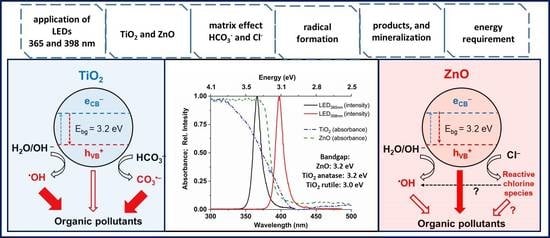
Impact of Reaction Parameters and Water Matrices on the Removal of Organic Pollutants by TiO2/LED and ZnO/LED Heterogeneous Photocatalysis Using 365 and 398 nm Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Photochemical Reactors and Light Sources
2.2. Photochemical Experiments and Analytical Methods
2.3. Chemicals and Solvents Used
3. Results and Discussion
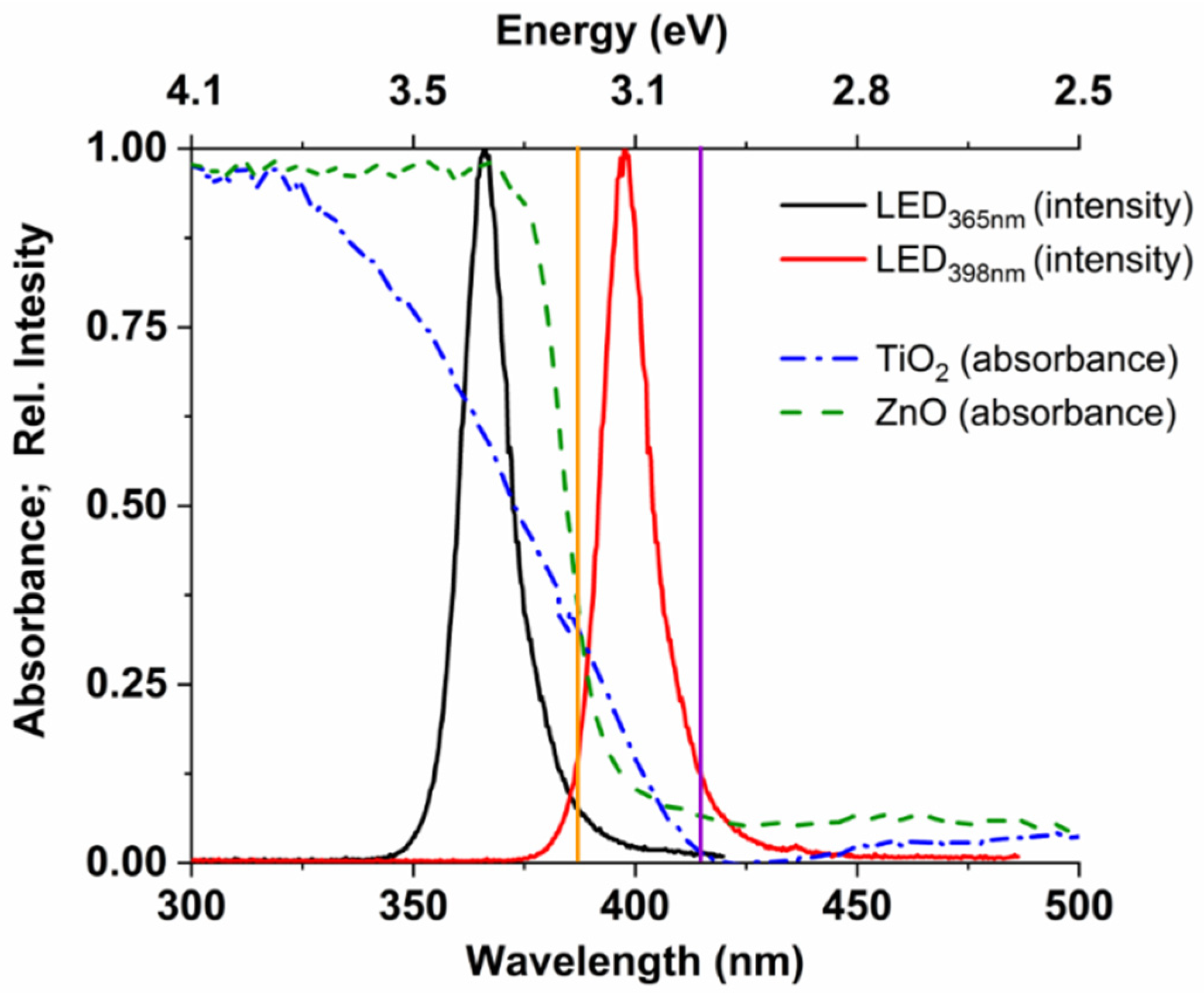
3.1. Photon Flux and Electrical Efficiency of the LEDs
3.2. Effect of Reaction Parameters on the •OH Formation
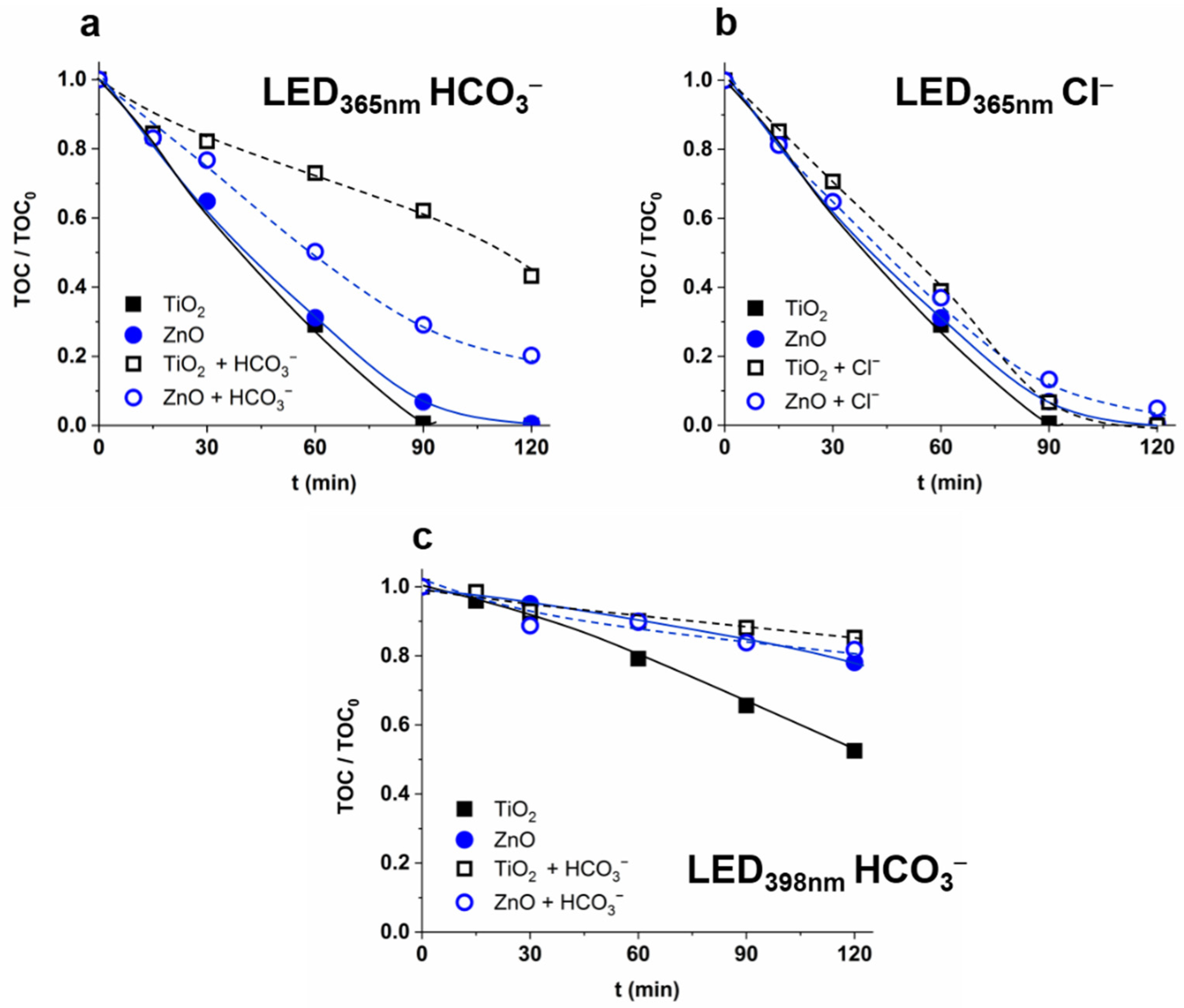
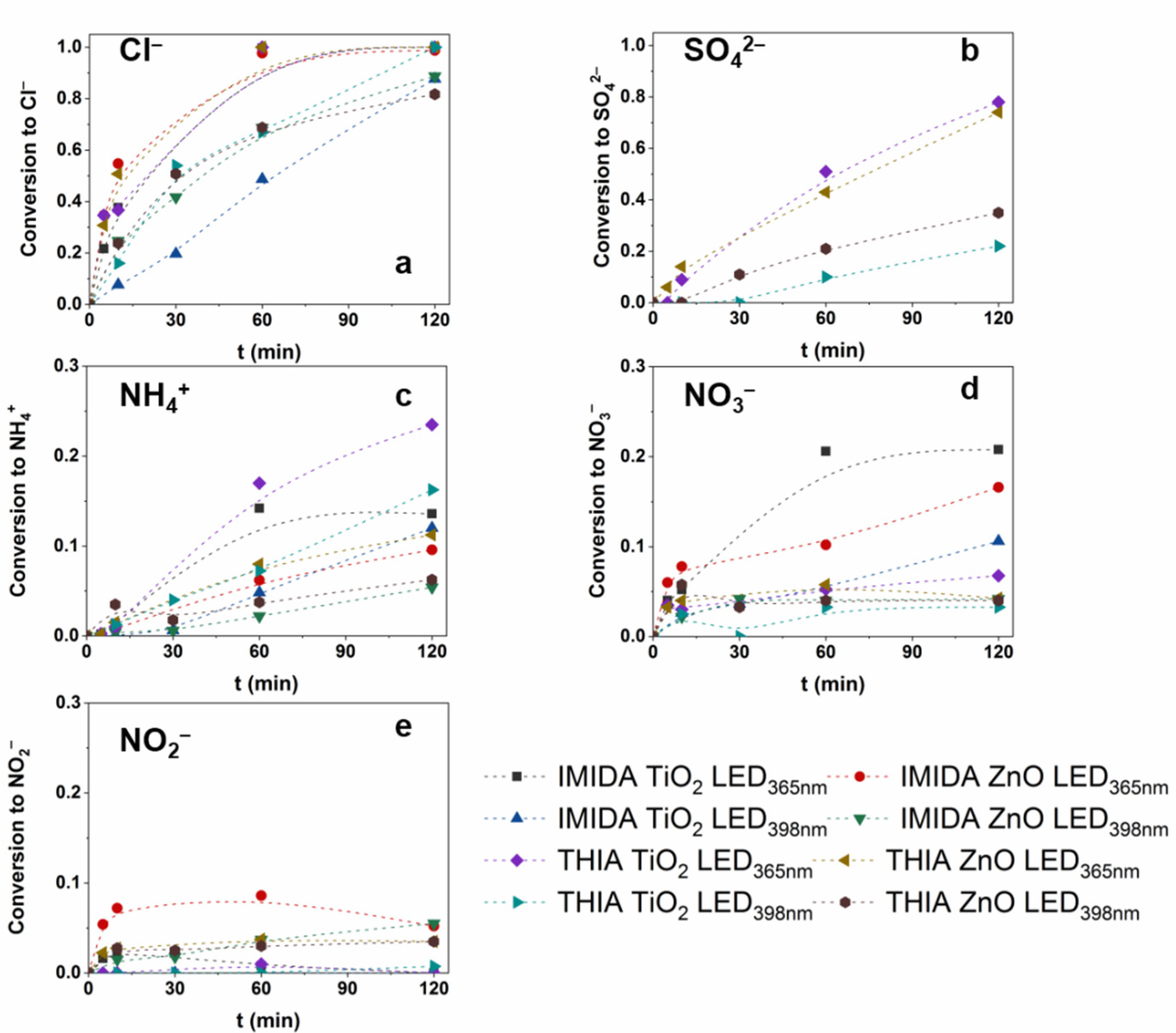
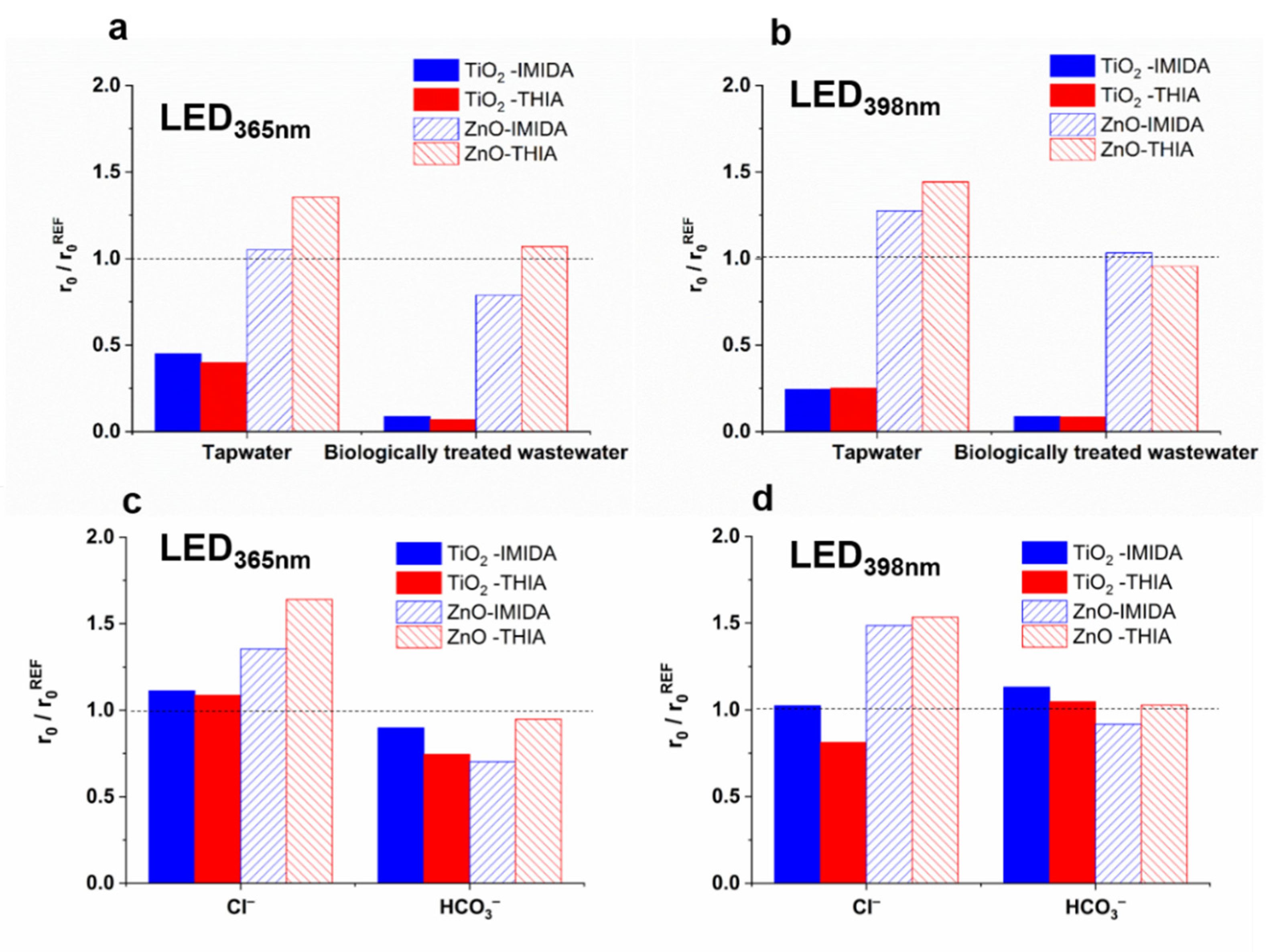
3.3. The Effect of Matrices and Matrix Components on the •OH Formation
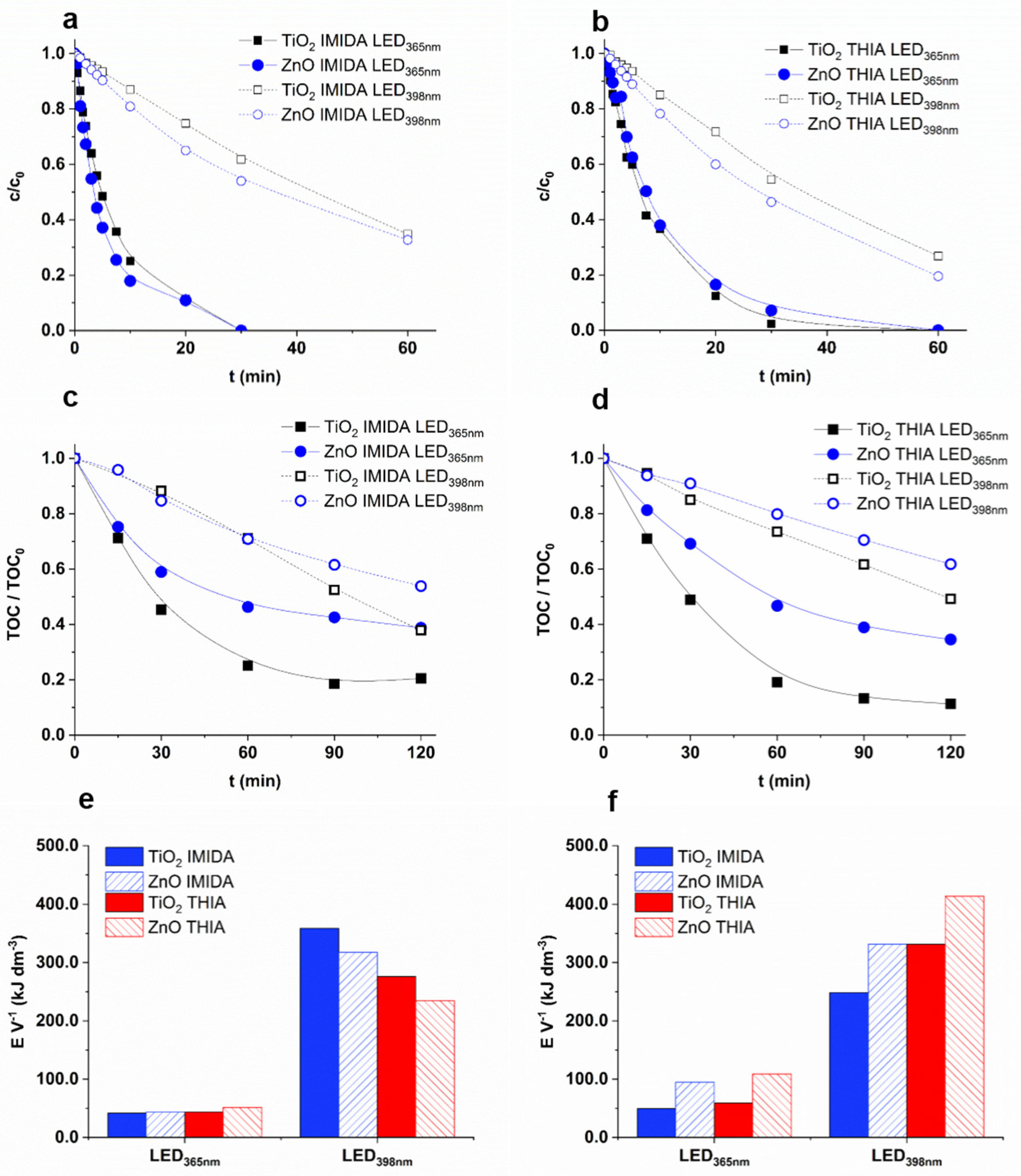
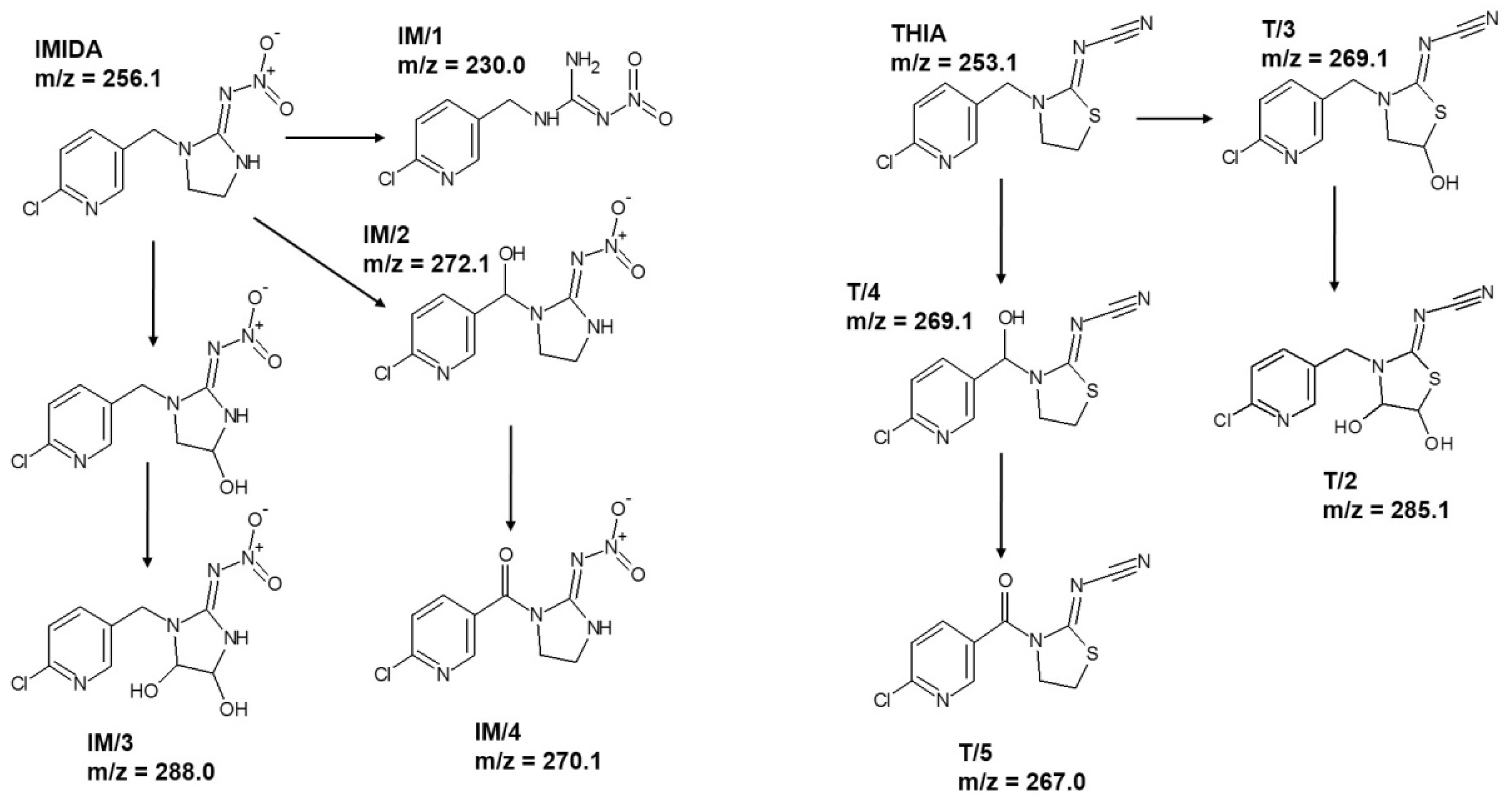
3.4. Removal Efficiency of Neonicotinoids
3.5. Effect of Matrices on the Removal of IMIDA and THIA
3.6. Ecotoxicity Change
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment-Fundamentals and Applications; IWA Publishing: London, UK, 2017; Volume 16, ISBN 9781780407180. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Colinge, J.P.; Colinge, C.A. Physics of Semiconductor Devices; Kluwer Academic Publishers-Springer International Publishing: Berlin/Heidelberg, Germany, 2002; ISBN 1-40207-018-7. [Google Scholar]
- Gaya, U.I. Heterogeneous Photocatalysis Using Inorganic Semiconductor Solids; Springer: Berlin, Germany, 2014; Volume 9789400777, ISBN 9789400777750. [Google Scholar]
- Nosaka, Y.; Nosaka, A.Y. Generation and Detection of Reactive Oxygen Species in Photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Srikant, V.; Clarke, D.R. On the Optical Band Gap of Zinc Oxide. J. Appl. Phys. 1998, 83, 5447–5451. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous Photocatalysis and Its Potential Applications in Water and Wastewater Treatment: A Review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef] [Green Version]
- Baxter, J.B.; Schmuttenmaer, C.A. Conductivity of ZnO Nanowires, Nanoparticles, and Thin Films Using Time-Resolved Terahertz Spectroscopy. J. Phys. Chem. B 2006, 110, 25229–25239. [Google Scholar] [CrossRef]
- Meulenkamp, E.A. Electron Transport in Nanoparticulate ZnO Films. J. Phys. Chem. B 1999, 103, 7831–7838. [Google Scholar] [CrossRef]
- Lee, K.M.; Lai, C.W.; Ngai, K.S.; Juan, J.C. Recent Developments of Zinc Oxide Based Photocatalyst in Water Treatment Technology: A Review. Water Res. 2016, 88, 428–448. [Google Scholar] [CrossRef]
- Yusoff, N.A.; Ho, L.N.; Ong, S.A.; Wong, Y.S.; Khalik, W.F. Photocatalytic Activity of Zinc Oxide (ZnO) Synthesized through Different Methods. Desalin. Water Treat. 2016, 57, 12496–12507. [Google Scholar] [CrossRef]
- Kumar, S.G.; Rao, K.S.R.K. Zinc Oxide Based Photocatalysis: Tailoring Surface-Bulk Structure and Related Interfacial Charge Carrier Dynamics for Better Environmental Applications. RSC Adv. 2015, 5, 3306–3351. [Google Scholar] [CrossRef]
- Spathis, P.; Poulios, I. The Corrosion and Photocorrosion of Zinc and Zinc Oxide Coatings. Corros. Sci. 1995, 37, 673–680. [Google Scholar] [CrossRef]
- Kositzi, M.; Poulios, I.; Samara, K.; Tsatsaroni, E.; Darakas, E. Photocatalytic Oxidation of Cibacron Yellow LS-R. J. Hazard. Mater. 2007, 146, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Tokode, O.; Prabhu, R.; Lawton, L.A.; Robertson, P.K.J. UV LED Sources for Heterogeneous Photocatalysis. Handb. Environ. Chem. 2015, 35, 159–179. [Google Scholar] [CrossRef] [Green Version]
- Bloh, J.Z. Intensification of Heterogeneous Photocatalytic Reactions Without Efficiency Losses: The Importance of Surface Catalysis. Catal. Lett. 2021, 151, 3105–3113. [Google Scholar] [CrossRef]
- Kuipers, J.; Bruning, H.; Yntema, D.; Rijnaarts, H. Wirelessly Powered Ultraviolet Light Emitting Diodes for Photocatalytic Oxidation. J. Photochem. Photobiol. A Chem. 2015, 299, 25–30. [Google Scholar] [CrossRef]
- Bertagna Silva, D.; Buttiglieri, G.; Babić, S. State-of-the-Art and Current Challenges for TiO2/UV-LED Photocatalytic Degradation of Emerging Organic Micropollutants. Environ. Sci. Pollut. Res. 2021, 28, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Sergejevs, A.; Clarke, C.T.; Allsopp, D.W.E.; Marugan, J.; Jaroenworaluck, A.; Singhapong, W.; Manpetch, P.; Timmers, R.; Casado, C.; Bowen, C.R. A Calibrated UV-LED Based Light Source for Water Purification and Characterisation of Photocatalysis. Photochem. Photobiol. Sci. 2017, 16, 1690–1699. [Google Scholar] [CrossRef]
- Eskandarian, M.R.; Choi, H.; Fazli, M.; Rasoulifard, M.H. Effect of UV-LED Wavelengths on Direct Photolytic and TiO2 Photocatalytic Degradation of Emerging Contaminants in Water. Chem. Eng. J. 2016, 300, 414–422. [Google Scholar] [CrossRef]
- Khademalrasool, M.; Farbod, M.; Talebzadeh, M.D. The Improvement of Photocatalytic Processes: Design of a Photoreactor Using High-Power LEDs. J. Sci. Adv. Mater. Devices 2016, 1, 382–387. [Google Scholar] [CrossRef] [Green Version]
- Natarajan, K.; Natarajan, T.S.; Bajaj, H.C.; Tayade, R.J. Photocatalytic Reactor Based on UV-LED/TiO2 Coated Quartz Tube for Degradation of Dyes. Chem. Eng. J. 2011, 178, 40–49. [Google Scholar] [CrossRef]
- Tapia-Tlatelpa, T.; Buscio, V.; Trull, J.; Sala, V. Performance Analysis and Methodology for Replacing Conventional Lamps by Optimized LED Arrays for Photocatalytic Processes. Chem. Eng. Res. Des. 2020, 156, 456–468. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of Parameters on the Heterogeneous Photocatalytic Degradation of Pesticides and Phenolic Contaminants in Wastewater: A Short Review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [Green Version]
- Al-Sayyed, G.; D’Oliveira, J.C.; Pichat, P. Semiconductor-Sensitized Photodegradation of 4-Chlorophenol in Water. J. Photochem. Photobiol. A Chem. 1991, 58, 99–114. [Google Scholar] [CrossRef]
- Ollis, D.F. Solar-Assisted Photocatalysis for Water Purification: Issues, Data, Questions. In Photochemical Conversion and Storage of Solar Energy; Pelizzetti, E., Schiavello, M., Eds.; Springer: Dordrecht, The Netherlands, 1991. [Google Scholar] [CrossRef]
- Herrmann, J.M. Heterogeneous Photocatalysis: Fundamentals and Applications to the Removal of Various Types of Aqueous Pollutants. Catal. Today 1999, 53, 115–129. [Google Scholar] [CrossRef]
- Ohtani, B. Titania Photocatalysis beyond Recombination: A Critical Review. Catalysts 2013, 3, 942–953. [Google Scholar] [CrossRef] [Green Version]
- Nosaka, Y.; Nosaka, A.Y. Langmuir-Hinshelwood and Light-Intensity Dependence Analyses of Photocatalytic Oxidation Rates by Two-Dimensional-Ladder Kinetic Simulation. J. Phys. Chem. C 2018, 122, 28748–28756. [Google Scholar] [CrossRef]
- Torimoto, T.; Aburakawa, Y.; Kawahara, Y.; Ikeda, S.; Ohtani, B. Light Intensity Dependence of the Action Spectra of Photocatalytic Reactions with Anatase Titanium(IV) Oxide. Chem. Phys. Lett. 2004, 392, 220–224. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.C.; Chu, W. The Direct Photolysis and Photocatalytic Degradation of Alachlor at Different TiO2 and UV Sources. Chemosphere 2003, 50, 981–987. [Google Scholar] [CrossRef]
- Zhu, X.; Yuan, C.; Bao, Y.; Yang, J.; Wu, Y. Photocatalytic Degradation of Pesticide Pyridaben on TiO2 Particles. J. Mol. Catal. A Chem. 2005, 229, 95–105. [Google Scholar] [CrossRef]
- McMurray, T.A.; Dunlop, P.S.M.; Byrne, J.A. The Photocatalytic Degradation of Atrazine on Nanoparticulate TiO2 Films. J. Photochem. Photobiol. A Chem. 2006, 182, 43–51. [Google Scholar] [CrossRef]
- Tao, Y.; Cheng, Z.; Ting, K.E.; Yin, X.J. Studies of Photocatalytic Kinetics on the Degradation of Bisphenol A (BPA) by Immobilized ZnO Nanoparticles in Aerated Photoreactors. J. Environ. Sci. Eng. A 2012, 1, 187–194. [Google Scholar]
- Bouanimba, N.; Laid, N.; Zouaghi, R.; Sehili, T. A Comparative Study of the Activity of TiO2 Degussa P25 and Millennium PCs in the Photocatalytic Degradation of Bromothymol Blue. Int. J. Chem. React. Eng. 2018, 16, 1–19. [Google Scholar] [CrossRef]
- Šojić Merkulov, D.V.; Lazarević, M.J.; Despotović, V.N.; Banić, N.D.; Finčur, N.L.; Maletić, S.P.; Abramović, B.F. The Effects of Inorganic Anions and Organic Matter on Mesotrione (Callisto®) Removal from Environmental Waters. J. Serb. Chem. Soc. 2017, 82, 343–355. [Google Scholar] [CrossRef]
- Farner Budarz, J.; Turolla, A.; Piasecki, A.F.; Bottero, J.Y.; Antonelli, M.; Wiesner, M.R. Influence of Aqueous Inorganic Anions on the Reactivity of Nanoparticles in TiO2 Photocatalysis. Langmuir 2017, 33, 2770–2779. [Google Scholar] [CrossRef]
- Lair, A.; Ferronato, C.; Chovelon, J.M.; Herrmann, J.M. Naphthalene Degradation in Water by Heterogeneous Photocatalysis: An Investigation of the Influence of Inorganic Anions. J. Photochem. Photobiol. A Chem. 2008, 193, 193–203. [Google Scholar] [CrossRef]
- Kaabeche, O.N.E.H.; Zouaghi, R.; Boukhedoua, S.; Bendjabeur, S.; Sehili, T. A Comparative Study on Photocatalytic Degradation of Pyridinium-Based Ionic Liquid by TiO2 and ZnO in Aqueous Solution. Int. J. Chem. React. Eng. 2019, 17, 1–14. [Google Scholar] [CrossRef]
- Lado Ribeiro, A.R.; Moreira, N.F.F.; Li Puma, G.; Silva, A.M.T. Impact of Water Matrix on the Removal of Micropollutants by Advanced Oxidation Technologies. Chem. Eng. J. 2019, 363, 155–173. [Google Scholar] [CrossRef] [Green Version]
- Tufail, A.; Price, W.E.; Mohseni, M.; Pramanik, B.K.; Hai, F.I. A Critical Review of Advanced Oxidation Processes for Emerging Trace Organic Contaminant Degradation: Mechanisms, Factors, Degradation Products, and Effluent Toxicity. J. Water Process. Eng. 2021, 40, 101778. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Generation of OH Radicals and Oxidation Mechanism in Photocatalysis of WO3 and BiVO4 Powders. J. Photochem. Photobiol. A Chem. 2015, 303–304, 53–58. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Quantitative Detection of OH Radicals for Investigating the Reaction Mechanism of Various Visible-Light TiO2 Photocatalysts in Aqueous Suspension. J. Phys. Chem. C 2013, 117, 1383–1391. [Google Scholar] [CrossRef]
- Ishibashi, K.I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of Active Oxidative Species in TiO2 Photocatalysis Using the Fluorescence Technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Nagarajan, S.; Skillen, N.C.; Fina, F.; Zhang, G.; Randorn, C.; Lawton, L.A.; Irvine, J.T.S.; Robertson, P.K.J. Comparative Assessment of Visible Light and UV Active Photocatalysts by Hydroxyl Radical Quantification. J. Photochem. Photobiol. A Chem. 2017, 334, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Černigoj, U.; Štangar, U.L.; Trebše, P.; Sarakha, M. Determination of Catalytic Properties of TiO2 Coatings Using Aqueous Solution of Coumarin: Standardization Efforts. J. Photochem. Photobiol. A Chem. 2009, 201, 142–150. [Google Scholar] [CrossRef]
- Kakuma, Y.; Nosaka, A.Y.; Nosaka, Y. Difference in TiO2 Photocatalytic Mechanism between Rutile and Anatase Studied by the Detection of Active Oxygen and Surface Species in Water. Phys. Chem. Chem. Phys. 2015, 17, 18691–18698. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Comment on “Coumarin as a Quantitative Probe for Hydroxyl Radical Formation in Heterogeneous Photocatalysis”. J. Phys. Chem. C 2019, 123, 20682–20684. [Google Scholar] [CrossRef] [Green Version]
- Czili, H.; Horváth, A. Applicability of Coumarin for Detecting and Measuring Hydroxyl Radicals Generated by Photoexcitation of TiO2 Nanoparticles. Appl. Catal. B Environ. 2008, 81, 295–302. [Google Scholar] [CrossRef]
- Wafi, A.; Szabó-Bárdos, E.; Horváth, O.; Makó, É.; Jakab, M.; Zsirka, B. Coumarin-Based Quantification of Hydroxyl Radicals and Other Reactive Species Generated on Excited Nitrogen-Doped TiO2. J. Photochem. Photobiol. A Chem. 2021, 404. [Google Scholar] [CrossRef]
- Wood, T.J.; Goulson, D. The Environmental Risks of Neonicotinoid Pesticides: A Review of the Evidence Post 2013. Environ. Sci. Pollut. Res. 2017, 24, 17285–17325. [Google Scholar] [CrossRef]
- Arce, A.N.; Rodrigues, A.R.; Yu, J.; Colgan, T.J.; Wurm, Y.; Gill, R.J. Foraging Bumblebees Acquire a Preference for Neonicotinoid-Treated Food with Prolonged Exposure. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180655. [Google Scholar] [CrossRef] [PubMed]
- Pisa, L.W.; Amaral-Rogers, V.; Belzunces, L.P.; Bonmatin, J.M.; Downs, C.A.; Goulson, D.; Kreutzweiser, D.P.; Krupke, C.; Liess, M.; Mcfield, M.; et al. Effects of Neonicotinoids and Fipronil on Non-Target Invertebrates. Environ. Sci. Pollut. Res. 2014, 22, 68–102. [Google Scholar] [CrossRef] [Green Version]
- Caron-Beaudoin, E.; Viau, R.; Hudon-Thibeault, A.A.; Vaillancourt, C.; Sanderson, J.T. The Use of a Unique Co-Culture Model of Fetoplacental Steroidogenesis as a Screening Tool for Endocrine Disruptors: The Effects of Neonicotinoids on Aromatase Activity and Hormone Production. Toxicol. Appl. Pharmacol. 2017, 332, 15–24. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2017/1925 of 12 October 2017 amending Annex I to Council Regulation (EEC) No 2658/87 on the tariff and statistical nomenclature and on the Common Customs Tariff. Off. J. Eur. Union 2018, 282, 1–958. [Google Scholar]
- Berberidou, C.; Kitsiou, V.; Lambropoulou, D.A.; Michailidou, D.; Kouras, A.; Poulios, I. Decomposition and Detoxification of the Insecticide Thiacloprid by TiO2-Mediated Photocatalysis: Kinetics, Intermediate Products and Transformation Pathways. J. Chem. Technol. Biotechnol. 2019, 94, 2475–2486. [Google Scholar] [CrossRef]
- Akbari Shorgoli, A.; Shokri, M. Photocatalytic Degradation of Imidacloprid Pesticide in Aqueous Solution by TiO2 Nanoparticles Immobilized on the Glass Plate. Chem. Eng. Commun. 2017, 204, 1061–1069. [Google Scholar] [CrossRef]
- Yari, K.; Seidmohammadi, A.; Khazaei, M.; Bhatnagar, A.; Leili, M. A Comparative Study for the Removal of Imidacloprid Insecticide from Water by Chemical-Less UVC, UVC/TiO2 and UVC/ZnO Processes. J. Environ. Heal. Sci. Eng. 2019, 17, 337–351. [Google Scholar] [CrossRef]
- Abramović, B.F.; Banić, N.D.; Krstić, J.B. Degradation of Thiacloprid by ZnO in a Laminar Falling Film Slurry Photocatalytic Reactor. Ind. Eng. Chem. Res. 2013, 52, 5040–5047. [Google Scholar] [CrossRef]
- Murillo-Acevedo, Y.; Bernal-Sanchez, J.; Giraldo, L.; Sierra-Ramirez, R.; Moreno-Piraján, J.C. Initial Approximation to the Design and Construction of a Photocatalysis Reactor for Phenol Degradation with TiO2 Nanoparticles. ACS Omega 2019, 4, 19605–19613. [Google Scholar] [CrossRef] [Green Version]
- Braslavsky, S.E. IUPAC Glossary of Terms Used in Photochemistry, 3rd Edition. Pure Appl. Chem. 2007, 79, 293–465. [Google Scholar] [CrossRef]
- Hatchard, C.G.; Parker, C.A. A New Sensitive Chemical Actinometer-II. Potassium Ferrioxalate as a Standard Chemical Actinometer. Proc. R. Soc. London. Ser. A. Math. Phys. Sci. 1956, 235, 518–536. [Google Scholar] [CrossRef]
- Goldstein, S.; Rabani, J. The Ferrioxalate and Iodide-Iodate Actinometers in the UV Region. J. Photochem. Photobiol. A Chem. 2008, 193, 50–55. [Google Scholar] [CrossRef]
- Bolton, J.R.; Stefan, M.I.; Shaw, P.S.; Lykke, K.R. Determination of the Quantum Yields of the Potassium Ferrioxalate and Potassium Iodide-Iodate Actinometers and a Method for the Calibration of Radiometer Detectors. J. Photochem. Photobiol. A Chem. 2011, 222, 166–169. [Google Scholar] [CrossRef]
- Kuhn, H.J.; Braslavsky, S.E.; Schmidt, R. ORGANIC AND BIOMOLECULAR CHEMISTRY DIVISION * ( IUPAC Technical Report ) Chemical Actinometry (IUPAC Technical Report). Pure Appl. Chem. 2004, 76, 2105–2146. [Google Scholar] [CrossRef]
- Lehóczki, T.; Józsa, É.; Osz, K. Ferrioxalate Actinometry with Online Spectrophotometric Detection. J. Photochem. Photobiol. A Chem. 2013, 251, 63–68. [Google Scholar] [CrossRef]
- Aillet, T.; Loubiere, K.; Dechy-Cabaret, O.; Prat, L. Accurate Measurement of the Photon Flux Received inside Two Continuous Flow Microphotoreactors by Actinometry. Int. J. Chem. React. Eng. 2014, 12, 257–269. [Google Scholar] [CrossRef] [Green Version]
- Rabani, J.; Mamane, H.; Pousty, D.; Bolton, J.R. Practical Chemical Actinometry—A Review. Photochem. Photobiol. 2021, 97, 873–902. [Google Scholar] [CrossRef]
- Jiang, X.; Manawan, M.; Feng, T.; Qian, R.; Zhao, T.; Zhou, G.; Kong, F.; Wang, Q.; Dai, S.; Pan, J.H. Anatase and Rutile in Evonik Aeroxide P25: Heterojunctioned or Individual Nanoparticles? Catal. Today 2018, 300, 12–17. [Google Scholar] [CrossRef]
- Ohtani, B.; Prieto-Mahaney, O.O.; Li, D.; Abe, R. What Is Degussa (Evonic) P25? Crystalline Composition Analysis, Reconstruction from Isolated Pure Particles and Photocatalytic Activity Test. J. Photochem. Photobiol. A Chem. 2010, 216, 179–182. [Google Scholar] [CrossRef] [Green Version]
- Tobaldi, D.M.; Pullar, R.C.; Seabra, M.P.; Labrincha, J.A. Fully Quantitative X-ray Characterisation of Evonik Aeroxide TiO2 P25®. Mater. Lett. 2014, 122, 345–347. [Google Scholar] [CrossRef]
- Náfrádi, M.; Farkas, L.; Alapi, T.; Hernádi, K.; Kovács, K.; Wojnárovits, L.; Takács, E. Application of Coumarin and Coumarin-3-Carboxylic Acid for the Determination of Hydroxyl Radicals during Different Advanced Oxidation Processes. Radiat. Phys. Chem. 2020, 170. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nishikawa, M.; Nosaka, A.Y. Spectroscopic Investigation of the Mechanism of Photocatalysis. Molecules 2014, 19, 18248–18267. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Durrant, J.R.; Klug, D.R. Mechanism of Photocatalytic Water Splitting in TiO2. Reaction of Water with Photoholes, Importance of Charge Carrier Dynamics, and Evidence for Four-Hole Chemistry. J. Am. Chem. Soc. 2008, 103, 13885–13891. [Google Scholar] [CrossRef]
- Sachs, M.; Pastor, E.; Kafizas, A.; Durrant, J.R. Evaluation of Surface State Mediated Charge Recombination in Anatase and Rutile TiO2. J. Phys. Chem. Lett. 2016, 7, 3742–3746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Li, Y. The Influence of Inorganic Anions on Photocatalytic CO2 Reduction. Catal. Sci. Technol. 2020, 10, 959–966. [Google Scholar] [CrossRef]
- Rioja, N.; Zorita, S.; Peñas, F.J. Effect of Water Matrix on Photocatalytic Degradation and General Kinetic Modeling. Appl. Catal. B Environ. 2016, 180, 330–335. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Moon, G.; Kim, B.; Tachikawa, T.; Majima, T.; Hong, S.; Cho, K.; Kim, W.; Choi, W. Crystal Phase-Dependent Generation of Mobile OH Radicals on TiO2: Revisiting the Photocatalytic Oxidation Mechanism of Anatase and Rutile. Appl. Catal. B Environ. 2021, 286, 119905. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical Review of Rate Constants for Reactions of Hydrated Electrons, Hydrogen Atoms and Hydroxyl Radicals (⋅OH/⋅O− in Aqueous Solution. J. Phys. Chem. Ref. Data 1988, 17, 513–886. [Google Scholar] [CrossRef] [Green Version]
- Reichman, B.; Byvik, C.E. Photoproduction of I2, Br2, and Cl2 on n-Semiconducting Powder. J. Phys. Chem. 1981, 85, 2255–2258. [Google Scholar] [CrossRef]
- Maji, T.K.; Bagchi, D.; Kar, P.; Karmakar, D.; Pal, S.K. Enhanced Charge Separation through Modulation of Defect-State in Wide Band-Gap Semiconductor for Potential Photocatalysis Application: Ultrafast Spectroscopy and Computational Studies. J. Photochem. Photobiol. A Chem. 2017, 332, 391–398. [Google Scholar] [CrossRef]
- Nguyen, V.N.H.; Amal, R.; Beydoun, D. Effect of Formate and Methanol on Photoreduction/Removal of Toxic Cadmium Ions Using TiO2 Semiconductor as Photocatalyst. Chem. Eng. Sci. 2003, 58, 4429–4439. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, T.; Ren, P.; Cao, D.; Lin, Y.; Wang, L.; Zhang, B.; Xiang, X. Doping of Chlorine from a Neoprene Adhesive Enhances Degradation Efficiency of Dyes by Structured TiO2-Coated Photocatalytic Fabrics. Catalysts 2020, 10, 69. [Google Scholar] [CrossRef] [Green Version]
- Iguchi, S.; Teramura, K.; Hosokawa, S.; Tanaka, T. Effect of the Chloride Ion as a Hole Scavenger on the Photocatalytic Conversion of CO2 in an Aqueous Solution over Ni-Al Layered Double Hydroxides. Phys. Chem. Chem. Phys. 2015, 17, 17995–18003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, R.; Miseki, Y.; Okunaka, S.; Sayama, K. Photocatalytic Production of Hypochlorous Acid over Pt/WO3 under Simulated Solar Light. ACS Sustain. Chem. Eng. 2020, 8, 8629–8637. [Google Scholar] [CrossRef]
- Long, C.A.; Blelski, B.H.J. Rate of Reaction of Superoxide Radical with Chloride-Containing Species. J. Phys. Chem. 1980, 84, 555–557. [Google Scholar] [CrossRef]
- Serrà, A.; Philippe, L.; Perreault, F.; Garcia-Segura, S. Photocatalytic Treatment of Natural Waters. Reality or Hype? The Case of Cyanotoxins Remediation. Water Res. 2021, 188, 116543. [Google Scholar] [CrossRef]
- Cheng, Z.; Ling, L.; Wu, Z.; Fang, J.; Westerhoff, P.; Shang, C. Novel Visible Light-Driven Photocatalytic Chlorine Activation Process for Carbamazepine Degradation in Drinking Water. Environ. Sci. Technol. 2020, 54, 11584–11593. [Google Scholar] [CrossRef]
- Mazellier, P.; Busset, C.; Delmont, A.; De Laat, J. A Comparison of Fenuron Degradation by Hydroxyl and Carbonate Radicals in Aqueous Solution. Water Res. 2007, 41, 4585–4594. [Google Scholar] [CrossRef] [PubMed]
- Dell’Arciprete, M.L.; Soler, J.M.; Santos-Juanes, L.; Arques, A.; Mártire, D.O.; Furlong, J.P.; Gonzalez, M.C. Reactivity of Neonicotinoid Insecticides with Carbonate Radicals. Water Res. 2012, 46, 3479–3489. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Duan, X.; Fu, Y.; Fatta-Kassinos, D.; Dionysiou, D.D. Significant Role of UV and Carbonate Radical on the Degradation of Oxytetracycline in UV-AOPs: Kinetics and Mechanism. Water Res. 2016, 95, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-Merit for the Technical Development and Application of Advanced Oxidation Technologies for Both Electric- and Solar-Driven Systems. Pure Appl. Chem. 2001, 73, 627–637. [Google Scholar] [CrossRef]
- Bourgin, M.; Violleau, F.; Debrauwer, L.; Albet, J. Ozonation of Imidacloprid in Aqueous Solutions: Reaction Monitoring and Identification of Degradation Products. J. Hazard. Mater. 2011, 190, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Bamba, D.; Coulibaly, M.; Robert, D. Nitrogen-Containing Organic Compounds: Origins, Toxicity and Conditions of Their Photocatalytic Mineralization over TiO2. Sci. Total Environ. 2017, 580, 1489–1504. [Google Scholar] [CrossRef]
- Piccinini, P.; Minero, C.; Vincenti, M.; Pelizzetti, E. Photocatalytic Mineralization of Nitrogen-Containing Benzene Derivatives. Catal. Today 1997, 39, 187–195. [Google Scholar] [CrossRef]
- Løgager, T.; Sehested, K. Formation and Decay of Peroxynitric Acid: A Pulse Radiolysis Study. J. Phys. Chem. 1993, 97, 10047–10052. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.J.; Kim, J.S.; Yoo, K.; Lee, J.C.; Anderson, W.A.; Lee, J.H. Photocatalytic Reduction of Nitrate in Wastewater Using ZnO Nanopowder Synthesized by Solution Combustion Method. J. Nanosci. Nanotechnol. 2007, 7, 4069–4072. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Deng, Y.; Liu, C.; He, Q.; Wei, Y.; Chen, S.; Liu, T.; Luo, S. Kinetics, Pathways and Toxicity Evaluation of Neonicotinoid Insecticides Degradation via UV/Chlorine Process. Chem. Eng. J. 2018, 346, 298–306. [Google Scholar] [CrossRef]
- Rózsa, G.; Náfrádi, M.; Alapi, T.; Schrantz, K.; Szabó, L.; Wojnárovits, L.; Takács, E.; Tungler, A. Photocatalytic, Photolytic and Radiolytic Elimination of Imidacloprid from Aqueous Solution: Reaction Mechanism, Efficiency and Economic Considerations. Appl. Catal. B Environ. 2019, 250, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Rózsa, G.; Szabó, L.; Schrantz, K.; Takács, E.; Wojnárovits, L. Mechanistic Study on Thiacloprid Transformation: Free Radical Reactions. J. Photochem. Photobiol. A Chem. 2017, 343, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Patra, S.G.; Mizrahi, A.; Meyerstein, D. The Role of Carbonate in Catalytic Oxidations. Acc. Chem. Res. 2020, 53, 2189–2200. [Google Scholar] [CrossRef]
- Chen, S.; Wang, L.W. Thermodynamic Oxidation and Reduction Potentials of Photocatalytic Semiconductors in Aqueous Solution. Chem. Mater. 2012, 24, 3659–3666. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, D.A.; Huie, R.E.; Koppenol, W.H.; Lymar, S.V.; Merenyi, G.; Neta, P.; Ruscic, B.; Stanbury, D.M.; Steenken, S.; Wardman, P. Standard Electrode Potentials Involving Radicals in Aqueous Solution: Inorganic Radicals (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1139–1150. [Google Scholar] [CrossRef]
- Dell’Arciprete, M.L.; Santos-Juanes, L.; Sanz, A.A.; Vicente, R.; Amat, A.M.; Furlong, J.P.; Mártire, D.O.; Gonzalez, M.C. Reactivity of Hydroxyl Radicals with Neonicotinoid Insecticides: Mechanism and Changes in Toxicity. Photochem. Photobiol. Sci. 2009, 8, 1016–1023. [Google Scholar] [CrossRef] [PubMed]
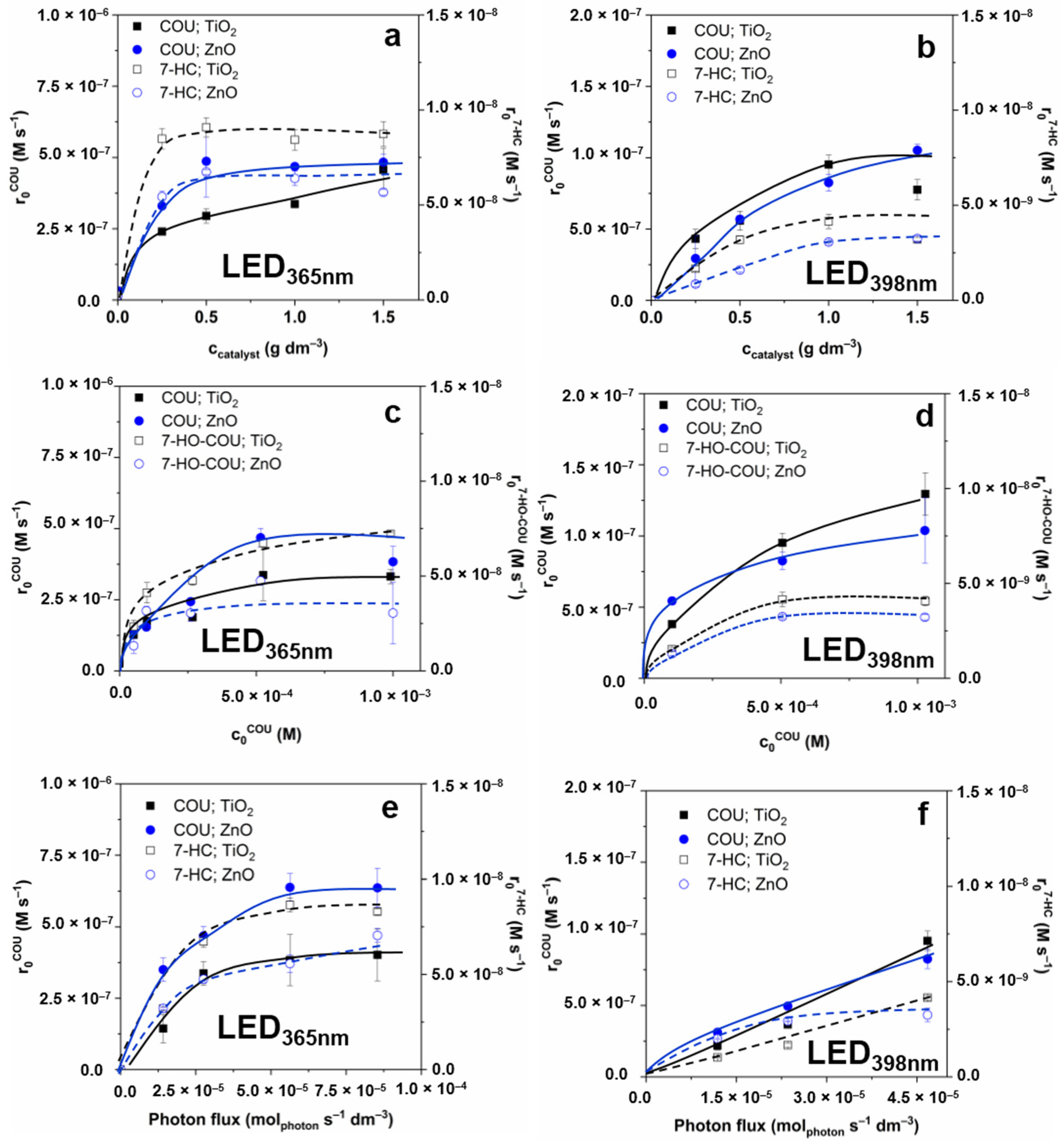


| LED | Pel (W) | TiO2 | ZnO | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r0COU (×10−7) | ΦappCOU (×10−2) | r07-HC (×10−9) | Φapp7-HC (×10−4) | r07-HC/r0COU | r0COU (×10−7) | ΦappCOU (×10−2) | r07-HC (×10−9) | Φapp7-HC (×10−4) | r07-HC/r0COU | ||
| LED365nm | 3.39 | 1.49 | 1.01 | 3.4 | 2.3 | 0.023 | 3.50 | 2.47 | 3.22 | 2.3 | 0.009 |
| 6.56 | 3.03 | 1.22 | 6.40 | 2.4 | 0.021 | 4.68 | 1.70 | 4.75 | 1.7 | 0.010 | |
| 13.60 | 3.83 | 0.68 | 8.25 | 1.5 | 0.022 | 6.21 | 1.13 | 5.57 | 1.0 | 0.009 | |
| 20.77 | 4.02 | 0.47 | 8.30 | 1.0 | 0.021 | 6.20 | 0.75 | 6.25 | 0.8 | 0.011 | |
| LED398nm | 0.96 | 0.22 | 0.18 | 1.02 | 0.85 | 0.044 | 0.31 | 0.26 | 2.00 | 1.7 | 0.039 |
| 2.16 | 0.47 | 0.19 | 1.97 | 0.82 | 0.045 | 0.50 | 0.21 | 2.93 | 1.3 | 0.059 | |
| 4.68 | 0.95 | 0.20 | 4.15 | 0.89 | 0.047 | 0.83 | 0.18 | 3.25 | 0.7 | 0.064 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Náfrádi, M.; Alapi, T.; Bencsik, G.; Janáky, C. Impact of Reaction Parameters and Water Matrices on the Removal of Organic Pollutants by TiO2/LED and ZnO/LED Heterogeneous Photocatalysis Using 365 and 398 nm Radiation. Nanomaterials 2022, 12, 5. https://doi.org/10.3390/nano12010005
Náfrádi M, Alapi T, Bencsik G, Janáky C. Impact of Reaction Parameters and Water Matrices on the Removal of Organic Pollutants by TiO2/LED and ZnO/LED Heterogeneous Photocatalysis Using 365 and 398 nm Radiation. Nanomaterials. 2022; 12(1):5. https://doi.org/10.3390/nano12010005
Chicago/Turabian StyleNáfrádi, Máté, Tünde Alapi, Gábor Bencsik, and Csaba Janáky. 2022. "Impact of Reaction Parameters and Water Matrices on the Removal of Organic Pollutants by TiO2/LED and ZnO/LED Heterogeneous Photocatalysis Using 365 and 398 nm Radiation" Nanomaterials 12, no. 1: 5. https://doi.org/10.3390/nano12010005