7574. [Google Scholar] [CrossRef] [PubMed]
- Fillot, F.; Morel, T.; Minoret, S.; Matko, I.; Maîtrejean, S.; Guillaumot, B.; Chenevier, B.; Billon, T. Investigations of titanium nitride as metal gate material, elaborated by metal organic atomic layer deposition using TDMAT and NH3. Microelectron. Eng. 2005, 82, 248–253. [Google Scholar] [CrossRef]
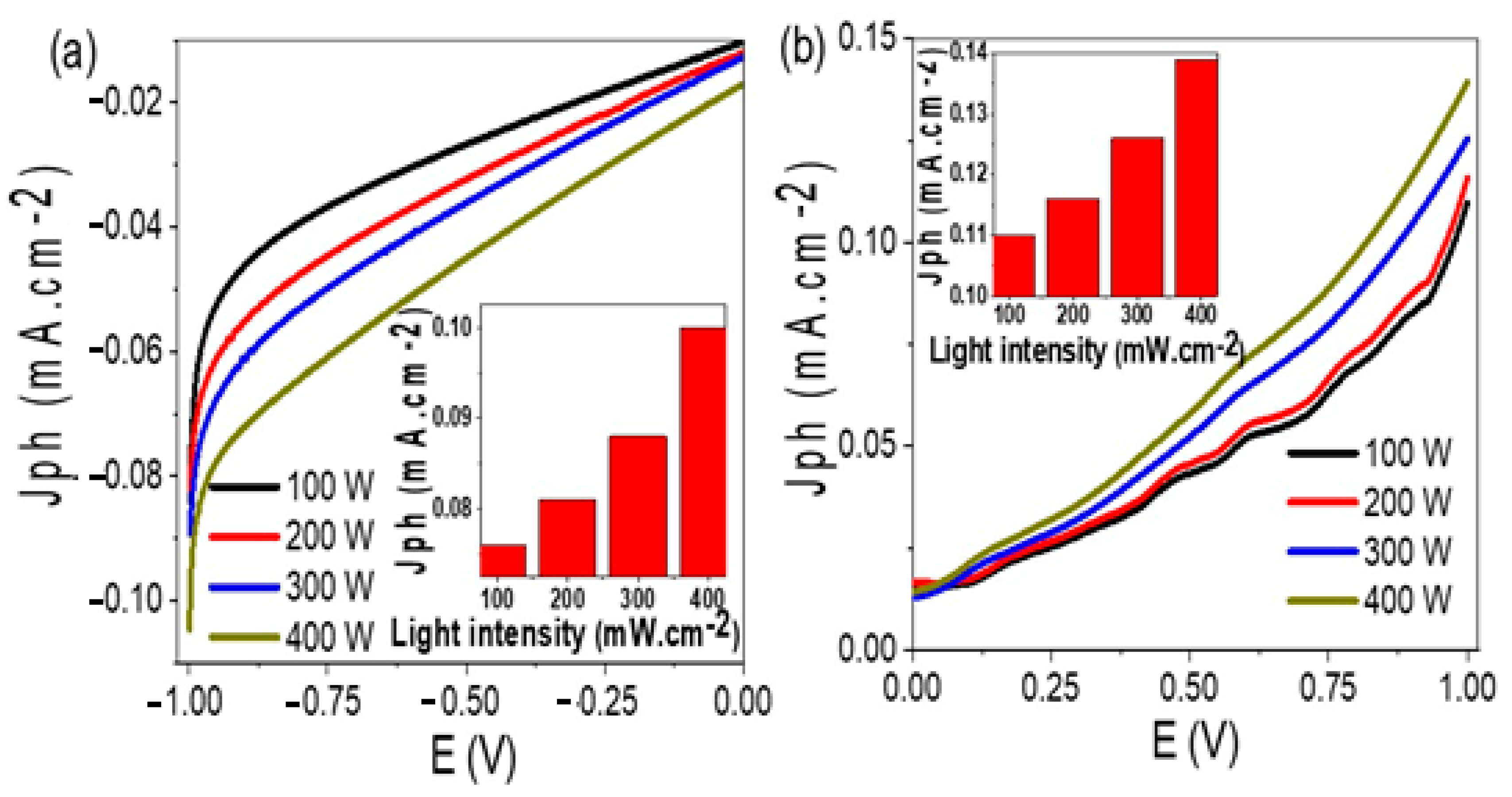
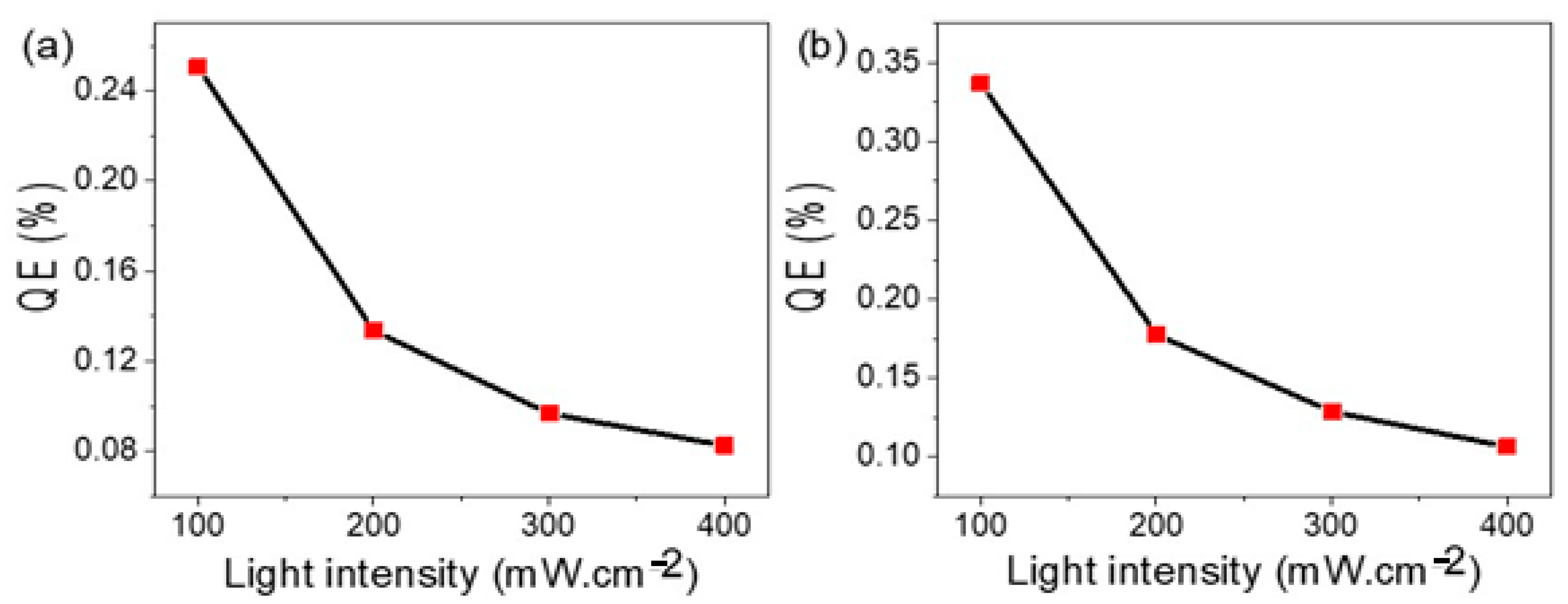
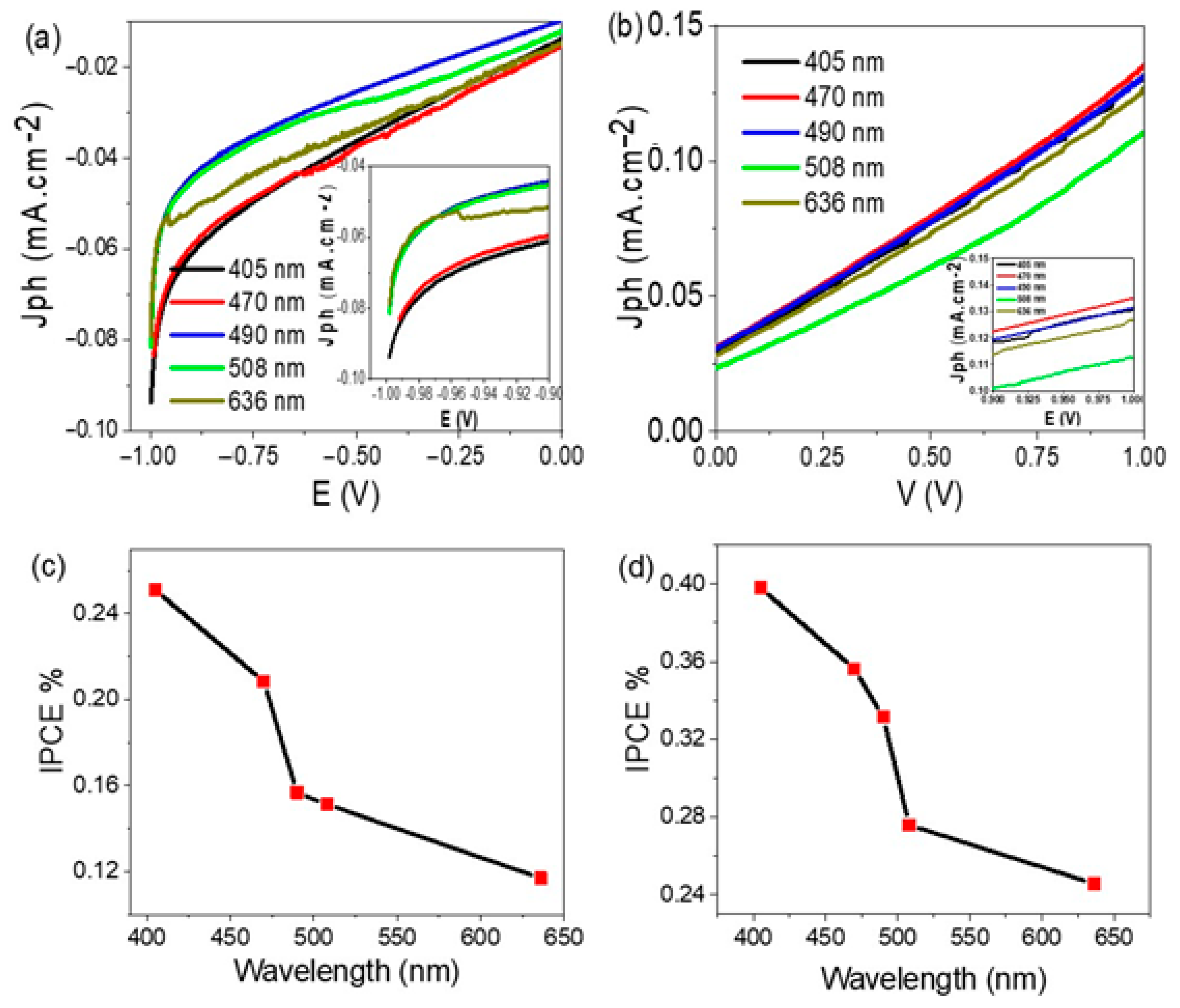
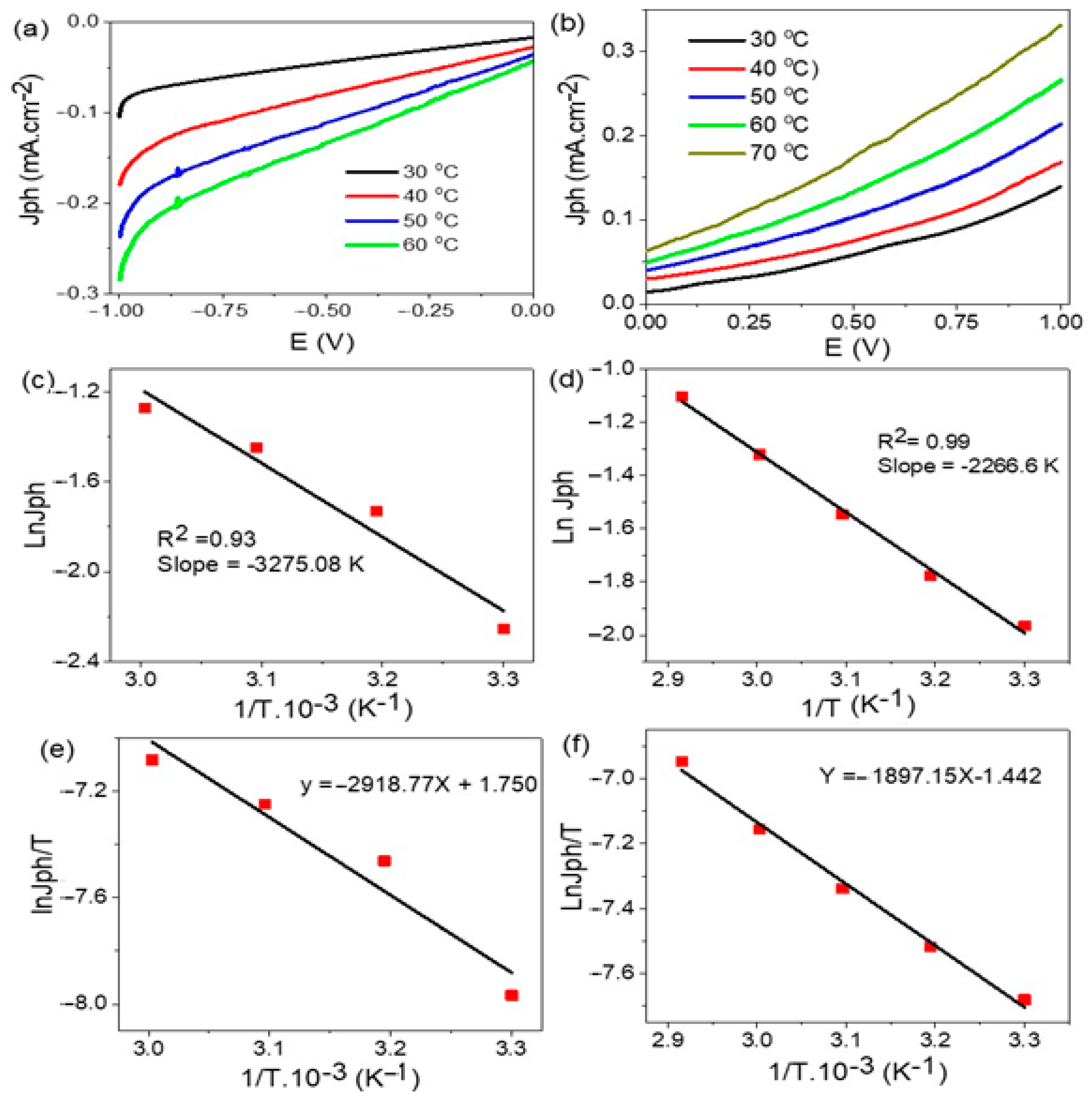
| Photoelectrode | Electrolyte | Jph | IPCE % (405 nm) | Ref. |
|---|---|---|---|---|
| BiFeO3 | H2SO4 | 0.1 mA·cm−2 | 0.21 | [28] |
| PrFeO | H2SO4 | −130 µA·cm−2 | - | [46] |
| Au/Pb(Zr, Ti)O3 | H2SO4 | 0.06 mA·cm−2 | 0.2 | [47] |
| Poly(3-aminobenzoic acid) frame | H2SO4 | 0.08 mA·cm−2 | - | [50] |
| TiN-TiO2 | H2SO4 | 3.0 nA·cm−2 | 0.03 | [64] |
| TiN/TiO2/Al2O3 Au/TiN/TiO2/Al2O3 | Sewage water Sewage water | 0.0.9 mA·cm−2 0.14 mA·cm−2 | 0.25 0.39 | Present work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almohammedi, A.; Shaban, M.; Mostafa, H.; Rabia, M. Nanoporous TiN/TiO2/Alumina Membrane for Photoelectrochemical Hydrogen Production from Sewage Water. Nanomaterials 2021, 11, 2617. https://doi.org/10.3390/nano11102617
Almohammedi A, Shaban M, Mostafa H, Rabia M. Nanoporous TiN/TiO2/Alumina Membrane for Photoelectrochemical Hydrogen Production from Sewage Water. Nanomaterials. 2021; 11(10):2617. https://doi.org/10.3390/nano11102617
Chicago/Turabian StyleAlmohammedi, Abdullah, Mohamed Shaban, Huda Mostafa, and Mohamed Rabia. 2021. "Nanoporous TiN/TiO2/Alumina Membrane for Photoelectrochemical Hydrogen Production from Sewage Water" Nanomaterials 11, no. 10: 2617. https://doi.org/10.3390/nano11102617







