Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy
Abstract
:1. Cancer
2. Photodynamic Therapy
3. Phthalocyanines as Therapeutic Agents in PDT
4. Polymeric Nanoparticle Delivery Systems
4.1. Polymeric Micelles
4.2. Polymersomes
4.3. Polymeric Nanoparticles
4.4. Dendrimers
5. Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy
5.1. Pc-PEGylated Delivery Systems in PDT
5.2. Pc-Polymeric Nanocarriers Based on Synthetic Polymers in PDT
5.3. Polymeric Nanocarriers Based on Natural Polymers
5.4. Polymeric Nanocarriers in PDT with Assistance of UCNPs
5.5. Pc-Polymeric Nanocarriers in Combination of PDT with Chemotherapy
5.6. Bioresponsive Pc-Polymeric Nanocarriers in PDT
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| λmax | maximum absorption |
| ΦΔ | singlet oxygen quantum yield |
| AlPc | aluminum-phthalocyanine chloride |
| AlPc-sulfo4 | tetrasulfonated aluminum phthalocyanine |
| CPT | camptothecin |
| DEGMA | di(ethylene glycol) methyl ether methacrylate |
| Dex-b-AcDex | dextran-block-acetalated dextran |
| DOX | doxorubicin |
| DTT | dithiothreitol |
| FDA | Food and Drug Administration |
| GaPc | gallium (III) phthalocyanine chloride |
| GSH | glutathione |
| HEMA | 2-hydroxyethyl methacrylate |
| InPc | indium(III) phthalocyanine chloride |
| MNP | magnetic nanoparticle |
| NP | nanoparticle |
| PEG-b-PLLA | poly(L-lactide)-b-poly(ethylene oxide) block copolymer |
| PEG-b-PNIPAAM | poly(ethylene glycol)-b-poly(N-isopropylacrylamide) |
| OEGMA | oligo(ethylene glycol) methyl ether methacrylate |
| PAH | polyalkylamine hydrochloride |
| PBLA | poly(β-benzyl-L-aspartate) |
| Pc | phthalocyanine |
| PCI | photochemical internalization |
| PDT | photodynamic therapy |
| PEG | polyethylene glycol |
| PEG-b-PCL | poly(ethylene glycol)-b-poly(ε-caprolactone) diblock copolymer |
| PEG-PMAN | poly(ethylene glycol)-poly[2-(methylacryloyl)ethylnicotinate] |
| PEG-b-PLGA | poly(ethylene glycol)-b-poly(lactide-co-glycolide) |
| PMMA | poly(methyl methacrylate) |
| pNIPAM | poly(N-isopropylacrylamide) |
| P(R)-b-PPEGA | poly(N-substituted acrylamide)-b-poly(polyethylene glycol monomethyl ether acrylate) |
| PS | photosensitizer |
| PS-b-PAA | poly(styrene)-b-poly(acrylic acid) |
| PSt-b-PPEGA | polystyrene-b-poly(polyethylene glycol monomethyl ether acrylate) |
| PSS | poly(4-styrene sulfonate) |
| PTT | photothermal therapy |
| RuPc(4–12 PEG) | (ruthenium(II) phthalocyanines functionalized with 4–12 PEG chains |
| SiPc | silicon (IV) phthalocyanine dichloride |
| ZnPc | zinc (II) phthalocyanine |
| ZnPcBCH3 | 2(3), 9(10), 16(17), 23(24)-tetrakis-(4′-methyl-benzyloxy) phthalocyanine zinc(II) |
| ZnPcF16 | zinc 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro29H,31H-phthalocyanine |
| ZnPc-sulfo4 | Zinc(II) phthalocyanine tetrasulfonic acid |
| ZnTAPc | zinc(II) tetra-aminophthalocyanine |
References
- Available online: https://www.who.int/en/news-room/fact-sheets/detail/cancer (accessed on 29 July 2021).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Carbone, A. Cancer Classification at the Crossroads. Cancers 2020, 12, 980. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar]
- Bonnett, R. Photosensitizers of the porphyrin and phthalocyanine series for photodynamic therapy. Chem. Soc. Rev. 1995, 24, 19–33. [Google Scholar] [CrossRef]
- Henderson, B.W.; Dougherty, T.J. How does photodynamic therapy work? Photochem. Photobiol. 1992, 55, 145–157. [Google Scholar] [CrossRef]
- Macdonald, I.J.; Dougherty, T.J. Basic principles of photodynamic therapy. J. Porphyr. Phthalocyanines 2001, 5, 105–129. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic Therapy of Cancer: An Update. Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef]
- Idris, N.M.; Gnanasammandhan, M.K.; Zhang, J.; Ho, P.C.; Mahendran, R.; Zhang, Y. In vivo photodynamic therapy using upconversion nanoparticles as remote-controlled nanotransducers. Nat. Med. 2012, 18, 1580–1586. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.S.; Guo, H.C.; Ho, P.C.-L.; Mahendran, R.; Zhang, Y. Mesoporous-Silica-Coated Up-Conversion Fluorescent Nanoparticles for Photodynamic Therapy. Small 2009, 5, 2285–2290. [Google Scholar] [CrossRef]
- Borzęcka, W.; Trindade, T.; Torres, T.; Tomé, J. Targeting Cancer Cells with Photoactive Silica Nanoparticles. Curr. Pharm. Des. 2016, 22, 6021–6038. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Ormond, A.; Freeman, H. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deda, D.K.; Araki, K. Nanotechnology, Light and Chemical Action: An Effective Combination to Kill Cancer Cells. J. Braz. Chem. Soc. 2015, 26, 2448–2470. [Google Scholar] [CrossRef]
- Allison, R.; Sibata, C. Oncologic photodynamic therapy photosensitizers: A clinical review. Photodiagn. Photodyn. Ther. 2010, 7, 61–75. [Google Scholar] [CrossRef]
- Yoon, I.; Li, J.Z.; Shim, Y.K. Advance in Photosensitizers and Light Delivery for Photodynamic Therapy. Clin. Endosc. 2013, 46, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Castano, A.P.; Demidova, T.N.; Hamblin, M.R. Mechanisms in photodynamic therapy: Part one—Photosensitizers, photochemistry and cellular localization. Photodiagn. Photodyn. Ther. 2004, 1, 279–293. [Google Scholar] [CrossRef] [Green Version]
- Detty, M.R.; Gibson, S.L.; Wagner, S.J. Current Clinical and Preclinical Photosensitizers for Use in Photodynamic Therapy. J. Med. Chem. 2004, 47, 3897–3915. [Google Scholar] [CrossRef]
- Al-Omari, S. Toward a molecular understanding of the photosensitizer-copper interaction for tumor destruction. Biophys. Rev. 2013, 5, 305–311. [Google Scholar] [CrossRef] [Green Version]
- Rio, Y.; Rodriguez-Morgade, M.S.; Torres, T. Modulating the electronic properties of porphyrinoids: A voyage from the violet to the infrared regions of the electromagnetic spectrum. Org. Biomol. Chem. 2008, 6, 1877–1894. [Google Scholar] [CrossRef]
- Lo, P.C.; Rodríguez-Morgade, M.S.; Pandey, R.K.; Ng, D.K.P.; Torres, T.; Dumoulin, F. The unique features and promises of phthalocyanines as advanced photosensitisers for photodynamic therapy of cancer. Chem. Soc. Rev. 2020, 49, 1041–1056. [Google Scholar] [CrossRef]
- De la Escosura, A.; Martínez-Díaz, M.V.; Thordarson, P.; Rowan, A.E.; Nolte, R.J.M.; Torres, T. Donor−Acceptor Phthalocyanine Nanoaggregates. J. Am. Chem. Soc. 2003, 125, 12300–12308. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Chen, J.; Xu, S.; Zhou, Y.; Zhu, L.; Xiang, Y.; Xia, D. The effect of a hydrogen bond on the supramolecular self-aggregation mode and the extent of metal-free benzoxazole-substituted phthalocyanines. New J. Chem. 2015, 39, 5750–5758. [Google Scholar] [CrossRef]
- Jing, C.; Wang, R.; Ou, H.; Li, A.; An, Y.; Guo, S.; Shi, L. Axial modification inhibited H-aggregation of phthalocyanines in polymeric micelles for enhanced PDT efficacy. Chem. Commun. 2018, 54, 3985–3988. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zheng, B.-D.; Peng, X.-H.; Li, S.-Z.; Ying, J.-W.; Zhao, Y.; Huang, J.-D.; Yoon, J. Phthalocyanines as medicinal photosensitizers: Developments in the last five years. Coord. Chem. Rev. 2019, 379, 147–160. [Google Scholar] [CrossRef]
- Li, Y.-S.; Zaidi, S.I.A.; Rodgers, M.A.J.; Mukhtar, H.; Kenney, M.E.; Oleinick, N.L.; He, J.; Larkin, H.E.; Rihter, B.D. The Synthesis, Photophysical and Photobiological Properties and in vitro Structure-Activity Relationships of a Set of Silicon Phthalocyanine PDT Photosensitizers. Photochem. Photobiol. 1997, 65, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Song, M.; Huang, J.; Chen, N.; Xue, J.; Huang, M. Photocyanine: A novel and effective phthalocyanine-based photosensitizer for cancer treatment. J. Innov. Opt. Health Sci. 2020, 13, 2030009. [Google Scholar] [CrossRef]
- Tuncel, S.; Dumoulin, F.; Gailer, J.; Sooriyaarachchi, M.; Atilla, D.; Durmuş, M.; Bouchu, D.; Savoie, H.; Boyle, R.W.; Ahsen, V. A set of highly water-soluble tetraethyleneglycol-substituted Zn(ii) phthalocyanines: Synthesis, photochemical and photophysical properties, interaction with plasma proteins and in vitro phototoxicity. Dalton Trans. 2011, 40, 4067–4079. [Google Scholar] [CrossRef]
- Sekkat, N.; Bergh, H.V.D.; Nyokong, T.; Lange, N. Like a Bolt from the Blue: Phthalocyanines in Biomedical Optics. Molecules 2012, 17, 98–144. [Google Scholar] [CrossRef] [Green Version]
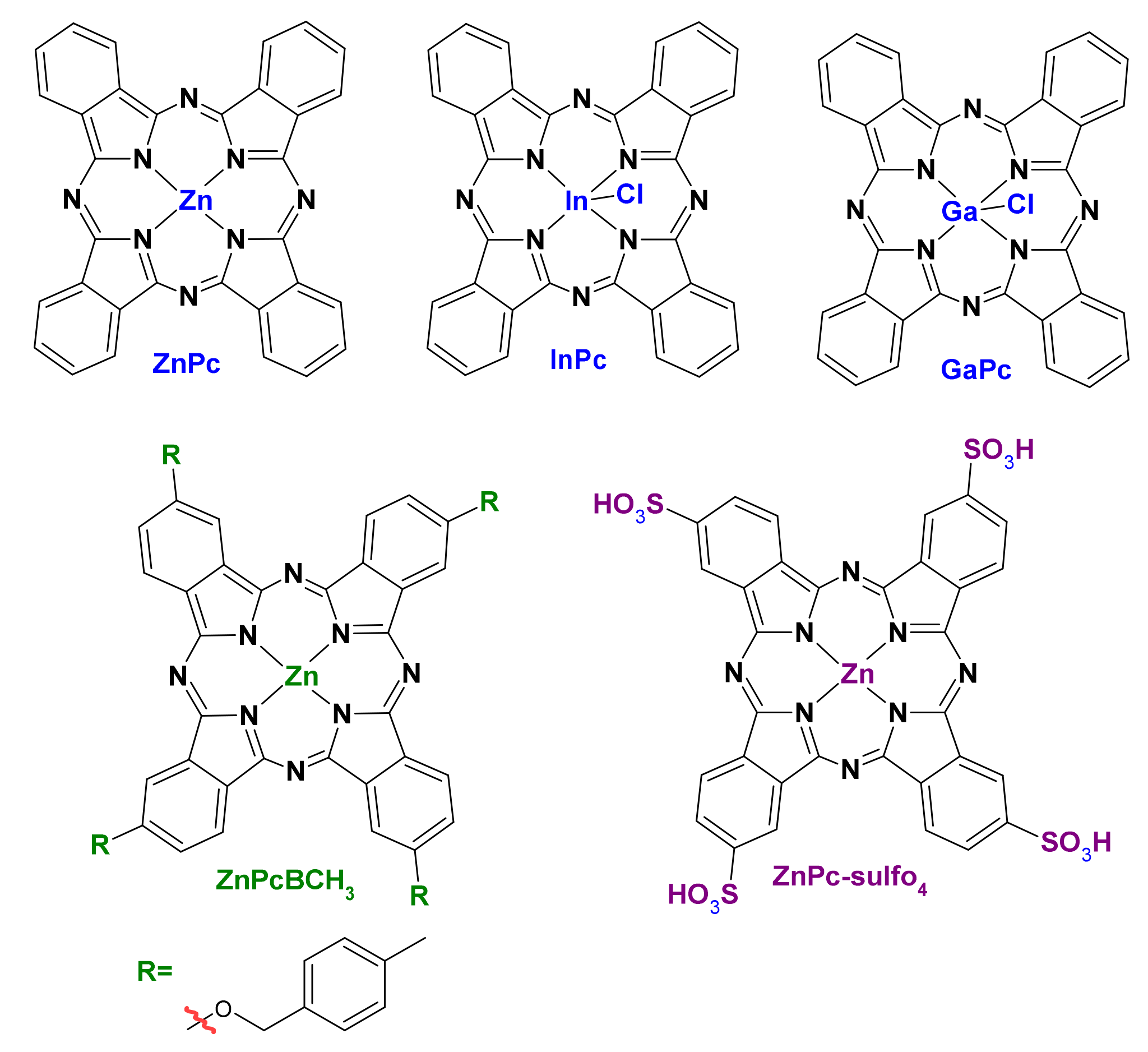
- Nyokong, T.; Antunes, E. Influence of nanoparticle materials on the photophysical behavior of phthalocyanines. Coord. Chem. Rev. 2013, 257, 2401–2418. [Google Scholar] [CrossRef]
- Idowu, M.; Nyokong, T. Photophysical and photochemical properties of zinc and aluminum phthalocyanines in the presence of magnetic fluid. J. Photochem. Photobiol. A Chem. 2007, 188, 200–206. [Google Scholar] [CrossRef]
- Alonso, L.; Sampaio, R.N.; Souza, T.F.M.; Silva, R.C.; Neto, N.M.B.; Ribeiro, A.O.; Alonso, A.; Gonçalves, P.J. Photodynamic evaluation of tetracarboxy-phthalocyanines in model systems. J. Photochem. Photobiol. B Biol. 2016, 161, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Pound-Lana, G.E.N.; Garcia, G.M.; Trindade, I.C.; Capelari-Oliveira, P.; Pontifice, T.G.; Vilela, J.M.C.; Andrade, M.S.; Nottelet, B.; Postacchini, B.B.; Mosqueira, V.C.F. Phthalocyanine photosensitizer in polyethylene glycol-block-poly(lactide-co-benzyl glycidyl ether) nanocarriers: Probing the contribution of aromatic donor-acceptor interactions in polymeric nanospheres. Mater. Sci. Eng. C 2019, 94, 220–233. [Google Scholar] [CrossRef]
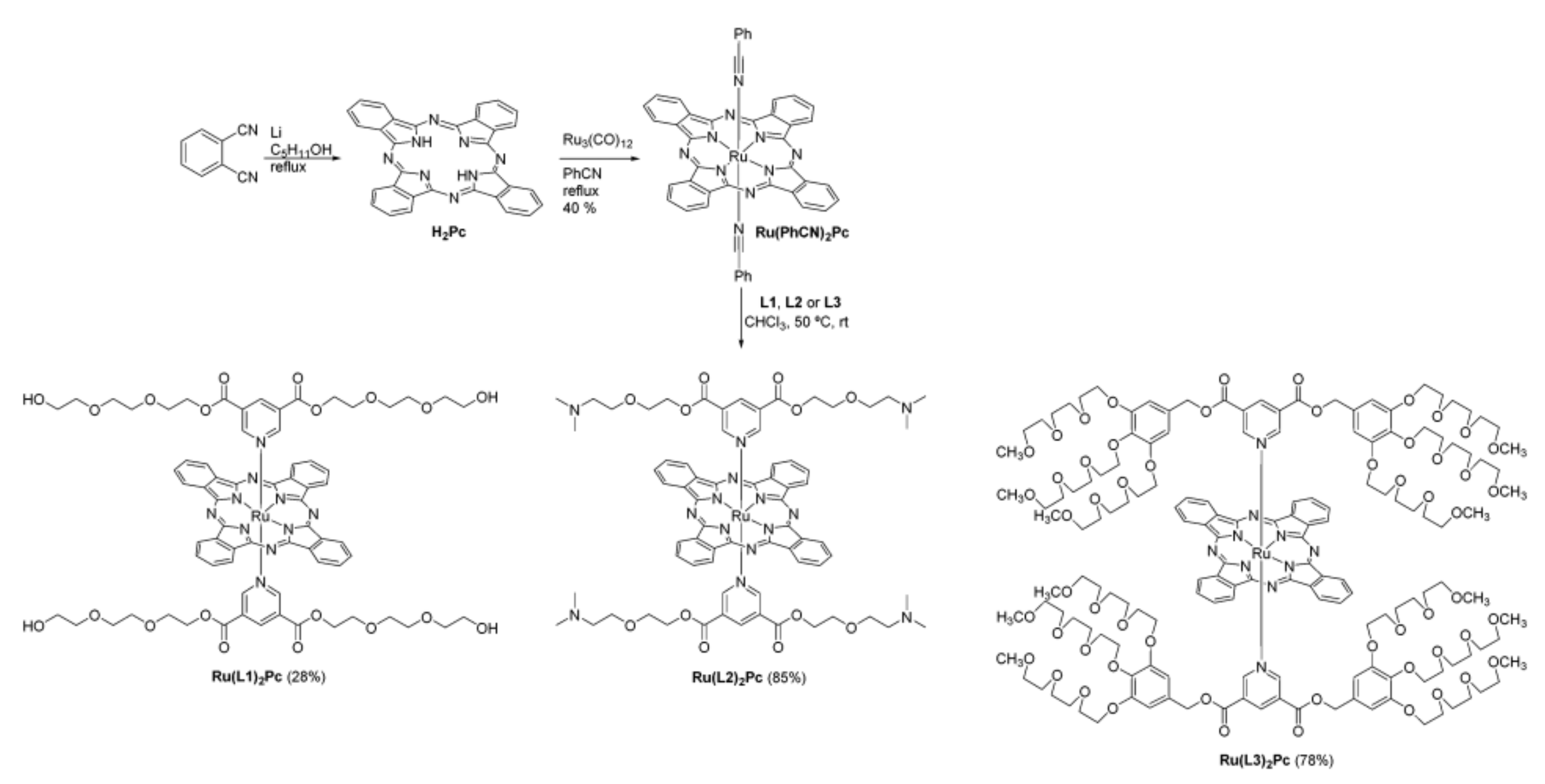
- Teles Ferreira, J.; Pina, J.; Alberto Fontes Ribeiro, C.; Fernandes, R.; Tomé, J.P.C.; Rodríguez-Morgade, M.S.; Torres, T. PEG-containing ruthenium phthalocyanines as photosensitizers for photodynamic therapy: Synthesis, characterization and in vitro evaluation. J. Mater. Chem. B 2017, 5, 5862–5869. [Google Scholar] [CrossRef]
- Ongarora, B.G.; Hu, X.; Verberne-Sutton, S.D.; Garno, J.C.; Vicente, M.G.H. Syntheses and Photodynamic Activity of Pegylated Cationic Zn(II)-Phthalocyanines in HEp2 Cells. Theranostics 2012, 2, 850–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Duan, W.; Lo, P.-C.; Duan, L.; Wu, C.; Ng, D.K.P. Mono-PEGylated Zinc(II) Phthalocyanines: Preparation, Nanoparticle Formation, and In Vitro Photodynamic Activity. Chem. Asian J. 2013, 8, 55–59. [Google Scholar] [CrossRef]
- Mehraban, N.; Musich, P.R.; Freeman, H.S. Synthesis and Encapsulation of a New Zinc Phthalocyanine Photosensitizer into Polymeric Nanoparticles to Enhance Cell Uptake and Phototoxicity. Appl. Sci. 2019, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Yu, H.; Lv, H.; Zhang, H.; Ma, D.; Yang, H.; Xie, S.; Peng, Y. Triblock copolymers encapsulated poly (aryl benzyl ether) dendrimer zinc(II) phthalocyanine nanoparticles for enhancement in vitro photodynamic efficacy. Photodiagnosis Photodyn. Ther. 2016, 16, 124–131. [Google Scholar] [CrossRef]
- Setaro, F.; Wennink, J.W.H.; Mäkinen, P.I.; Holappa, L.; Trohopoulos, P.N.; Ylä-Herttuala, S.; van Nostrum, C.F.; de la Escosura, A.; Torres, T. Amphiphilic phthalocyanines in polymeric micelles: A supramolecular approach toward efficient third-generation photosensitizers. J. Mater. Chem. B 2020, 8, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Chen, X.; Wang, Y.; Guo, Q.; Ye, Q.; Guo, R.; Xiao, S.; Ye, Q.; Huang, Y.; Peng, Y. Benzyl ester dendrimer silicon phthalocyanine based polymeric nanoparticle for in vitro photodynamic therapy of glioma. J. Lumin. 2019, 207, 597–601. [Google Scholar] [CrossRef]
- Simioni, A.R.; Primo, F.L.; Tedesco, A. Silicon(IV) phthalocyanine-loaded-nanoparticles for application in photodynamic process. J. Laser Appl. 2012, 24, 012004. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-Organic Framework Nanoparticles in Photodynamic Therapy: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Cai, X.; Xie, Z.; Li, D.; Kassymova, M.; Zang, S.-Q.; Jiang, H.-L. Nano-sized metal-organic frameworks: Synthesis and applications. Coord. Chem. Rev. 2020, 417, 213366. [Google Scholar] [CrossRef]
- Maeda, H. Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects. Bioconj. Chem. 2010, 21, 797–802. [Google Scholar] [CrossRef]
- Kobayashi, H.; Watanabe, R.; Choyke, P.L. Improving Conventional Enhanced Permeability and Retention (EPR) Effects; What Is the Appropriate Target? Theranostics 2014, 4, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Chawla, J.S.; Amiji, M.M. Biodegradable poly(epsilon-caprolactone) nanoparticles for tumor-targeted delivery of tamoxifen. Int. J. Pharm. 2002, 249, 127–138. [Google Scholar] [CrossRef]
- Ricci-Júnior, E.; Marchetti, J.M. Zinc(II) phthalocyanine loaded PLGA nanoparticles for photodynamic therapy use. Int. J. Pharm. 2006, 310, 187–195. [Google Scholar] [CrossRef]
- Souto, C.A.Z.; Madeira, K.P.; Rettori, D.; Baratti, M.O.; Rangel, L.B.A.; Razzo, D.; da Silva, A.R. Improved photodynamic action of nanoparticles loaded with indium (III) phthalocyanine on MCF-7 breast cancer cells. J. Nanoparticle Res. 2013, 15, 1879. [Google Scholar] [CrossRef]
- Lorenzoni, D.; Souto, C.A.Z.; Araujo, M.B.; de Souza Berger, C.; da Silva, L.C.D.; Baratti, M.O.; Ribeiro, J.N.; Endringer, D.C.; Guimarães, M.C.C.; da Silva, A.R. PLGA-PEG nanoparticles containing gallium phthalocyanine: Preparation, optimization and analysis of its photodynamic efficiency on red blood cell and Hepa-1C1C7. J. Photochem. Photobiol. B Biol. 2019, 198, 111582. [Google Scholar] [CrossRef]
- De Toledo, M.C.M.C.; Abreu, A.D.S.; Carvalho, J.A.; Ambrósio, J.A.R.; Godoy, D.d.S.; dos Santos Pinto, B.C.; Beltrame, M., Jr.; Simioni, A.R. Zinc phthalocyanine tetrasulfonate-loaded polyelectrolytic PLGA nanoparticles for photodynamic therapy applications. Photodiagn. Photodyn. Ther. 2020, 32, 101966. [Google Scholar] [CrossRef]
- Gao, D.; Wong, R.C.H.; Wang, Y.; Guo, X.; Yang, Z.; Lo, P.-C. Shifting the absorption to the near-infrared region and inducing a strong photothermal effect by encapsulating zinc(II) phthalocyanine in poly(lactic-co-glycolic acid)-hyaluronic acid nanoparticles. Acta Biomater. 2020, 116, 329–343. [Google Scholar] [CrossRef]
- Lamch, Ł.; Kulbacka, J.; Pietkiewicz, J.; Rossowska, J.; Dubińska-Magiera, M.; Choromańska, A.; Wilk, K.A. Preparation and characterization of new zinc(II) phthalocyanine—Containing poly(l-lactide)-b-poly(ethylene glycol) copolymer micelles for photodynamic therapy. J. Photochem. Photobiol. B Biol. 2016, 160, 185–197. [Google Scholar] [CrossRef]
- Lamch, Ł.; Kulbacka, J.; Dubińska-Magiera, M.; Saczko, J.; Wilk, K.A. Folate-directed zinc (II) phthalocyanine loaded polymeric micelles engineered to generate reactive oxygen species for efficacious photodynamic therapy of cancer. Photodiagn. Photodyn. Ther. 2019, 25, 480–491. [Google Scholar] [CrossRef]
- Lamch, Ł.; Tsirigotis-Maniecka, M.; Kulbacka, J.; Wilk, K.A. Synthesis of new zinc (II) phthalocyanine conjugates with block copolymers for cancer therapy. ARKIVOC 2016, 2017, 433–445. [Google Scholar] [CrossRef]
- Keyal, U.; Luo, Q.; Bhatta, A.K.; Luan, H.; Zhang, P.; Wu, Q.; Zhang, H.; Liu, P.; Zhang, L.; Wang, P.; et al. Zinc pthalocyanine-loaded chitosan/mPEG-PLA nanoparticles-mediated photodynamic therapy for the treatment of cutaneous squamous cell carcinoma. J. Biophotonics 2018, 11, e201800114. [Google Scholar] [CrossRef]
- Conte, C.; Costabile, G.; d’Angelo, I.; Pannico, M.; Musto, P.; Grassia, G.; Ialenti, A.; Tirino, P.; Miro, A.; Ungaro, F.; et al. Skin transport of PEGylated poly(ε-caprolactone) nanoparticles assisted by (2-hydroxypropyl)-β-cyclodextrin. J. Colloid Interface Sci. 2015, 454, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Chen, X.; Ye, Q.; Chen, K.; Xiao, W.; Guan, X.; Huang, B.; Liu, G.; Wei, H.; Peng, Y. Prop-2-ynyloxybenzyloxy substituted phthalocyanine-based polymeric nanoparticles: Synthesis, photophysical properties and in vitro PDT efficacy. J. Coord. Chem. 2020, 73, 1232–1244. [Google Scholar] [CrossRef]
- Asem, H.; El-Fattah, A.A.; Nafee, N.; Zhao, Y.; Khalil, L.; Muhammed, M.; Hassan, M.; Kandil, S. Development and biodistribution of a theranostic aluminum phthalocyanine nanophotosensitizer. Photodiagn. Photodyn. Ther. 2016, 13, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Py-Daniel, K.R.; Namban, J.S.; de Andrade, L.R.; de Souza, P.E.N.; Paterno, L.G.; Azevedo, R.B.; Soler, M.A.G. Highly efficient photodynamic therapy colloidal system based on chloroaluminum phthalocyanine/pluronic micelles. Eur. J. Pharm. Biopharm. 2016, 103, 23–31. [Google Scholar] [CrossRef]
- Mike Motloung, B.; Babu, B.; Prinsloo, E.; Nyokong, T. The photophysicochemical properties and photodynamic therapy activity of In and Zn phthalocyanines when incorporated into individual or mixed Pluronic® micelles. Polyhedron 2020, 188, 114683. [Google Scholar] [CrossRef]
- Chiarante, N.; García Vior, M.C.; Awruch, J.; Marino, J.; Roguin, L.P. Phototoxic action of a zinc(II) phthalocyanine encapsulated into poloxamine polymeric micelles in 2D and 3D colon carcinoma cell cultures. J. Photochem. Photobiol. B Biol. 2017, 170, 140–151. [Google Scholar] [CrossRef]
- Li, L.; Yang, Q.; Shi, L.; Zheng, N.; Li, Z.; Li, K.; Qiao, S.; Jia, T.; Sun, T.; Wang, Y. Novel phthalocyanine-based polymeric micelles with high near-infrared photothermal conversion efficiency under 808 nm laser irradiation for in vivo cancer therapy. J. Mater. Chem. B 2019, 7, 2247–2251. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, W.; Qu, Z.; Shi, L.; Tan, S.; Ha, E.; Jia, T.; Sun, T. Novel phthalocyanine-based micelles/PNIPAM composite hydrogels: Spatially/temporally controlled drug release triggered by NIR laser irradiation. New J. Chem. 2020, 44, 8705–8709. [Google Scholar] [CrossRef]
- Feuser, P.E.; Gaspar, P.C.; Jacques, A.V.; Tedesco, A.C.; Santos Silva, M.C.D.; Ricci-Júnior, E.; Sayer, C.; de Araújo, P.H.H. Synthesis of ZnPc loaded poly(methyl methacrylate) nanoparticles via miniemulsion polymerization for photodynamic therapy in leukemic cells. Mater. Sci. Eng. C 2016, 60, 458–466. [Google Scholar] [CrossRef]
- Obata, M.; Tanaka, S.; Mizukoshi, H.; Ishihara, E.; Takahashi, M.; Hirohara, S. RAFT synthesis of polystyrene-block-poly(polyethylene glycol monomethyl ether acrylate) for zinc phthalocyanine-loaded polymeric micelles as photodynamic therapy photosensitizers. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 560–570. [Google Scholar] [CrossRef]
- Obata, M.; Masuda, S.; Takahashi, M.; Yazaki, K.; Hirohara, S. Effect of the hydrophobic segment of an amphiphilic block copolymer on micelle formation, zinc phthalocyanine loading, and photodynamic activity. Eur. Polym. J. 2021, 147, 110325. [Google Scholar] [CrossRef]
- Vilsinski, B.H.; Witt, M.A.; Barbosa, P.M.; Montanha, M.C.; Nunes, C.S.; Bellettini, I.C.; de Castro, L.V.; Sato, F.; Baesso, M.L.; Muniz, E.C.; et al. Formulation of chloroaluminum phthalocyanine incorporated into PS-b-PAA diblock copolymer nanomicelles. J. Mol. Liq. 2018, 271, 949–958. [Google Scholar] [CrossRef]
- Yu, W.; Ye, M.; Zhu, J.; Wang, Y.; Liang, C.; Tang, J.; Tao, H.; Shen, Y. Zinc phthalocyanine encapsulated in polymer micelles as a potent photosensitizer for the photodynamic therapy of osteosarcoma. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1099–1110. [Google Scholar] [CrossRef]
- Master, A.M.; Livingston, M.; Oleinick, N.L.; Sen Gupta, A. Optimization of a Nanomedicine-Based Silicon Phthalocyanine 4 Photodynamic Therapy (Pc 4-PDT) Strategy for Targeted Treatment of EGFR-Overexpressing Cancers. Mol. Pharm. 2012, 9, 2331–2338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Master, A.M.; Qi, Y.; Oleinick, N.L.; Gupta, A.S. EGFR-mediated intracellular delivery of Pc 4 nanoformulation for targeted photodynamic therapy of cancer: In vitro studies. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 655–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Master, A.M.; Rodriguez, M.E.; Kenney, M.E.; Oleinick, N.L.; Gupta, A.S. Delivery of the photosensitizer Pc 4 in PEG–PCL micelles for in vitro PDT studies. J. Pharm. Sci. 2010, 99, 2386–2398. [Google Scholar] [CrossRef]
- Chen, K.; Pan, S.; Zhuang, X.; Lv, H.; Que, S.; Xie, S.; Yang, H.; Peng, Y. Effect of diblock copolymer properties on the photophysical properties of dendrimer silicon phthalocyanine nanoconjugates. J. Nanoparticle Res. 2016, 18, 197. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, D.; Pan, S.; Lin, P.; Lin, Y.; Yang, H.; Peng, Y. Comparative study of aluminum phthalocyanine incorporating into two types of block copolymer: Photo-physical property, size, and in vitro photodynamic therapy efficacy. J. Nanoparticle Res. 2015, 17, 41. [Google Scholar] [CrossRef]
- Conte, C.; Ungaro, F.; Maglio, G.; Tirino, P.; Siracusano, G.; Sciortino, M.T.; Leone, N.; Palma, G.; Barbieri, A.; Arra, C.; et al. Biodegradable core-shell nanoassemblies for the delivery of docetaxel and Zn(II)-phthalocyanine inspired by combination therapy for cancer. J. Control. Release 2013, 167, 40–52. [Google Scholar] [CrossRef]
- Dag, A.; Cakilkaya, E.; Omurtag Ozgen, P.S.; Atasoy, S.; Yigit Erdem, G.; Cetin, B.; Çavuş Kokuroǧlu, A.; Gürek, A.G. Phthalocyanine-Conjugated Glyconanoparticles for Chemo-photodynamic Combination Therapy. Biomacromolecules 2021, 22, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Q.; Sun, X.; Zhang, B.; Kang, H.; Zhang, F.; Jin, Y. Doxorubicin-Loaded Photosensitizer-Core pH-Responsive Copolymer Nanocarriers for Combining Photodynamic Therapy and Chemotherapy. ACS Biomater. Sci. Eng. 2017, 3, 1008–1016. [Google Scholar] [CrossRef]
- De Souza, T.D.; Ziembowicz, F.I.; Müller, D.F.; Lauermann, S.C.; Kloster, C.L.; Santos, R.C.V.; Lopes, L.Q.S.; Ourique, A.F.; Machado, G.; Villetti, M.A. Evaluation of photodynamic activity, photostability and in vitro drug release of zinc phthalocyanine-loaded nanocapsules. Eur. J. Pharm. Sci. 2016, 83, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Li, P.; Chen, Y.; Dong, E.; Feng, Z.; He, Z.; Zhou, C.; Wang, C.; Liu, Y.; Feng, C. Preparation of zinc phthalocyanine-loaded amphiphilic phosphonium chitosan nanomicelles for enhancement of photodynamic therapy efficacy. Colloids Surf. B Biointerfaces 2021, 202, 111693. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Chen, H.; Zhu, H.; Tian, J.; Chi, X.; Qian, Z.; Achilefu, S.; Gu, Y. Amphiphilic chitosan modified upconversion nanoparticles for in vivo photodynamic therapy induced by near-infrared light. J. Mater. Chem. 2012, 22, 4861–4873. [Google Scholar] [CrossRef]
- Cui, S.; Yin, D.; Chen, Y.; Di, Y.; Chen, H.; Ma, Y.; Achilefu, S.; Gu, Y. In Vivo Targeted Deep-Tissue Photodynamic Therapy Based on Near-Infrared Light Triggered Upconversion Nanoconstruct. ACS Nano 2013, 7, 676–688. [Google Scholar] [CrossRef]
- Gao, W.; Wang, Z.; Lv, L.; Yin, D.; Chen, D.; Han, Z.; Ma, Y.; Zhang, M.; Yang, M.; Gu, Y. Photodynamic Therapy Induced Enhancement of Tumor Vasculature Permeability Using an Upconversion Nanoconstruct for Improved Intratumoral Nanoparticle Delivery in Deep Tissues. Theranostics 2016, 6, 1131–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yue, C.; Yang, Y.; Zhang, C.; Alfranca, G.; Cheng, S.; Ma, L.; Liu, Y.; Zhi, X.; Ni, J.; Jiang, W.; et al. ROS-Responsive Mitochondria-Targeting Blended Nanoparticles: Chemo- and Photodynamic Synergistic Therapy for Lung Cancer with On-Demand Drug Release upon Irradiation with a Single Light Source. Theranostics 2016, 6, 2352–2366. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Lo, P.-C. Polymeric micelles encapsulating pH-responsive doxorubicin prodrug and glutathione-activated zinc(II) phthalocyanine for combined chemotherapy and photodynamic therapy. J. Control. Release 2018, 282, 46–61. [Google Scholar] [CrossRef]
- Breitenbach, B.B.; Steiert, E.; Konhäuser, M.; Vogt, L.-M.; Wang, Y.; Parekh, S.H.; Wich, P.R. Double stimuli-responsive polysaccharide block copolymers as green macrosurfactants for near-infrared photodynamic therapy. Soft Matter 2019, 15, 1423–1434. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Hu, Z.; Jiang, X.-J.; Ngai, T.; Lo, P.-C.; Zhang, W.; Chen, G. Novel phthalocyanine and PEG-methacrylates based temperature-responsive polymers for targeted photodynamic therapy. Polym. Chem. 2013, 4, 782–788. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Zhang, Z.; Weng, Y.; Chen, G.; Yuan, B.; Yang, K.; Ma, Y. Encapsulation of Hydrophobic Phthalocyanine with Poly(N-isopropylacrylamide)/Lipid Composite Microspheres for Thermo-Responsive Release and Photodynamic Therapy. Materials 2014, 7, 3481–3493. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhao, D.; Li, Y.; Liu, Y.; Duan, Q.; Kakuchi, T. Synthesis of water-soluble and thermoresponsive phthalocyanine ended block copolymers as potential photosensitizer. Dye. Pigment. 2017, 142, 88–99. [Google Scholar] [CrossRef]
- Feuser, P.E.; Fernandes, A.C.; Nele, M.; Viegas, A.D.C.; Ricci-Junior, E.; Tedesco, A.C.; Sayer, C.; de Araújo, P.H.H. Simultaneous encapsulation of magnetic nanoparticles and zinc phthalocyanine in poly(methyl methacrylate) nanoparticles by miniemulsion polymerization and in vitro studies. Colloids Surfaces B Biointerfaces 2015, 135, 357–364. [Google Scholar] [CrossRef]
- Duchi, S.; Ramos-Romero, S.; Dozza, B.; Guerra-Rebollo, M.; Cattini, L.; Ballestri, M.; Dambruoso, P.; Guerrini, A.; Sotgiu, G.; Varchi, G.; et al. Development of near-infrared photoactivable phthalocyanine-loaded nanoparticles to kill tumor cells: An improved tool for photodynamic therapy of solid cancers. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 1885–1897. [Google Scholar] [CrossRef]
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-Installed Nanocarriers toward Precision Therapy. Adv. Mater. 2020, 32, 1902604. [Google Scholar] [CrossRef]
- Alibolandi, M.; Ramezani, M.; Abnous, K.; Sadeghi, F.; Hadizadeh, F. Comparative evaluation of polymersome versus micelle structures as vehicles for the controlled release of drugs. J. Nanoparticle Res. 2015, 17, 76. [Google Scholar] [CrossRef]
- Chidanguro, T.; Simon, Y.C. Bent out of shape: Towards non-spherical polymersome morphologies. Polym. Int. 2021, 70, 951–957. [Google Scholar] [CrossRef]
- Yorulmaz Avsar, S.; Kyropoulou, M.; Di Leone, S.; Schoenenberger, C.-A.; Meier, W.P.; Palivan, C.G. Biomolecules Turn Self-Assembling Amphiphilic Block Co-polymer Platforms Into Biomimetic Interfaces. Front. Chem. 2019, 6, 645. [Google Scholar] [CrossRef] [PubMed]
- Smart, T.; Lomas, H.; Massignani, M.; Flores-Merino, M.V.; Perez, L.R.; Battaglia, G. Block copolymer nanostructures. Nano Today 2008, 3, 38–46. [Google Scholar] [CrossRef]
- Cho, H.K.; Cheong, I.W.; Lee, J.M.; Kim, J.H. Polymeric nanoparticles, micelles and polymersomes from amphiphilic block copolymer. Korean J. Chem. Eng. 2010, 27, 731–740. [Google Scholar] [CrossRef]
- Choucair, A.; Eisenberg, A. Control of amphiphilic block copolymer morphologies using solution conditions. Eur. Phys. J. E 2003, 10, 37–44. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, L.; Eisenberg, A. Morphogenic Effect of Solvent on Crew-Cut Aggregates of Apmphiphilic Diblock Copolymers. Macromolecules 1998, 31, 1144–1154. [Google Scholar] [CrossRef]
- Shen, H.; Zhang, L.; Eisenberg, A. Multiple pH-Induced Morphological Changes in Aggregates of Polystyrene-block-poly(4-vinylpyridine) in DMF/H2O Mixtures. J. Am. Chem. Soc. 1999, 121, 2728–2740. [Google Scholar] [CrossRef]
- Meng, F.; Zhong, Z.; Feijen, J. Stimuli-Responsive Polymersomes for Programmed Drug Delivery. Biomacromolecules 2009, 10, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, R.; Sponchioni, M.; Morbidelli, M.; Moscatelli, D. Polymer nanoparticles for the intravenous delivery of anticancer drugs: The checkpoints on the road from the synthesis to clinical translation. Nanoscale 2018, 10, 22701–22719. [Google Scholar] [CrossRef]
- Letchford, K.; Burt, H. A review of the formation and classification of amphiphilic block copolymer nanoparticulate structures: Micelles, nanospheres, nanocapsules and polymersomes. Eur. J. Pharm. Biopharm. 2007, 65, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Crucho, C.I.C.; Barros, M.T. Polymeric nanoparticles: A study on the preparation variables and characterization methods. Mater. Sci. Eng. C 2017, 80, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, R.; Patravale, V.; Joshi, M. Polymeric nanoparticles for targeted treatment in oncology: Current insights. Int. J. Nanomed. 2015, 10, 1001–1018. [Google Scholar]
- Riley, T.; Stolnik, S.; Heald, C.R.; Xiong, C.D.; Garnett, M.C.; Illum, L.; Davis, S.S.; Purkiss, S.C.; Barlow, R.J.; Gellert, P.R. Physicochemical Evaluation of Nanoparticles Assembled from Poly(lactic acid)−Poly(ethylene glycol) (PLA−PEG) Block Copolymers as Drug Delivery Vehicles. Langmuir 2001, 17, 3168–3174. [Google Scholar] [CrossRef]
- Heald, C.R.; Stolnik, S.; Kujawinski, K.S.; De Matteis, C.; Garnett, M.C.; Illum, L.; Davis, S.S.; Purkiss, S.C.; Barlow, R.J.; Gellert, P.R. Poly(lactic acid)−Poly(ethylene oxide) (PLA−PEG) Nanoparticles: NMR Studies of the Central Solidlike PLA Core and the Liquid PEG Corona. Langmuir 2002, 18, 3669–3675. [Google Scholar] [CrossRef]
- Sharma, S.; Parmar, A.; Kori, S.; Sandhir, R. PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC Trends Anal. Chem. 2016, 80, 30–40. [Google Scholar] [CrossRef]
- Abbasi, E.; Aval, S.F.; Akbarzadeh, A.; Milani, M.; Nasrabadi, H.T.; Joo, S.W.; Hanifehpour, Y.; Nejati-Koshki, K.; Pashaei-Asl, R. Dendrimers: Synthesis, applications, and properties. Nanoscale Res. Lett. 2014, 9, 247. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.C.; MacKay, J.A.; Fréchet, J.M.J.; Szoka, F.C. Designing dendrimers for biological applications. Nat. Biotechnol. 2005, 23, 1517–1526. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Fréchet, J.M.J. Discovery of dendrimers and dendritic polymers: A brief historical perspective. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 2719–2728. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers: Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef] [Green Version]
- Svenson, S.; Tomalia, D.A. Dendrimers in biomedical applications—Reflections on the field. Adv. Drug Deliv. Rev. 2005, 57, 2106–2129. [Google Scholar] [CrossRef]
- Langer, R.; Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 1976, 263, 797. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Chitkara, D.; Kumar, N.; Pawar, R.; Domb, A.; Corn, B. Polymeric Carriers for Regional Drug Therapy. In Smart Polymers: Applications in Biotechnology and Biomedicine; CRC: Boca Raton, FL, USA, 2007. [Google Scholar]
- Farokhzad, O.C.; Langer, R. Impact of Nanotechnology on Drug Delivery. ACS Nano 2009, 3, 16–20. [Google Scholar] [CrossRef]
- Patel, T.; Zhou, J.; Piepmeier, J.M.; Saltzman, W.M. Polymeric nanoparticles for drug delivery to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 701–705. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Yan, L. Functional Polymer Nanocarriers for Photodynamic Therapy. Pharmaceuticals 2018, 11, 133. [Google Scholar] [CrossRef] [Green Version]
- Sutton, D.; Nasongkla, N.; Blanco, E.; Gao, J. Functionalized Micellar Systems for Cancer Targeted Drug Delivery. Pharm. Res. 2007, 24, 1029–1046. [Google Scholar] [CrossRef]
- Li, L.; Huh, K.M. Polymeric nanocarrier systems for photodynamic therapy. Biomater. Res. 2014, 18, 19. [Google Scholar] [CrossRef] [Green Version]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
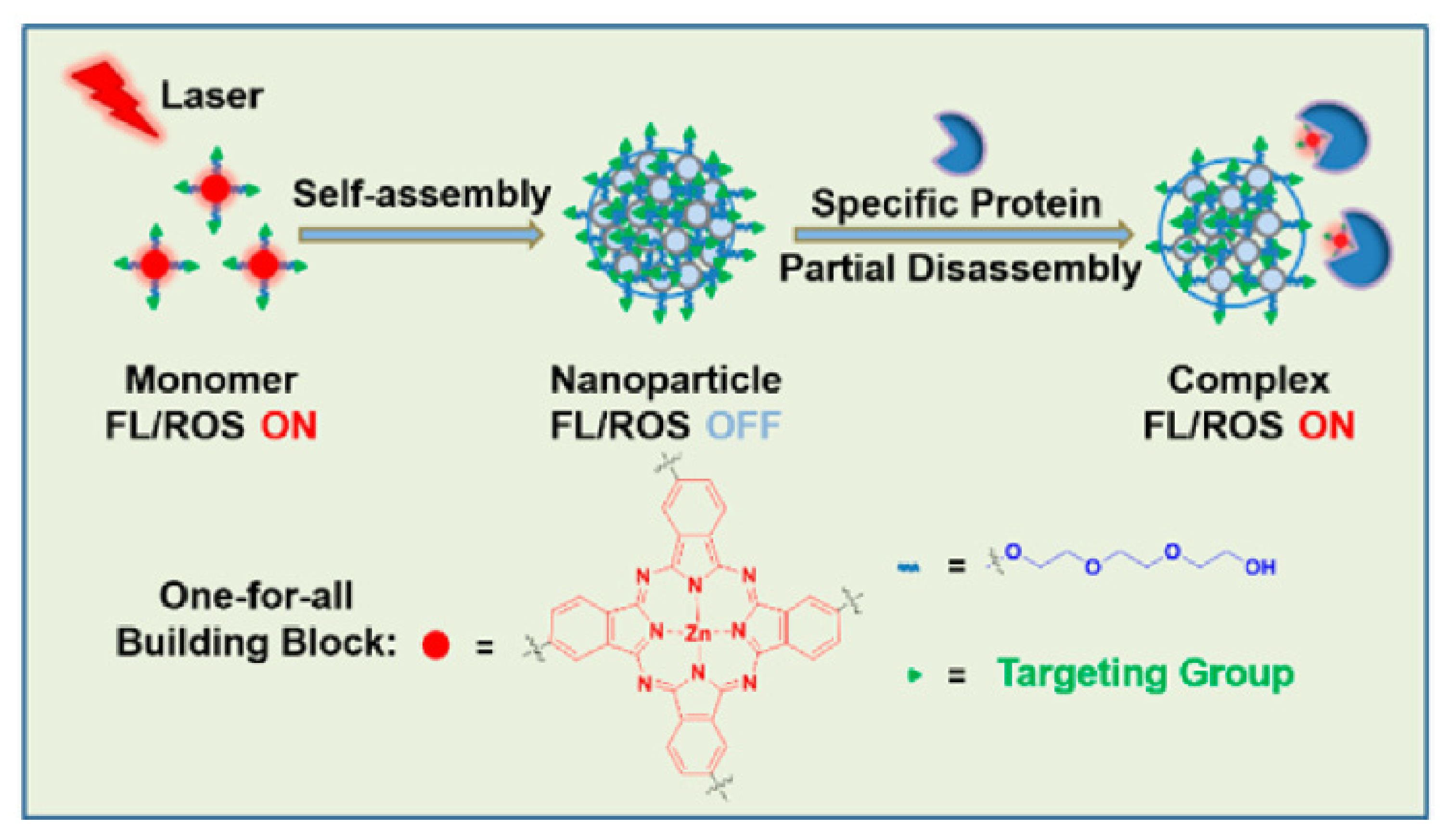
- Li, X.; Kim, C.Y.; Lee, S.; Lee, D.; Chung, H.-M.; Kim, G.; Heo, S.-H.; Kim, C.; Hong, K.-S.; Yoon, J. Nanostructured Phthalocyanine Assemblies with Protein-Driven Switchable Photoactivities for Biophotonic Imaging and Therapy. J. Am. Chem. Soc. 2017, 139, 10880–10886. [Google Scholar] [CrossRef] [PubMed]
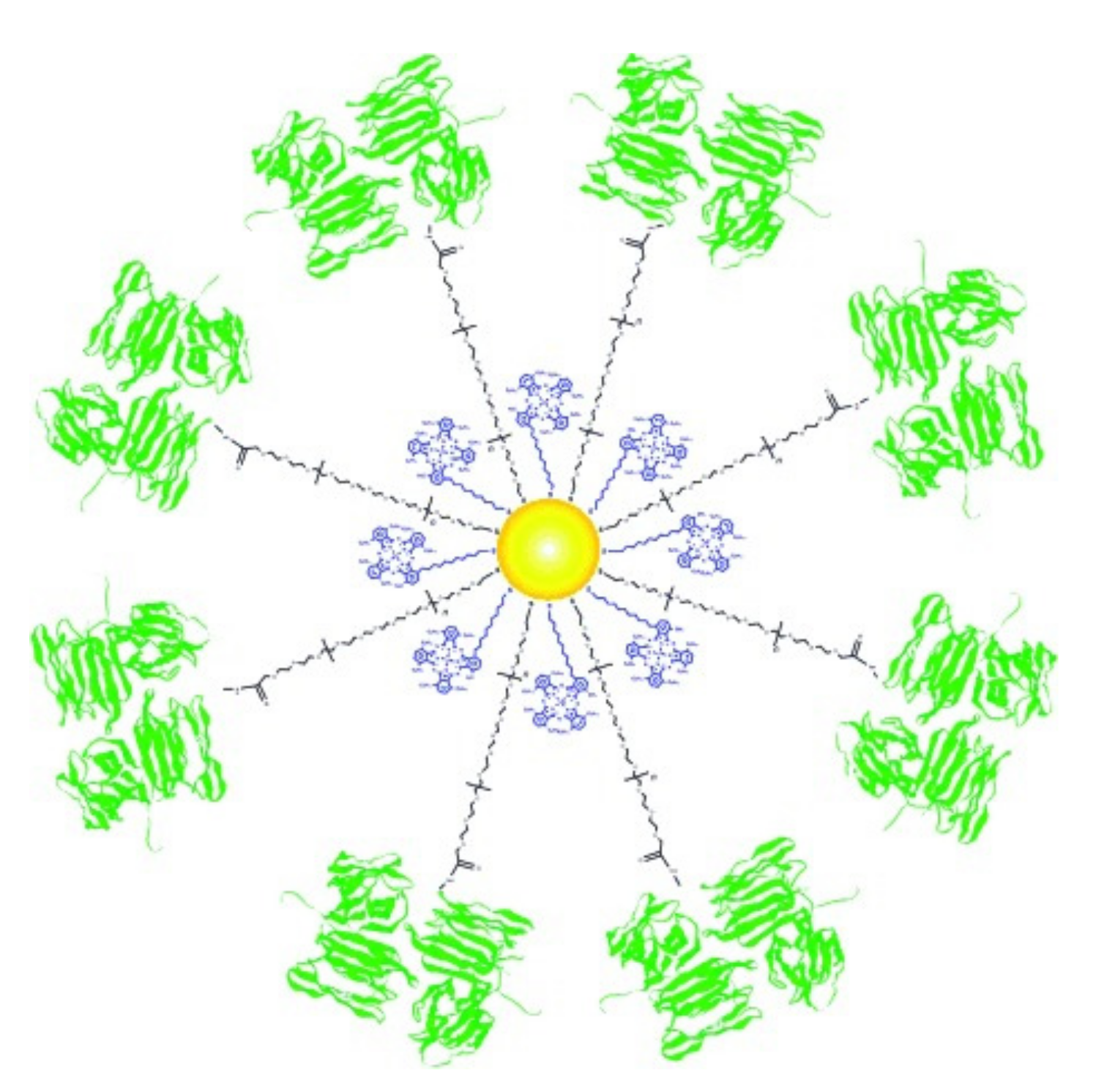
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Targeting the Oncofetal Thomsen–Friedenreich Disaccharide Using Jacalin-PEG Phthalocyanine Gold Nanoparticles for Photodynamic Cancer Therapy. Angew. Chem. Int. Ed. 2012, 51, 6158–6162. [Google Scholar] [CrossRef]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Cancer targeting with biomolecules: A comparative study of photodynamic therapy efficacy using antibody or lectin conjugated phthalocyanine-PEG gold nanoparticles. Photochem. Photobiol. Sci. 2015, 14, 737–747. [Google Scholar] [CrossRef] [Green Version]
- García Calavia, P.; Chambrier, I.; Cook, M.J.; Haines, A.H.; Field, R.A.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using lactose-phthalocyanine functionalized gold nanoparticles. J. Colloid Interface Sci. 2018, 512, 249–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwish, W.M.; Bayoumi, N.A.; El-Shershaby, H.M.; Allahloubi, N.M. Targeted photoimmunotherapy based on photosensitizer-antibody conjugates for multiple myeloma treatment. J. Photochem. Photobiol. B Biol. 2020, 203, 111777. [Google Scholar] [CrossRef] [PubMed]
- Darwish, W.M.; Al-Ashkar, E.A. Synthesis, photochemical and photophysical properties of a sulfo-pegylated zinc-phthalocyanine star polymer potential for biomedical applications. Adv. Appl. Sci. 2015, 9, 126–134. [Google Scholar]
- Domiński, A.; Konieczny, T.; Duale, K.; Krawczyk, M.; Pastuch-Gawołek, G.; Kurcok, P. Stimuli-Responsive Aliphatic Polycarbonate Nanocarriers for Tumor-Targeted Drug Delivery. Polymers 2020, 12, 2890. [Google Scholar] [CrossRef] [PubMed]
- Lamch, Ł.; Tylus, W.; Jewgiński, M.; Latajka, R.; Wilk, K.A. Location of Varying Hydrophobicity Zinc(II) Phthalocyanine-Type Photosensitizers in Methoxy Poly(ethylene oxide) and Poly(l-lactide) Block Copolymer Micelles Using 1H NMR and XPS Techniques. J. Phys. Chem. B 2016, 120, 12768–12780. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Zhang, W.; Chen, G.; Zhang, W.; Zhu, X. A novel approach to synthesize polymers for potential photodynamic therapy: From benzenedinitrile to phthalocyanine. Polym. Chem. 2014, 5, 2872–2879. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Li, C.; Li, Y.; Liu, J.; Tu, Y.; Zhang, W.; Zhou, N.; Zhu, X. Preparation and characterization of solution processable phthalocyanine-containing polymers via a combination of RAFT polymerization and post-polymerization modification techniques. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 691–698. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, H.; Wu, H.; Huang, B.; Gan, L.; Chen, Z. The synthesis and photophysical properties of zinc (II) phthalocyanine bearing poly(aryl benzyl ether) dendritic substituents. Dye. Pigment. 2010, 87, 10–16. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Souza, P.R.; Vilsinski, B.H.; Nunes, C.S.; Bonkovoski, L.C.; Garcia, F.; Nakamura, C.V.; Caetano, W.; Valente, A.J.M.; Martins, A.F.; Muniz, E.C. Application of a polyelectrolyte complex based on biocompatible polysaccharides for colorectal cancer inhibition. Carbohydr. Res. 2021, 499, 108194. [Google Scholar] [CrossRef]
- Liang, G.; Wang, H.; Shi, H.; Wang, H.; Zhu, M.; Jing, A.; Li, J.; Li, G. Recent progress in the development of upconversion nanomaterials in bioimaging and disease treatment. J. Nanobiotechnol. 2020, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, F.; Chen, X. Integrin alpha(v)beta(3)-Targeted Cancer Therapy. Drug Dev. Res. 2008, 69, 329–339. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.L.; Huang, X.; Chen, J.Y. Conjugation of sulfonated aluminum phthalocyanine to doxorubicin can improve the efficacy of photodynamic cancer therapy. Anti Cancer Drugs 2012, 23, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Thapa, P.; Li, M.; Bio, M.; Rajaputra, P.; Nkepang, G.; Sun, Y.; Woo, S.; You, Y. Far-Red Light-Activatable Prodrug of Paclitaxel for the Combined Effects of Photodynamic Therapy and Site-Specific Paclitaxel Chemotherapy. J. Med. Chem. 2016, 59, 3204–3214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Aimetti, A.A.; Langer, R.; Gu, Z. Bioresponsive materials. Nat. Rev. Mater. 2016, 2, 16075. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting Tumor Microenvironment for Cancer Therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [Green Version]
- Gao, D.; Lo, P.-C. Combined pH-responsive chemotherapy and glutathione-triggered photosensitization to overcome drug-resistant hepatocellular carcinoma—A SPP/JPP Young Investigator Award paper. J. Porphyr. Phthalocyanines 2020, 24, 1387–1401. [Google Scholar] [CrossRef]
- Guo, X.; Jin, H.; Lo, P.-C. Encapsulating an acid-activatable phthalocyanine–doxorubicin conjugate and the hypoxia-sensitive tirapazamine in polymeric micelles for multimodal cancer therapy. Biomater. Sci. 2021, 9, 4936–4951. [Google Scholar] [CrossRef]
- Rijcken, C.J.F.; Hofman, J.-W.; van Zeeland, F.; Hennink, W.E.; van Nostrum, C.F. Photosensitiser-loaded biodegradable polymeric micelles: Preparation, characterisation and in vitro PDT efficacy. J. Control. Release 2007, 124, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Grzywa, T.M.; Paskal, W.; Włodarski, P.K. Intratumor and Intertumor Heterogeneity in Melanoma. Transl. Oncol. 2017, 10, 956–975. [Google Scholar] [CrossRef] [PubMed]
- Kiew, L.V.; Cheah, H.Y.; Voon, S.H.; Gallon, E.; Movellan, J.; Ng, K.H.; Alpugan, S.; Lee, H.B.; Dumoulin, F.; Vicent, M.J.; et al. Near-infrared activatable phthalocyanine-poly-L-glutamic acid conjugate: Increased cellular uptake and light-dark toxicity ratio toward an effective photodynamic cancer therapy. Nanomedicine 2017, 13, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Cheah, H.Y.; Gallon, E.; Dumoulin, F.; Hoe, S.Z.; Japundžić-Žigon, N.; Glumac, S.; Lee, H.B.; Anand, P.; Chung, L.Y.; Vicent, M.J.; et al. Near-Infrared Activatable Phthalocyanine–Poly-L-Glutamic Acid Conjugate: Enhanced in Vivo Safety and Antitumor Efficacy toward an Effective Photodynamic Cancer Therapy. Mol. Pharm. 2018, 15, 2594–2605. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, X.; Liu, Y.; Xu, Y.; Zhang, J.; Huang, F.; Li, B.; Miao, Y.; Sun, Y.; Li, Y. Dual-light triggered metabolizable nano-micelles for selective tumor-targeted photodynamic/hyperthermia therapy. Acta Biomater. 2021, 119, 323–336. [Google Scholar] [CrossRef]




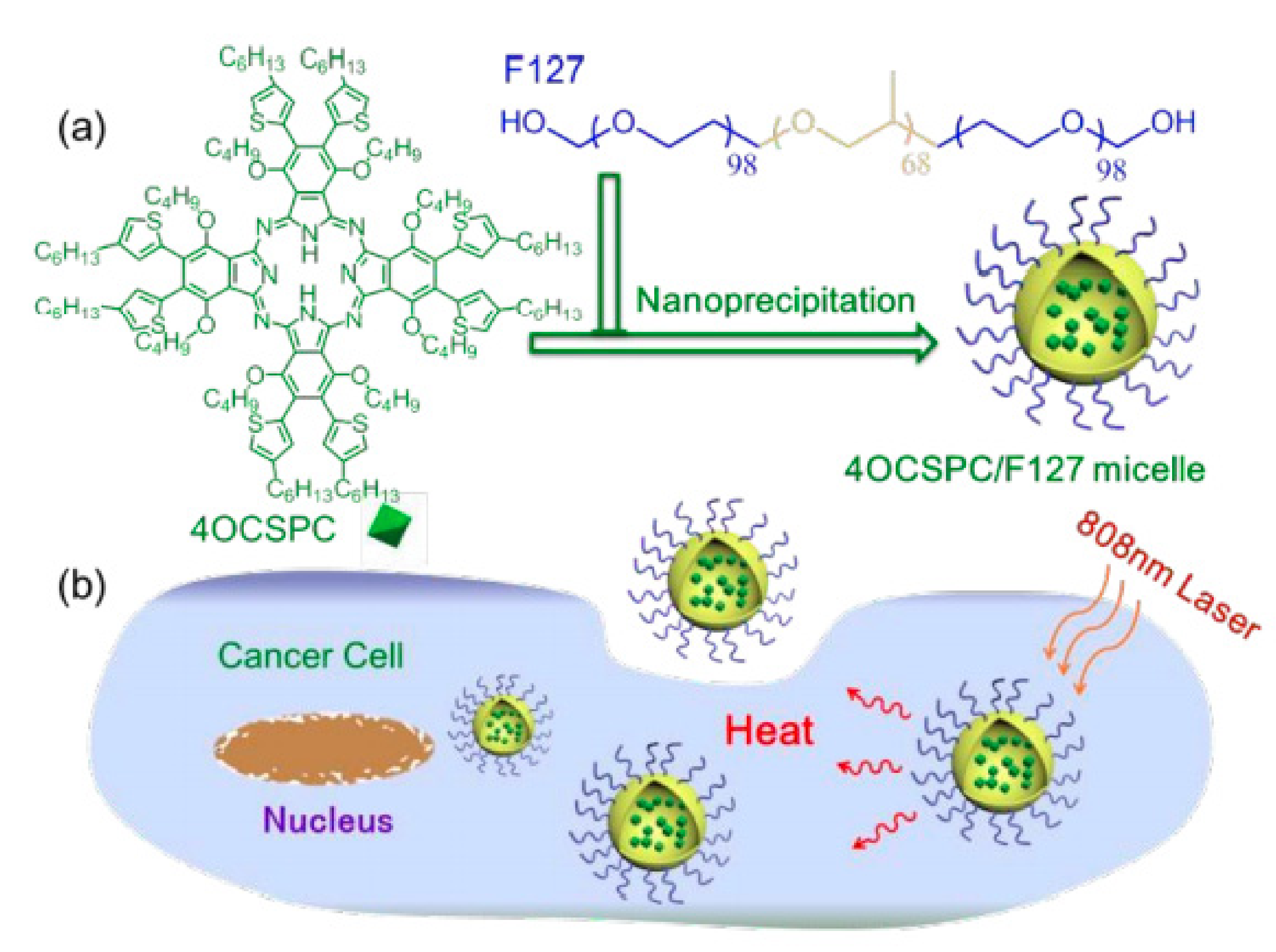


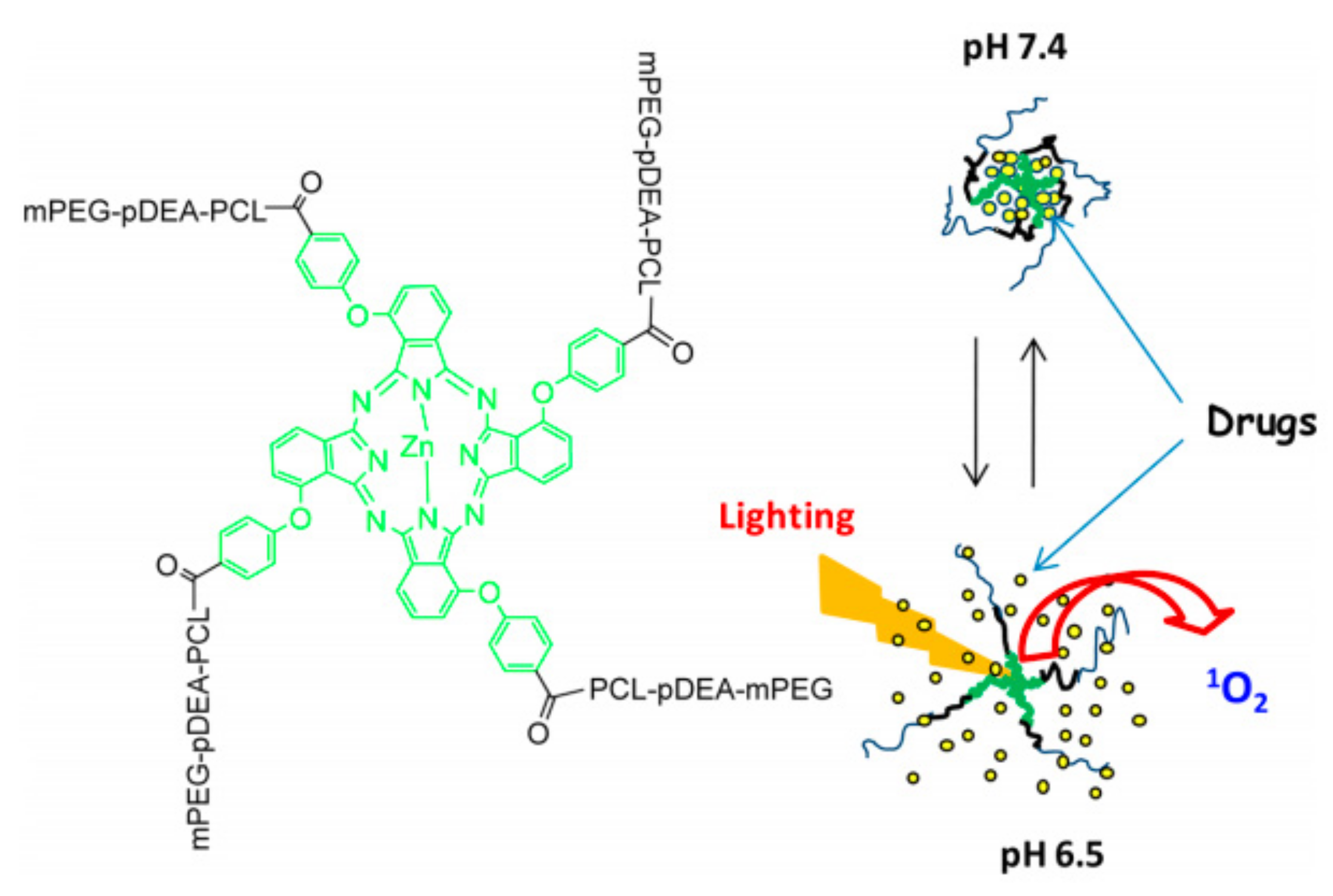

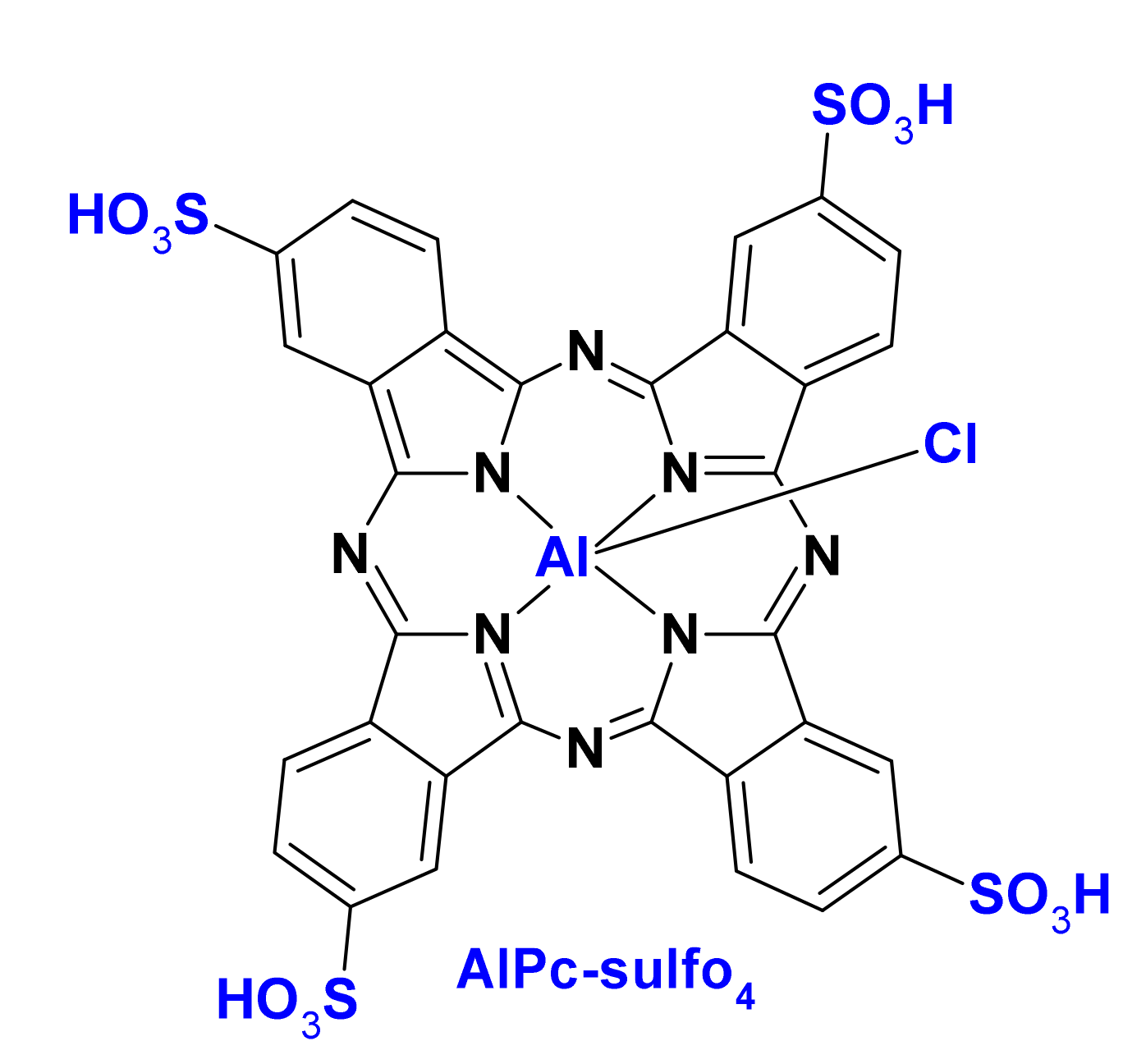

| Name | λmax (nm) | ΦΔ | Cancer Type/Cell Line/Animal Model |
|---|---|---|---|
| Photosens (sulfonated AlPcs) [14,26] | 676 (DMF) | 0.38 (DMF) | skin, stomach, lip, breast, and oral cancer (2) |
| Pc 4 (silicon phthalocyanine 4) [14,26] | 675 (CH3CN) | 0.43 [27] (CH3CN) | actinic keratosis, Bowen’s disease, skin cancer, mycosis fungoides (1) |
| Photocyanine [22,28] | 675 (DMSO) | 0.53 (DMSO) | HepG2 (1) |
| ZnPc [29,30,31] | 672 (DMSO) | 0.67 (DMSO) | cutaneous and subcutaneous lesions from diverse solid tumor origins (1,3) |
| AlPc [32,33] | 680 (DMSO) | 0.29 (DMSO) | J774A.1, Vero cells [34] (3) |
| RuPc-(4–12 PEG) [35] | 638–642 (H2O/DMSO 99: 1) | 0.76 (DMSO) | HT-1376 (3) |
| PEG-ZnPc [36] | 679–686 (DMF) | - | HEp2 (3) |
| Tetraethyleneglycol-substituted ZnPc [29] | 676–702 (DMSO) | 0.34–0.72 (DMSO) | HT-29 (3) |
| Mono-PEGylated ZnPc [37] | 672 (DMF0 | 0.53–0.56 (DMF) | HepG2 (3) |
| ZnPcBCH3 [38] | 681 (DMF) | 0.51 (DMSO) | A549 (3) |
| Poly(aryl benzyl ether)dendrimer ZnPc [39] | 620–630 (PBS) | 0.43–0.56 (DMSO) | HeLa (3) |
| Amphiphilic SiPc deriv [40]. | 686 (CHCl3) | 0.27 (DMSO) | RAW 264.7 (3) |
| Benzyl ester dendrimer SiPc [41] | 678 (DMF) | 0.31 (DMF) | - |
| NzPc [42] | 682 (EtOH) | 0.63 (EtOH) | - |
| Material Description | Nanocarrier Type | Pc-Type | NP Size [nm] | Cell Line/Animal Model | Active Targeting |
|---|---|---|---|---|---|
| PLGA [49] | NP | ZnPc | 285 ± 5.1 | P388-D1 | - |
| PLGA-PEG [50] | NP | InPc | 61–243 | MCF-7 | - |
| PLGA-PEG [51] | NP | GaPc | >200 | Hepa-1C1C7, blood red cell | - |
| PEG-b-PLGA [38] | NP | ZnPcBCH3 | 90.02 ± 0.07 | A549 | - |
| PEG-PLA-BGE [34] | M | AlPc | 60–130 | J774A.1, Vero cells | - |
| PLGA [52] | M | ZnPc-sulfo4 | 384.7 ± 138.6 | B16-F10 | - |
| PLGA-HA [53] | NP | ZnPc- | 259 | HT29, A549, LO2/HT29 tumor-bearing nude mice | HA |
| PEG-b-PLLA [54] | M | ZnPc | 32–35 | Me45, HaCaT, P388/D1, HUV-EC-C | - |
| FA-PEG-b-PLLA [55] | M | ZnPc | <150 | SKOV3, Me45 | FA |
| ZnPc-PEGylated Pluronic P123/PLLA [56] | M | ZnPc | 15–89 | MeWo | - |
| chitosan/mPEG-PLA [57] | NP | ZnPc | 189.7–3.5 | SCC, A431/SKH-1 hairless mice | - |
| PEG-b-PCL [58] | NP | ZnPc | 60 | SC | HPβCD |
| PEG-b-PCL [40] | M | SiPc deriv. | - | RAW 264.7 | - |
| PEG-b-PCL [59] | M | SiPc/ZnPc deriv. | 111/77 | MCF-7 | - |
| PEG-b-PCL [60] | M | AlPc | 66.5–99.1 | female Balb/c mice | |
| Pluronic F127 [61] | M | AlPc | 6 | A549 | - |
| Pluronic F127 [62] | M | InPc/ZnPc deriv. | 27.1–37.8 | MCF-7 | - |
| Tetronic 1107 [63] | M | ZnPc deriv. | 10–100 | CT26 | - |
| Pluronic F127 [64] | M | 4OCSPC | 193.2 | HeLa/mice bearing 4T1 tumor | - |
| Pluronic F127, pNIPAM [65] | M | 4OCSPC | 193.2 | HeLa | - |
| PMMA [66] | NP | ZnPc | 97 ± 2.5 | L929, HPBL, K562, Jurkat | - |
| PSt-b-PPEGA [67] | M | ZnPc | 190–210 | HeLa | - |
| P(R)-b-PPEGA [68] | M | ZnPc | 167–230 | RGK-1 | - |
| PS-b-PAA [69] | M | AlPc | 139.9 ± 0.8 | Caco-2 | - |
| PEG-PMAN [70] | M | ZnPc | 30 | MNNG/Hos, U2OS, Saos-2, MG-63/subcutaneous mouse | - |
| PEG-PCL [71,72,73] | M | Pc 4 | 80–100 | A431, MCF-7c3 | EGFR |
| PEG-b-PCL [25] | M | BtPc | 95–110 | HeLa cells | - |
| PEG-b-PCL [74] | M | SiPc deriv. | 45–70 | - | - |
| PLL-b-PEG-b-PLL, PEG-b-PLL [75] | M | S-AlPc | 10–70 | HUVECs | - |
| PEG5000-b-PLA3000 [41] | M | D-SiPc | 100 | U251 | - |
| PLL-b-PEG-b-PLL [39] | M | ZnPc-dendrimers | 80–150 | HeLa | - |
| PLGA [42] | NP | NzPc | 435 | WS-21 | - |
| PEO2000–b-PCL4300 PEO2000–PCL6800–b-PEO2000 [76] | NP | ZnPc/DTX | 60–100 | HeLa | - |
| P(MMA-b-MAEBA-b-FrucMA)-ZnPc/Dox [77] | NP | ZnPc/DTX | 30 | 3T3, MCF7, MDA-MB-231 | GLUT5 |
| mPEG-pDEA-PCL)4-ZnPc4 (PDCZP) [78] | M | PDCZP | 51–342 | MCF-7, SW480, HepG2/H22 tumor-bearing mice | pH |
| SOC, PCL [79] | NP | ZnPc | 100 | - | |
| phosphonium chitosan [80] | M | ZnPc | 103 ± 5 | Panc-1 | |
| SOC with UPNPs [81] | NP | ZnPc | 45 | HELF, MCF-7/S180 tumor-bearing mice | |
| folate-modified SOC UPNPs [82] | NP | ZnPc | 50 | HELF, MDA-MB-231/S180 tumor-bearing mice, Bel-7402 tumor bearing mice | FA |
| c(RGDyK) modified SOC with UPNPs [83] | NP | ZnPc | 52 | PC-3, WPMY-1/PC-3 tumor-bearing mice | αvβ3 |
| TL-CPT-PEG1K-TPP [84] | N | ZnPc/CPT | 77.1–149. | NCI-H460/Female BALB/c athymic nude mice | n |
| PEG-b-PBLA [85] | M | ZnPc/Dox | 160–180 | HepG2/HepG2 tumor-bearing nude mice | GSH |
| Dex-b-AcDex [86] | M | ZnPc | 120 | HeLa | pH |
| poly(OEGMA-co-DEGMA-co-HEMA) [87] | M | SiPc | 70 | - | temp. |
| pNIPAM/lipid [88] | microgelparticles | SiPc | 1000 | HeLa | temp. |
| PEG-b-PNIPAAM [89] | M | ZnTAPc | 45 | HeLa | temp. |
| PMMA, MNP [90] | NP | ZnPc | 104 ± 2.5 | U87MG | magnetic field |
| PMMA [91] | NP | AlPc-sulfo4 | 80 | MSC, PC3, SCID mice withPC3 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzęcka, W.; Domiński, A.; Kowalczuk, M. Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy. Nanomaterials 2021, 11, 2426. https://doi.org/10.3390/nano11092426
Borzęcka W, Domiński A, Kowalczuk M. Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy. Nanomaterials. 2021; 11(9):2426. https://doi.org/10.3390/nano11092426
Chicago/Turabian StyleBorzęcka, Wioleta, Adrian Domiński, and Marek Kowalczuk. 2021. "Recent Progress in Phthalocyanine-Polymeric Nanoparticle Delivery Systems for Cancer Photodynamic Therapy" Nanomaterials 11, no. 9: 2426. https://doi.org/10.3390/nano11092426






