Colorimetric, Naked-Eye Detection of Lysozyme in Human Urine with Gold Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
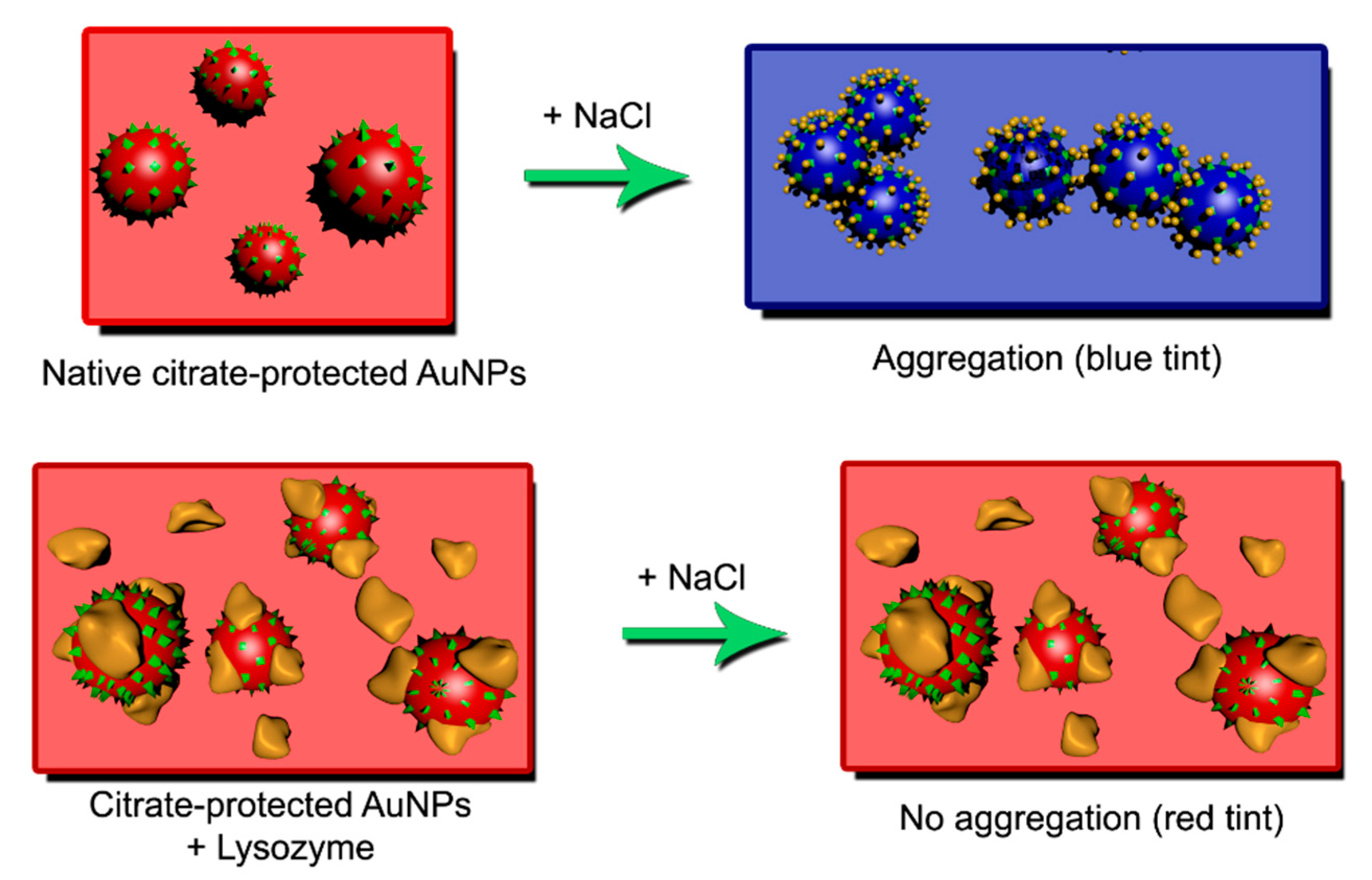
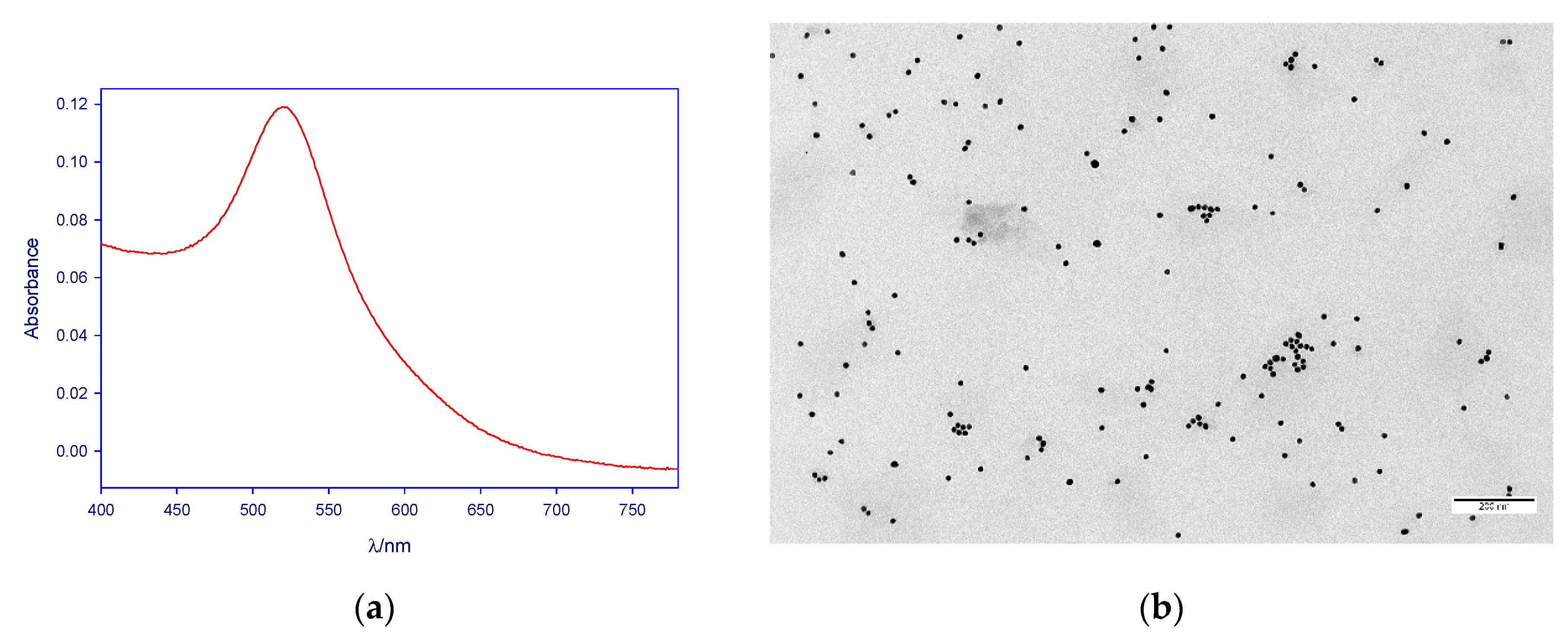
2.1. Nanoparticle Synthesis and Stabilization
2.2. Preliminary Testing
2.2.1. Sample Collection and Processing for Preliminary Testing
2.2.2. Preliminary Testing Protocols
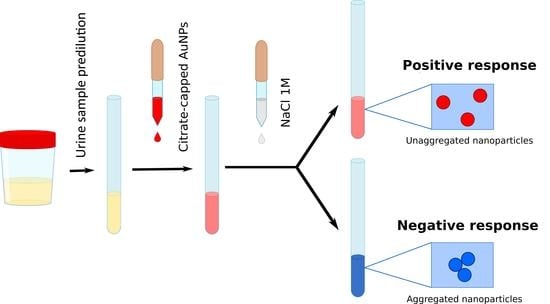
2.3. Working Protocol
2.3.1. Urine Collection and Processing
2.3.2. Sample Preparation
2.4. Sample Measurement and Obtainment of CIEL*a*b* Parameters
- Preliminary tests: referenced to L*a*b* parameters of a blank sample, which does contain aggregated AuNPs without urine or lysozyme and, therefore, can be employed as a neutral reference point. However, in doing so, it was observed that zero-lysozyme samples could also show subtle color differences from the urine-free blank.
- Working tests: referenced to the mean L*a*b* parameters obtained for all zero (lysozyme-free) urine samples. In this way, a “neutral” reference that was not directly related to any of the samples was created, and residual matrix effects of urine that could induce color changes were accounted for and compensated.
3. Results
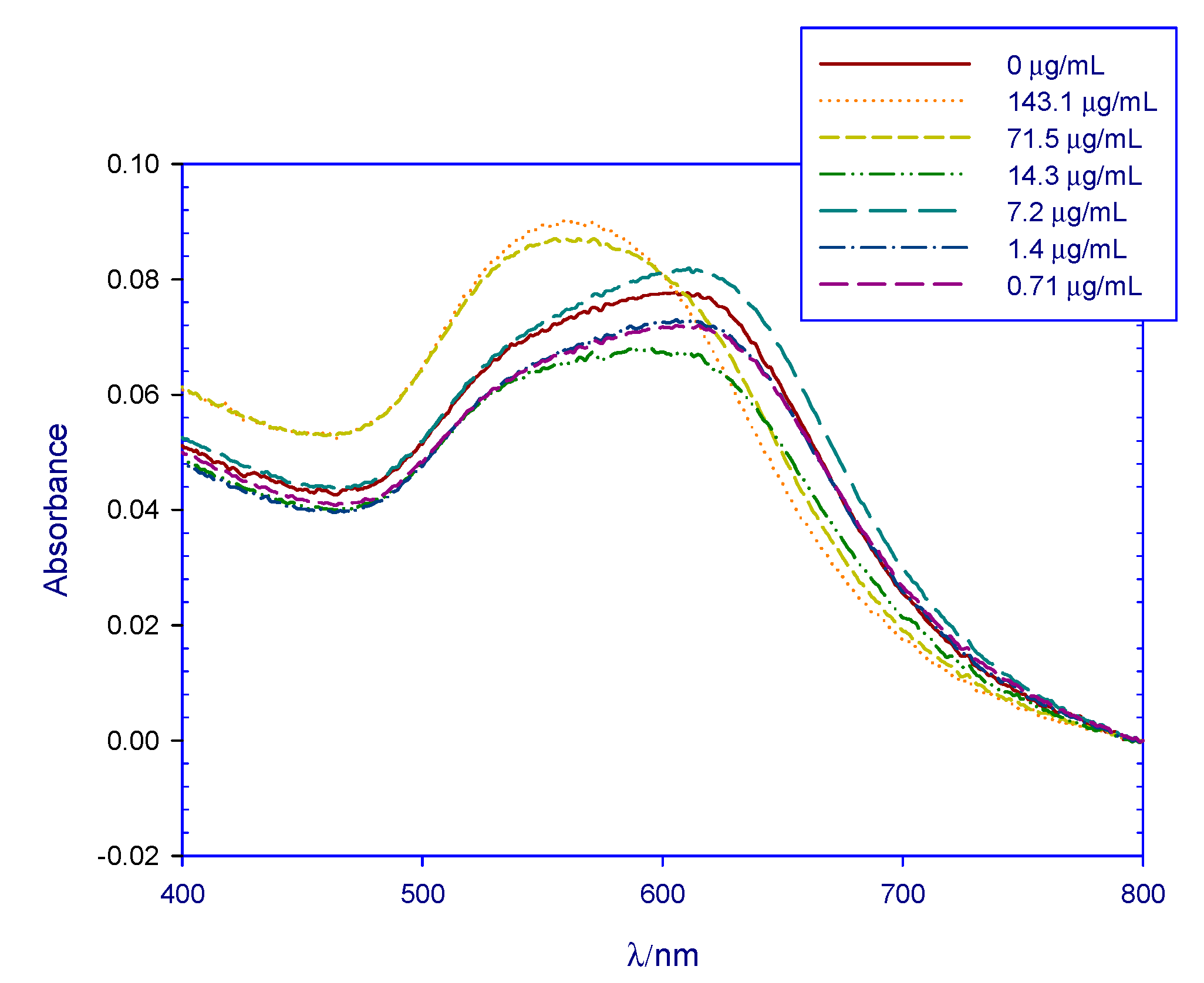
3.1. Preliminary Test Results
3.2. Working Test Results
4. Discussion
4.1. Preliminary Test Results
4.2. Working Test Results
4.3. Result Evaluation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polson, A. Immune Globulin and its Use in Prophylaxis and Therapy of Infectious Diseases. S. Afr. Med. J. 1957, 31, 1242–1244. [Google Scholar] [PubMed]
- Prin, C.; Bene, M.C.; Gobert, B.; Montagne, P.; Faure, G.C. Isoelectric restriction of human immunoglobulin isotypes. BBA Gen. Subj. 1995, 1243, 287–289. [Google Scholar] [CrossRef]
- Osserman, E.F.; Lawlor, D.P. Serum and Urinary Lysozyme (Muramidase) in Monocytic and Monomyelocytic Leukemia. J. Exp. Med. 1966, 124, 921–952. [Google Scholar] [CrossRef]
- Noble, R.E.; Fudenberg, H.H. Leukocyte lysozyme activity in myelocytic leukemia. Blood 1967, 30, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Harrison, J.F.; Parker, R.W.; de Silva, K.L. Lysozymuria and acute disorders of renal function. J. Clin. Pathol. 1973, 26, 278–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petryayeva, E.; Krull, U.J. Localized surface plasmon resonance: Nanostructures, bioassays and biosensing—A review. Anal. Chim. Acta 2011, 706, 8–24. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, E.; Pradhan, N. Gold nanoparticles as efficient sensors in colorimetric detection of toxic metal ions: A review. Sens. Actuators B Chem. 2017, 238, 888–902. [Google Scholar] [CrossRef]
- Zhao, W.; Chiuman, W.; Lam, J.C.F.; Brook, M.A.; Li, Y. Simple and rapid colorimetric enzyme sensing assays using non-crosslinking gold nanoparticle aggregation. Chem. Commun. 2007, 8, 3729. [Google Scholar] [CrossRef]
- Jazayeri, M.H.; Aghaie, T.; Avan, A.; Vatankhah, A.; Ghaffari, M.R.S. Colorimetric detection based on gold nano particles (GNPs): An easy, fast, inexpensive, low-cost and short time method in detection of analytes (protein, DNA, and ion). Sens. Bio-Sens. Res. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Gui, G.-F.; Zhuo, Y.; Chai, Y.-Q.; Xiang, Y.; Yuan, R. A novel {ECL} biosensor for β-lactamase detection: Using RU(II) linked-ampicillin complex as the recognition element. Biosens. Bioelectron. 2015, 70, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, P.; Ceyhan, B.; Niemeyer, C.M. Sensitive detection of proteins using difunctional DMA-gold nanoparticles. Small 2005, 1, 844–848. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, K.; Ma, H. Electrochemical DNA Biosensors Based on Gold Nanoparticles/Cysteamine/Poly(glutamic acid) Modified Electrode. Am. J. Biomed. Sci. 2009, 1, 115–125. [Google Scholar] [CrossRef]
- Chen, C.; Luo, M.; Ye, T.; Li, N.; Ji, X.; He, Z. Sensitive colorimetric detection of protein by gold nanoparticles and rolling circle amplification. Analyst 2015, 140, 4515–4520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; McKelvie, I.D.; Cattrall, R.W.; Kolev, S.D. Colorimetric detection based on localised surface plasmon resonance of gold nanoparticles: Merits, inherent shortcomings and future prospects. Talanta 2016, 152, 410–422. [Google Scholar] [CrossRef]
- Nirala, N.R.; Saxena, P.S.; Srivastava, A. Colorimetric detection of cholesterol based on enzyme modified gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 190, 506–512. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-Y.; Chuang, T.-L.; Wu, T.-H.; Wei, S.-C.; Liao, H.; Lin, C.-W. Aptamer-based colorimetric detection of proteins using a branched DNA cascade amplification strategy and unmodified gold nanoparticles. Biosens. Bioelectron. 2016, 78, 200–205. [Google Scholar] [CrossRef]
- Yang, J.A.; Johnson, B.J.; Wu, S.; Woods, W.S.; George, J.M.; Murphy, C.J. Study of wild-type α-synuclein binding and orientation on gold nanoparticles. Langmuir 2013, 29, 4603–4615. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Xu, X.; Hu, K.; Xiang, M.; Li, L.; Liu, F.; Li, N. Direct determination of urinary lysozyme using surface plasmon resonance light-scattering of gold nanoparticles. Talanta 2010, 82, 693–697. [Google Scholar] [CrossRef]
- Fu, F.; Li, L.; Luo, Q.; Li, Q.; Guo, T.; Yu, M.; Song, Y.; Song, E. Selective and sensitive detection of lysozyme based on plasmon resonance light-scattering of hydrolyzed peptidoglycan stabilized-gold nanoparticles. Analyst 2018, 143, 1133–1140. [Google Scholar] [CrossRef]
- Wai, J.L.; New, S.Y. Cysteamine-coated gold nanoparticles for bimodal colorimetric detection with inverse sensitivity: A proof-of-concept with lysozyme. RSC Adv. 2019, 10, 1088–1094. [Google Scholar] [CrossRef] [Green Version]
- Prado-Gotor, R.; Jimenez-Ruiz, A.; Carnerero, J.M.; Grueso, E.; Villa, I. CIELab chromaticity evolution to measure the binding free energy of non-colored biomolecules to gold nanoparticles. RSC Adv. 2015, 5, 85039–85045. [Google Scholar] [CrossRef]
- Chen, T.; Xie, N.; Viglianti, L.; Zhou, Y.; Tan, H.; Tang, B.Z.; Tang, Y. Quantitative urinalysis using aggregation-induced emission bioprobes for monitoring chronic kidney disease. Faraday Discuss. 2017, 196, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Chan, D.S.-H.; Kwong, D.W.J.; He, H.-Z.; Leung, C.-H.; Ma, D.-L. Detection of nicking endonuclease activity using a G-quadruplex-selective luminescent switch-on probe. Chem. Sci. 2014, 5, 4561–4568. [Google Scholar] [CrossRef]
- Mishra, R.K.; Hayat, A.; Mishra, G.K.; Catanante, G.; Sharma, V.; Marty, J.-L. A novel colorimetric competitive aptamer assay for lysozyme detection based on superparamagnetic nanobeads. Talanta 2017, 165, 436–441. [Google Scholar] [CrossRef]
- Ortiz-Aguayo, D.; Del Valle, M. Label-Free Aptasensor for Lysozyme Detection Using Electrochemical Impedance Spectroscopy. Sensors 2018, 18, 354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tohidi Moghadam, T.; Ranjbar, B. Heat induced aggregation of gold nanorods for rapid visual detection of lysozyme. Talanta 2015, 144, 778–787. [Google Scholar] [CrossRef]
- Rezaei, B.; Jamei, H.R.; Ensafi, A.A. An ultrasensitive and selective electrochemical aptasensor based on rGO-MWCNTs/Chitosan/carbon quantum dot for the detection of lysozyme. Biosens. Bioelectron. 2018, 115, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Qin, G.; Lan, Y.; Wei, Y.; Dong, C. A turn-on phosphorescence aptasensor for ultrasensitive detection of lysozyme in humoral samples. Sens. Actuators B Chem. 2019, 289, 100–105. [Google Scholar] [CrossRef]
- Qiu, Z.; Shu, J.; He, Y.; Lin, Z.; Zhang, K.; Lv, S.; Tang, D. CdTe/CdSe quantum dot-based fluorescent aptasensor with hemin/G-quadruplex DNzyme for sensitive detection of lysozyme using rolling circle amplification and strand hybridization. Biosens. Bioelectron. 2017, 87, 18–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrödinger, E. Über das Verhältnis der Vierfarben zur Dreifarbentheorie. Sitz. Akad. Wiss. Math. Kl. 1925, 134, 471–490. [Google Scholar]
- Kuehni, R.G. Color Space and Its Divisions; John Wiley and Sons: Hoboken, NJ, USA, 2003; ISBN 3175723993. [Google Scholar]
- Mahy, M.; Van Eycken, L.; Oosterlinck, A. Evaluation of Uniform Color Spaces Developed after the Adoption of CIELAB and CIELUV. Color Res. Appl. 1994, 19, 105–121. [Google Scholar] [CrossRef]
- Wojciech, M.; Maciej, T. Color difference Delta E—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Magubane, S.E.; Mlambo, M.; Mabaso, M.H.; Muthwa, S.F.; Kruger, H.G.; Mdluli, P.S. Optimization of CIEL*a*b*/Yxy colour system for colorimetric devices fabricated with gold nanoparticles. J. Mol. Struct. 2019, 1191, 271–277. [Google Scholar] [CrossRef]
- Mbambo, A.T.; Kruger, H.G.; Mdluli, P.S.; Madikizela, L.M. Fabrication and application of a gold nanoparticle-based colorimetric device for the determination of NaCl in seawater and estuarine water. J. Nanoparticle Res. 2019, 21, 135. [Google Scholar] [CrossRef]
- Chaisiwamongkhol, K.; Labaidae, S.; Pon-in, S.; Pinsrithong, S.; Bunchuay, T.; Phonchai, A. Smartphone-based colorimetric detection using gold nanoparticles of sibutramine in suspected food supplement products. Microchem. J. 2020, 158, 105273. [Google Scholar] [CrossRef]
- Lee, P.C.; Meisel, D. Adsorption and surface-enhanced Raman of dyes on silver and gold sols. J. Phys.Chem. 1982, 86, 3391–3395. [Google Scholar] [CrossRef]
- OIV. Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2014. [Google Scholar]
- Hunt, R.W.G.; Pointer, M.R. Measuring Colour; John Wiley and Sons: Chichester, UK, 2011. [Google Scholar]
- McDonald, R.; Smith, K.J. CIE94—A new colour-difference formula. J. Soc. Dye. Colour. 1995, 111, 376–379. [Google Scholar] [CrossRef]
- ISO. INTERNATIONAL STANDARD, Graphic Technology—Process Control for the Production of Half-Tone Colour Separations, Proof and Production Prints; ISO 12647-2; ISO: Geneva, Switzerland, 2004. [Google Scholar]
- Wiernik, P.H.; Serpick, A.A. Clinical Significance of Serum and Urinary Muraminidase Activity in Leukemia and Other Hematologic Malignancies. Am. J. Med. 1969, 46, 330–343. [Google Scholar] [CrossRef]
- Farr, M.; Wardle, E.N.; Wilkinson, K. Lysozymuria in diabetes. Br. Med. J. 1976, 1, 624–625. [Google Scholar] [CrossRef] [Green Version]
- Edwards, J.J.; Tollaksen, S.L.; Anderson, N.G. Proteins of human urine. III. Identification and two-dimensional electrophoretic map positions of some major urinary proteins. Clin. Chem. 1982, 28, 941–948. [Google Scholar] [CrossRef]
- De Paoli Lacerda, S.H.; Park, J.J.; Meuse, C.; Pristinski, D.; Becker, M.L.; Karim, A.; Douglas, J.F. Interaction of gold nanoparticles with common human blood proteins. ACS Nano 2010, 4, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Mandal, S.; Haldar, S.; Chattopadhyay, K.; Patra, A. Interaction of gold nanoparticle with human serum albumin (HSA) protein using surface energy transfer. J. Phys. Chem. C 2011, 115, 24037–24044. [Google Scholar] [CrossRef]
- Berggard, I. The plasma proteins in normal urine. Nature 1960, 187, 776–777. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, T.G.; Kariya, K.P.; Prasad, P.K.V.; Chaube, S.K.; Kumar, D. Development of enzyme-linked immunosorbent assay for estimation of urinary albumin. J. Immunoass. Immunochem. 2014, 35, 300–313. [Google Scholar] [CrossRef] [PubMed]

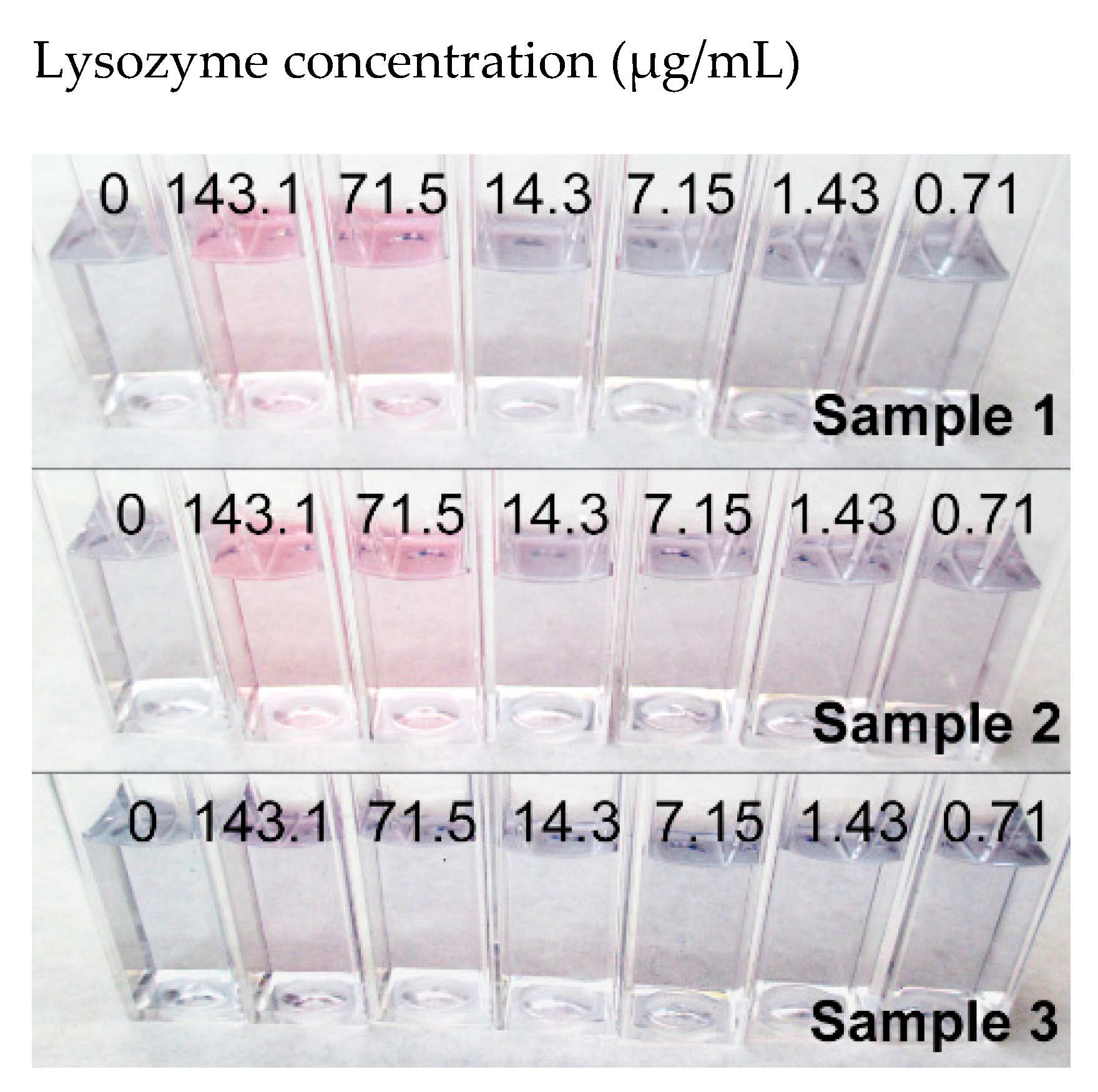

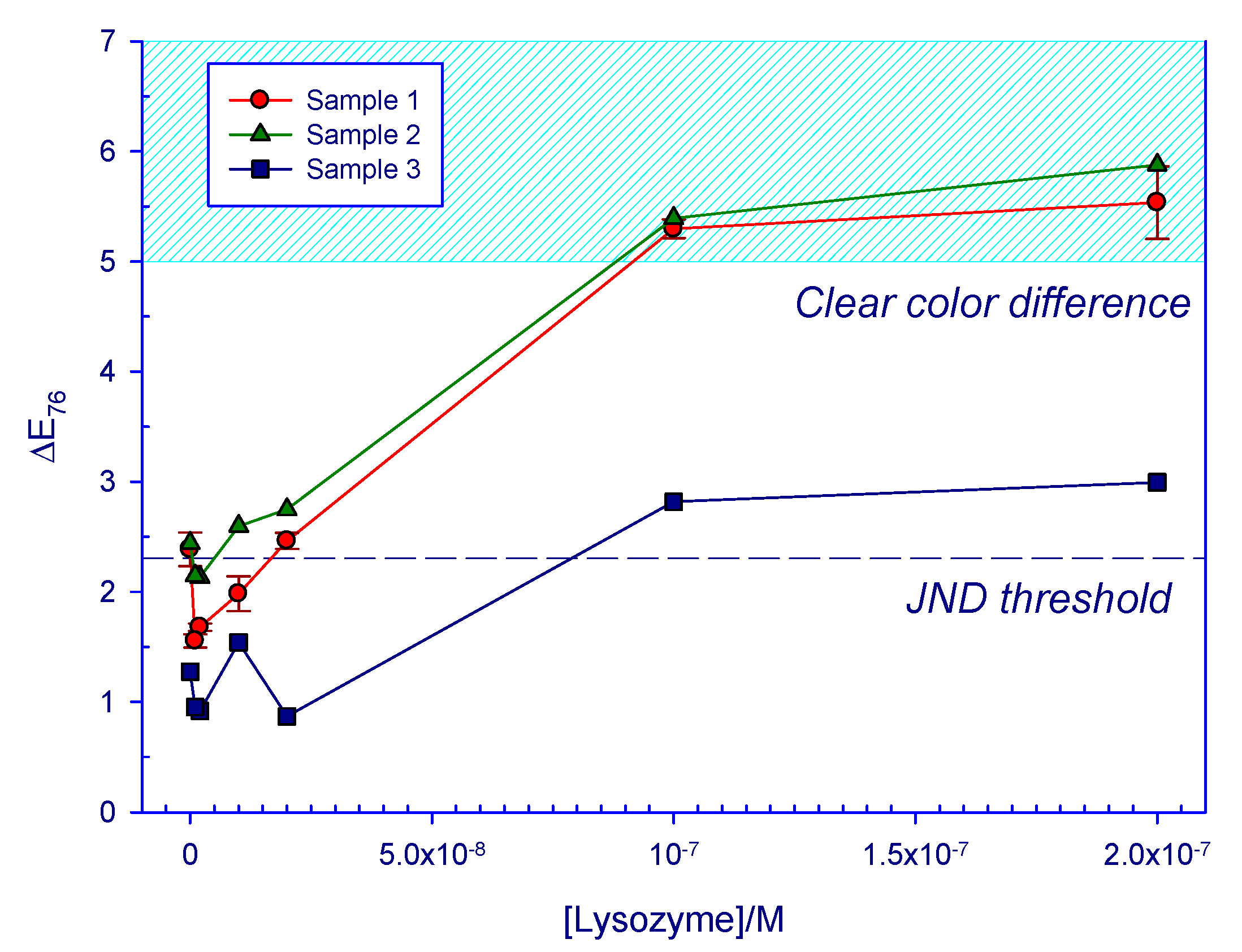
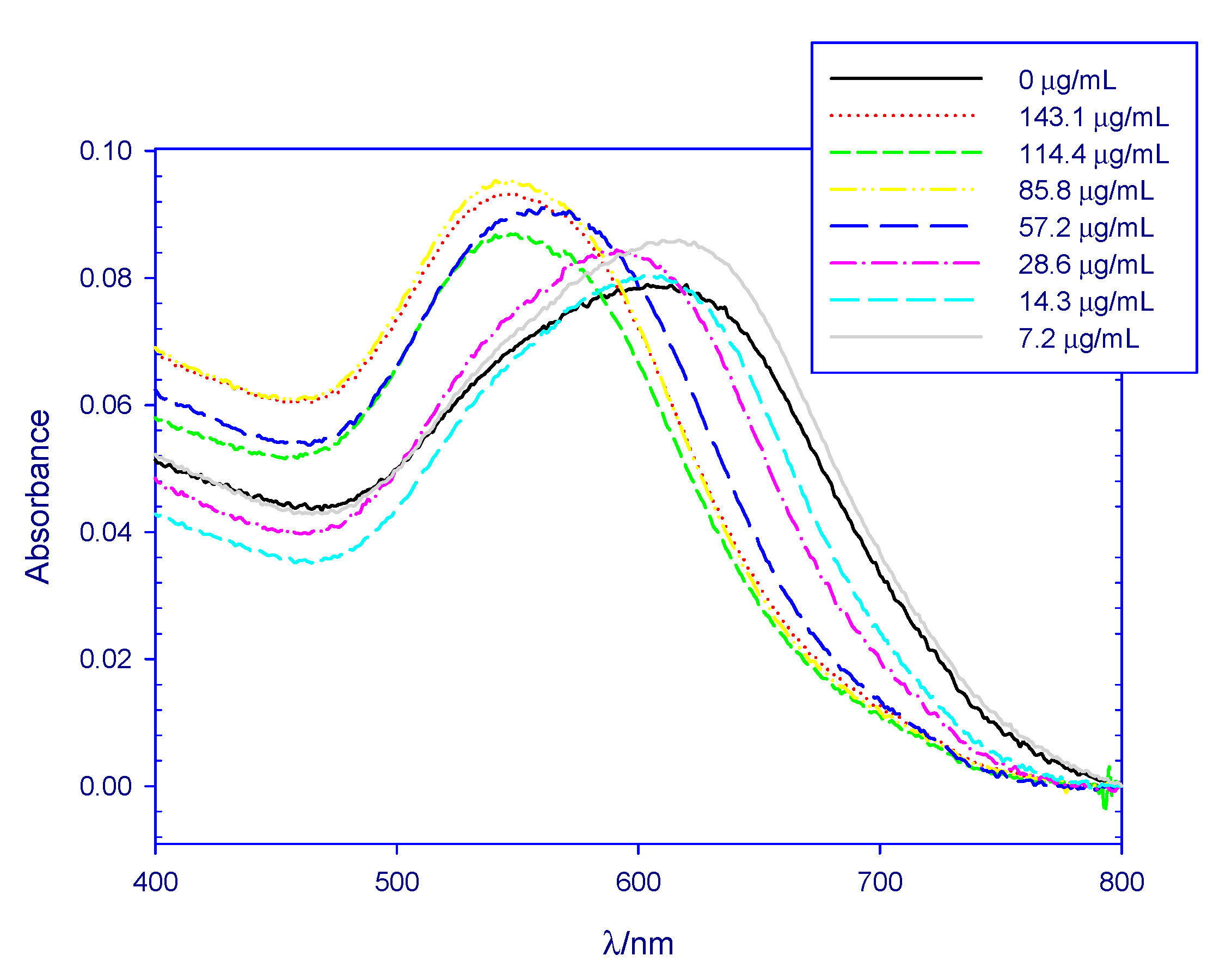

| Sample 1.1 | Sample 1.2 | Sample 1.3 | Sample 2 | Sample 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [Lys]/µg·mL−1 | ΔE76 | ΔE94 | ΔE76 | ΔE94 | ΔE76 | ΔE94 | ΔE76 | ΔE94 | ΔE76 | ΔE94 |
| 143.07 | 5.7 | 5.6 | 5.7 | 5.6 | 5.2 | 5.1 | 5.9 | 5.8 | 3.0 | 3.0 |
| 71.54 | 5.4 | 5.3 | 5.2 | 5.1 | 5.3 | 5.2 | 5.4 | 5.3 | 2.8 | 2.8 |
| 14.31 | 2.5 | 2.5 | 2.5 | 2.4 | 2.4 | 2.4 | 2.7 | 2.7 | 0.9 | 0.9 |
| 7.15 | 2.1 | 2.1 | 2.0 | 2.0 | 1.8 | 1.8 | 2.6 | 2.6 | 1.5 | 1.5 |
| 1.43 | 1.7 | 1.6 | 1.7 | 1.7 | 1.7 | 1.6 | 2.1 | 2.1 | 0.9 | 0.9 |
| 0.72 | 1.5 | 1.5 | 1.6 | 1.6 | 1.6 | 1.6 | 2.1 | 2.1 | 1.0 | 1.0 |
| 0 | 2.6 | 2.5 | 2.3 | 2.3 | 2.3 | 2.2 | 2.4 | 2.4 | 1.3 | 1.3 |
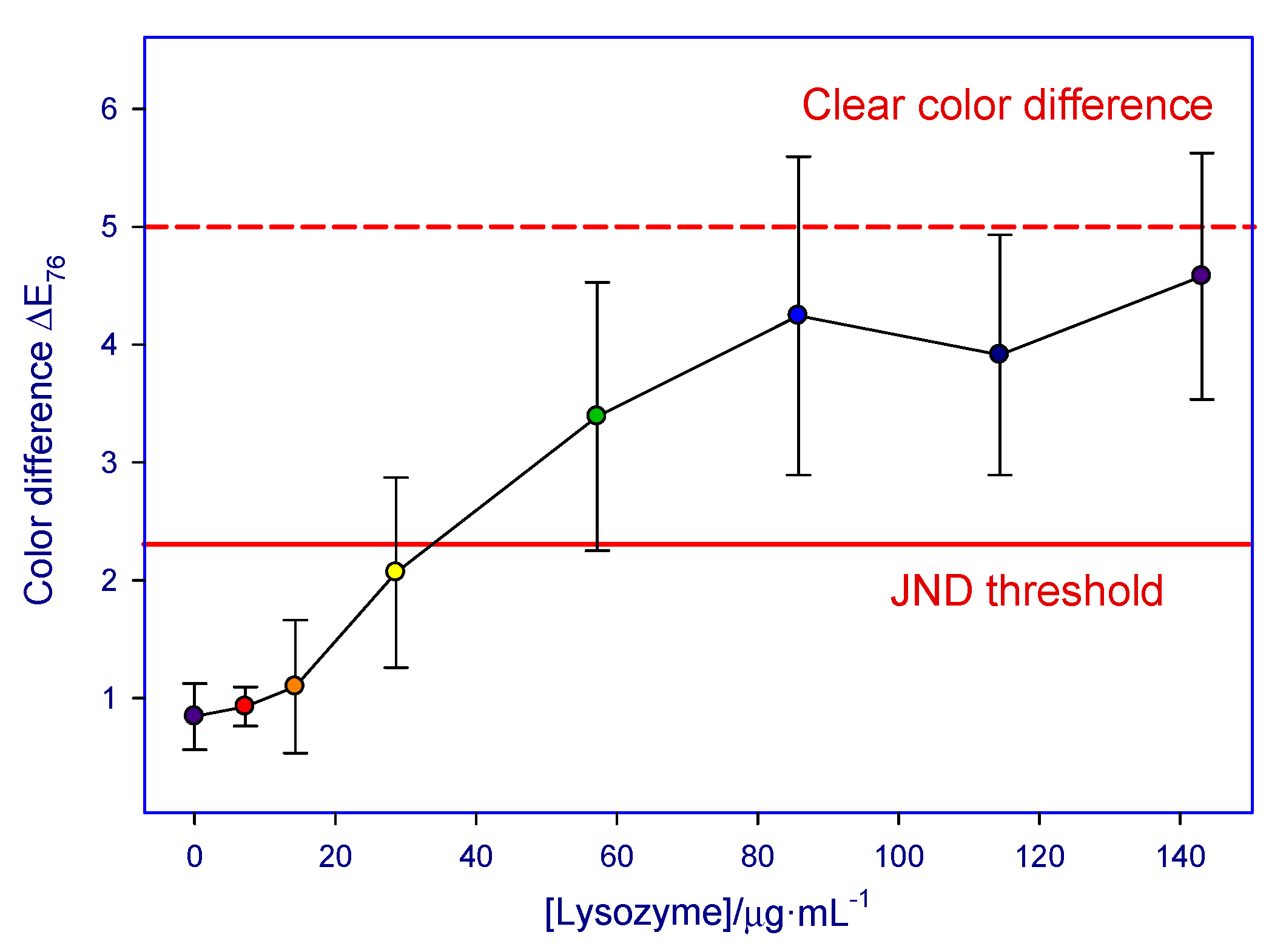
| Lysozyme (µg/mL) | A | B | C | D | E | F | G | H | I | J | Medium Value |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 143.1 | 3.1 | 3.5 | 5.2 | 6.1 | 5.3 | 4.0 | 5.9 | 4.9 | 3.7 | 3.9 | 4.7 ± 1.0 |
| 114.4 | 2.4 | 3.7 | 6.1 | 2.8 | 3.8 | 3.8 | 4.6 | 4.3 | 3.3 | 4.3 | 4.0 ± 1.0 |
| 85.8 | 1.7 | 4.3 | 4.4 | 6.3 | 4.2 | 4.3 | 5.8 | 4.1 | 3.1 | - | 4.4 ± 1.4 |
| 57.2 | 1.1 | 3.6 | 3.1 | 4.7 | 2.9 | 2.9 | 5.0 | 3.6 | 2.8 | 4.3 | 3.5 ± 1.1 |
| 28.6 | 0.5 | 2.0 | 1.8 | 3.0 | 2.6 | 1.2 | 3.0 | 2.0 | 1.8 | 2.8 | 2.2 ± 0.8 |
| 14.3 | 0.5 | 0.9 | 1.1 | 0.5 | 1.5 | 1.1 | 2.3 | 1.1 | 0.9 | - | 1.6 ± 0.6 |
| 7.2 | 1.0 | - | 0.9 | 0.6 | 1.1 | 1.0 | 1.0 | 0.9 | 0.8 | 1.1 | 1.3 ± 0.2 |
| 0 | 0.7 | 0.8 | 1.1 | 1.3 | 1.0 | 0.3 | 1.1 | 0.9 | 0.6 | 0.7 | 1.1 ± 0.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, P.M.; Fernández-Acejo, F.J.; Carnerero, J.M.; Prado-Gotor, R.; Jimenez-Ruiz, A. Colorimetric, Naked-Eye Detection of Lysozyme in Human Urine with Gold Nanoparticles. Nanomaterials 2021, 11, 612. https://doi.org/10.3390/nano11030612
Castillo PM, Fernández-Acejo FJ, Carnerero JM, Prado-Gotor R, Jimenez-Ruiz A. Colorimetric, Naked-Eye Detection of Lysozyme in Human Urine with Gold Nanoparticles. Nanomaterials. 2021; 11(3):612. https://doi.org/10.3390/nano11030612
Chicago/Turabian StyleCastillo, Paula M., Francisco J. Fernández-Acejo, Jose M. Carnerero, Rafael Prado-Gotor, and Aila Jimenez-Ruiz. 2021. "Colorimetric, Naked-Eye Detection of Lysozyme in Human Urine with Gold Nanoparticles" Nanomaterials 11, no. 3: 612. https://doi.org/10.3390/nano11030612