Solar-Driven Soil Remediation along with the Generation of Water Vapor and Electricity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pretreatment of Soil
2.2. Preparation of Soil Contaminated by Heavy Metals or Acid
2.3. ISUM Manufacturing
2.4. Characterization
3. Results
3.1. Composition and Characteristics of Six Typical Soils
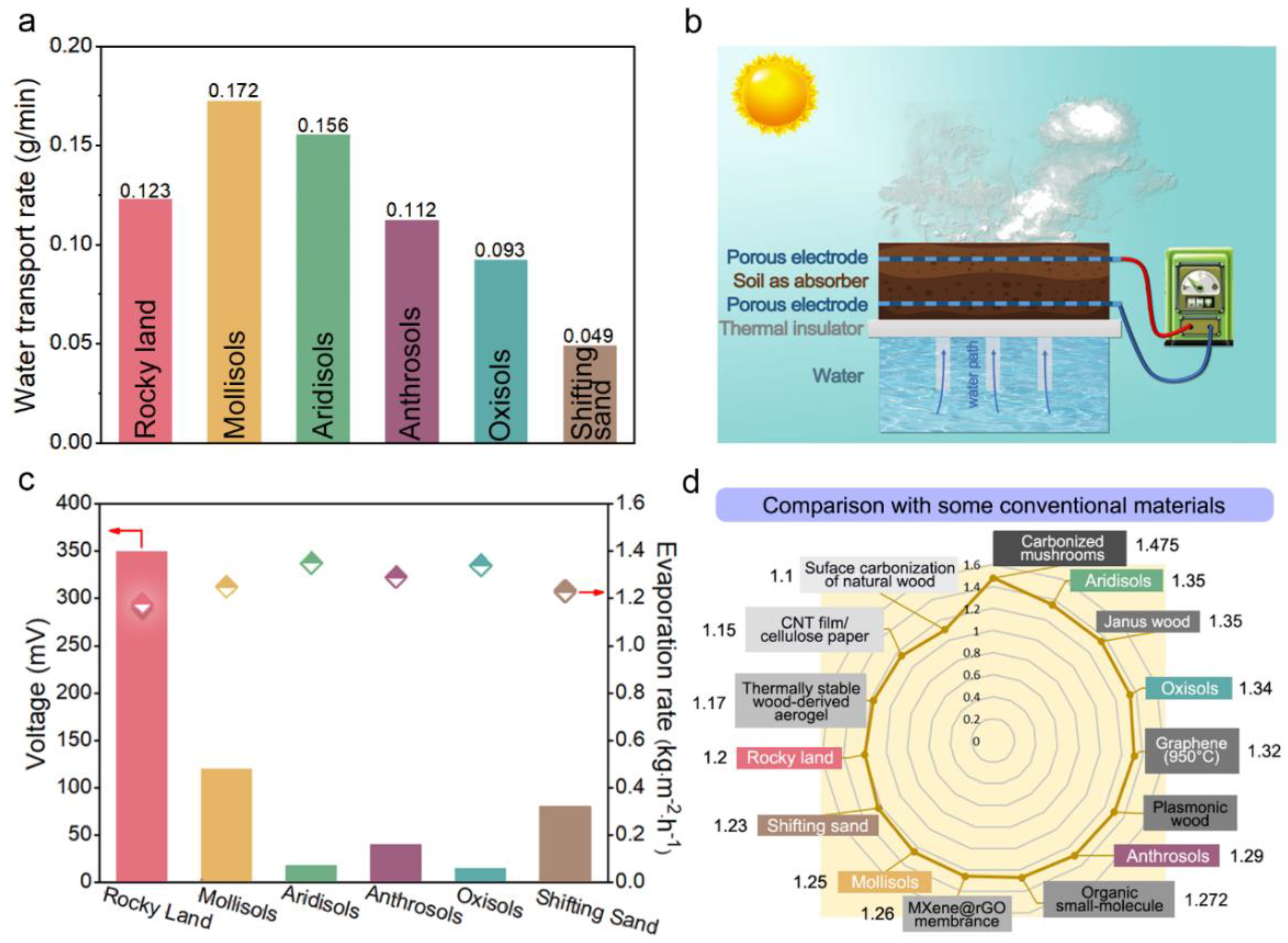
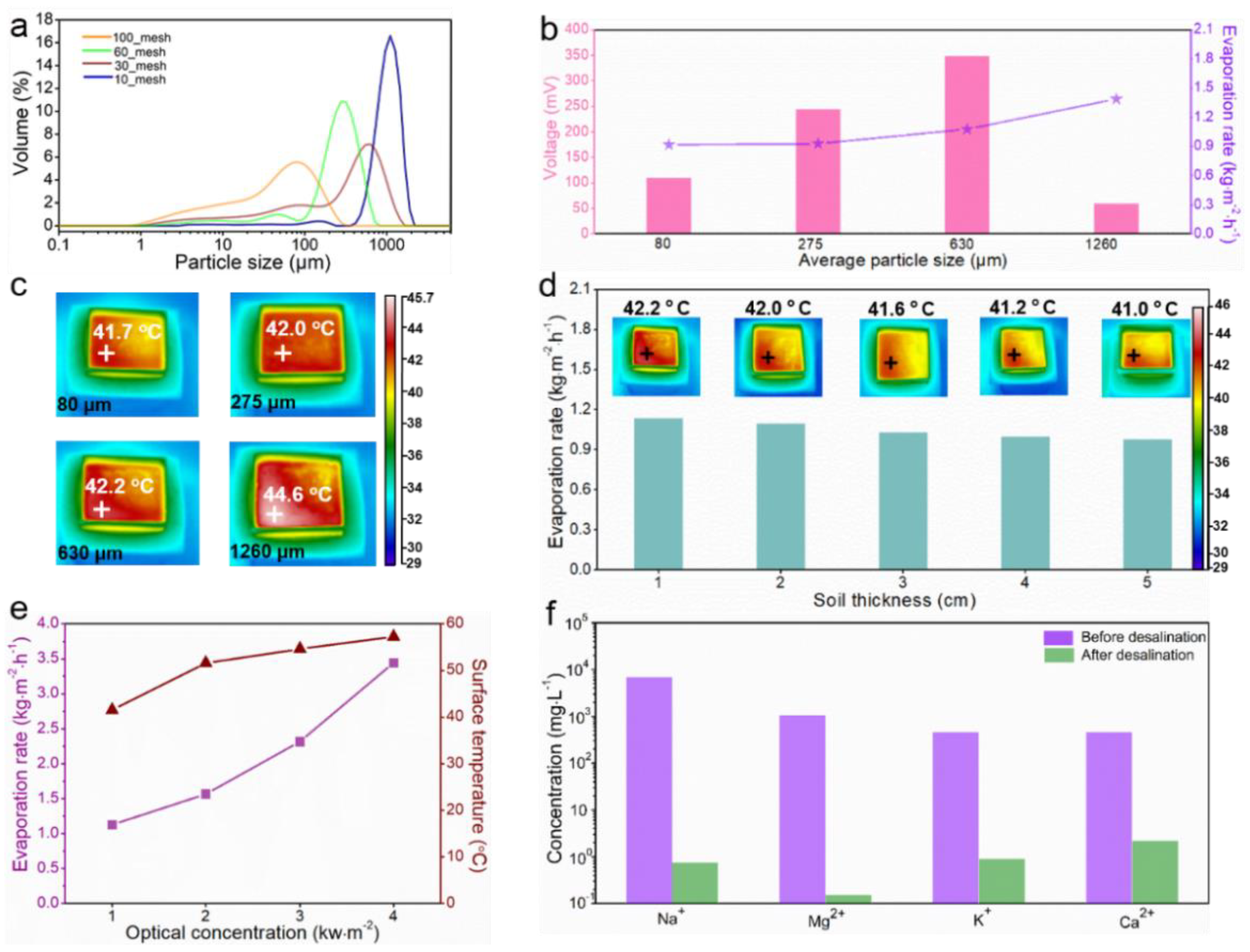
3.2. Water Vapor Generation Performance of ISUM
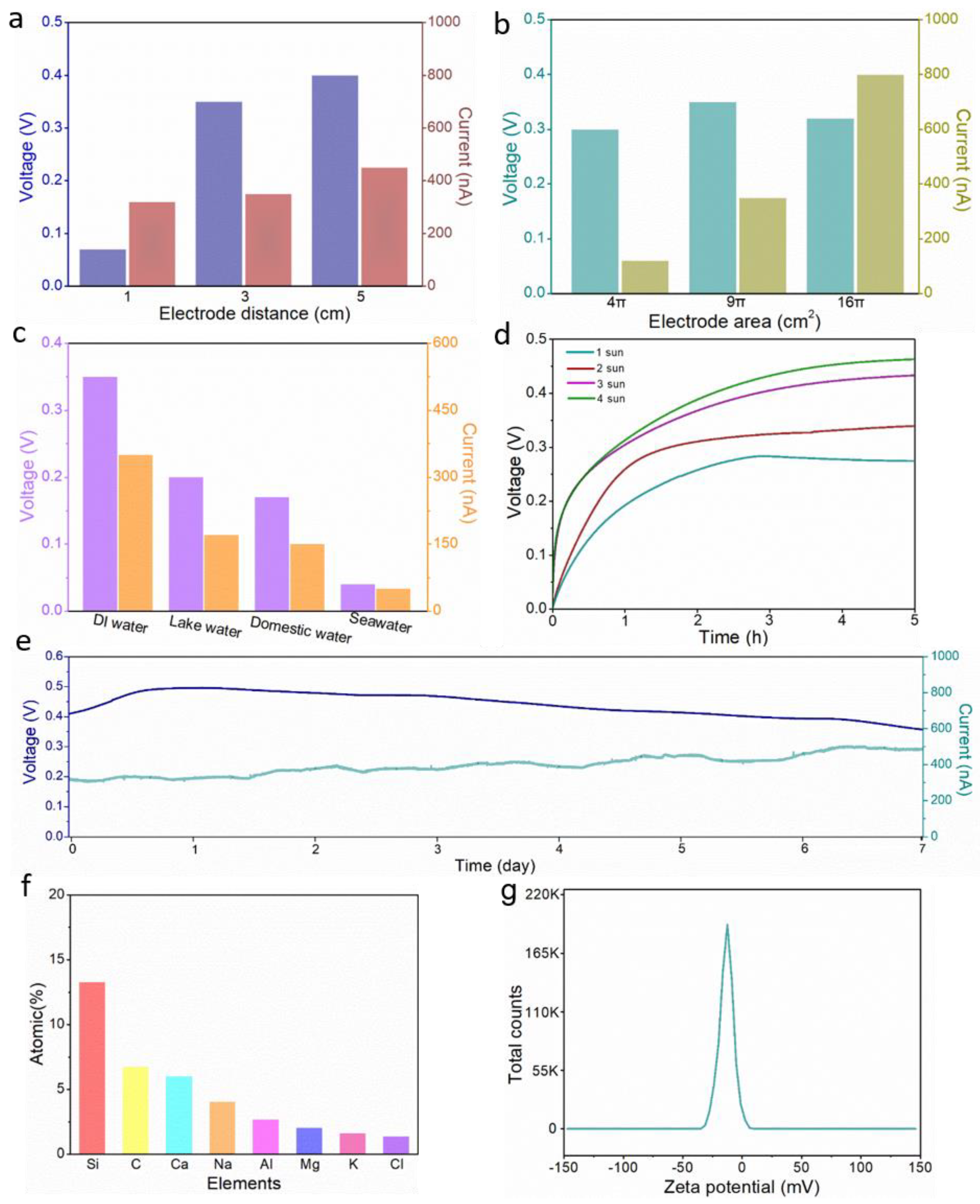
3.3. Electricity Generation Performance of ISUM
3.4. Soil Remediation Performance of ISUM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ager, J.W.; Lapkin, A.A. Chemical storage of renewable energy. Science 2018, 360, 707–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, S.N. Research opportunities to advance solar energy utilization. Science 2016, 351, 1920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Kuang, Y.; Hu, L. Challenges and Opportunities for Solar Evaporation. Joule 2019, 3, 683–718. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Li, Z.; Jiang, Y.; Xu, G.; Zhu, M.; Law, W.; Yong, K.; Wang, Y.; Yang, C.; Dong, B.; et al. Recent advances in solar-driven evaporation systems. J. Mater. Chem. A 2020, 8, 25571–25600. [Google Scholar] [CrossRef]
- Tao, P.; Ni, G.; Song, C.Y.; Shang, W.; Wu, J.B.; Zhu, J.; Chen, G.; Deng, T. Solar driven interfacial evaporation. Nat. Energy 2018, 3, 1031–1041. [Google Scholar] [CrossRef]
- Ghasemi, H.; Ni, G.; Marconnet, A.M.; Loomis, J.; Yerci, S.; Miljkovic, N.; Chen, G. Solar steam generation by heat localization. Nat. Commun. 2014, 5, 4449. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.L.; Gao, M.M.; Peh, C.K.N.; Wang, X.Q.; Ho, G.W. Self-Contained Monolithic Carbon Sponges for Solar-Driven Interfacial Water Evaporation Distillation and Electricity Generation. Adv. Energy Mater. 2018, 8, 1702149. [Google Scholar] [CrossRef]
- Cui, L.F.; Zhang, P.P.; Xiao, Y.K.; Liang, Y.; Liang, H.X.; Cheng, Z.H.; Qu, L.T. High Rate Production of Clean Water Based on the Combined Photo-Electro-Thermal Effect of Graphene Architecture. Adv. Mater. 2018, 30, 1706805. [Google Scholar] [CrossRef]
- Liang, H.X.; Liao, Q.H.; Chen, N.; Liang, Y.; Lv, G.Q.; Zhang, P.P.; Lu, B.; Qu, L.T. Thermal Efficiency of Solar Steam Generation Approaching 100% through Capillary Water Transport. Angew. Chem. Int. Ed. 2019, 58, 19041–19046. [Google Scholar] [CrossRef]
- Ren, H.Y.; Tang, M.; Guan, B.L.; Wang, K.X.; Yang, J.W.; Wang, F.F.; Wang, M.Z.; Shan, J.Y.; Chen, Z.L.; Wei, D.; et al. Hierarchical Graphene Foam for Efficient Omnidirectional Solar–Thermal Energy Conversion. Adv. Mater. 2017, 29, 1702590. [Google Scholar] [CrossRef]
- Hu, X.Z.; Xu, W.C.; Zhou, L.; Tan, Y.L.; Wang, Y.; Zhu, S.N.; Zhu, J. Tailoring Graphene Oxide-Based Aerogels for Efficient Solar Steam Generation under One Sun. Adv. Mater. 2016, 29, 1604031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Liao, Q.H.; Zhang, T.; Cheng, H.H.; Huang, Y.X.; Yang, C.; Li, C.; Jiang, L.; Qu, L.T. High throughput of clean water excluding ions, organic media, and bacteria from defect-abundant graphene aerogel under sunlight. Nano Energy 2018, 46, 415–422. [Google Scholar] [CrossRef]
- Zhang, P.P.; Li, J.; Lv, L.X.; Zhao, Y.; Qu, L.T. Vertically Aligned Graphene Sheets Membrane for Highly Efficient Solar Thermal Generation of Clean Water. ACS Nano 2017, 11, 5087–5093. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.P.; Liao, Q.; Yao, H.; Cheng, H.H.; Huang, Y.X.; Yang, C.; Jiang, L.; Qu, L.T. Three-dimensional water evaporation on a macroporous vertically aligned graphene pillar array under one sun. J. Mater. Chem. A 2018, 6, 15303. [Google Scholar] [CrossRef]
- Xiong, Z.C.; Zhu, Y.J.; Qin, D.D.; Chen, F.F.; Yang, R.L. Flexible Fire-Resistant Photothermal Paper Comprising Ultralong Hydroxyapatite Nanowires and Carbon Nanotubes for Solar Energy-Driven Water Purification. Small 2018, 14, 1803387. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Yao, J.F.; Horri, B.A.; Wang, K.; Wu, Y.Z.; Lia, D.; Wang, H.T. Solar evaporation enhancement using floating light-absorbing magnetic particles. Energy Environ. Sci. 2011, 4, 4074–4078. [Google Scholar] [CrossRef]
- Yang, W.J.; Zhao, J.H.; Tian, H.; Wang, L.Z.; Wang, X.Y.; Ye, S.; Liu, J.; Huang, J. Solar-Driven Carbon Nanoreactor Coupling Gold and Platinum Nanocatalysts for Alcohol Oxidations. Small 2020, 16, 2002236. [Google Scholar] [CrossRef]
- Guo, Y.H.; Lu, H.Y.; Zhao, F.; Zhou, X.Y.; Shi, W.; Yu, G.H. Biomass-Derived Hybrid Hydrogel Evaporators for Cost-Effective Solar Water Purification. Adv. Mater. 2020, 32, 1907061. [Google Scholar] [CrossRef]
- Xu, N.; Hu, X.Z.; Xu, W.C.; Li, X.Q.; Zhou, L.; Zhu, S.N.; Zhu, J. Mushrooms as Efficient Solar Steam-Generation Devices. Adv. Mater. 2017, 29, 1606762. [Google Scholar] [CrossRef]
- Bae, K.; Kang, G.; Cho, S.K.; Park, W.; Kim, K.; Padilla, W.J. Flexible thin-film black gold membranes with ultrabroadband plasmonic nanofocusing for efficient solar vapour generation. Nat. Commun. 2015, 6, 10103. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Tan, Y.L.; Ji, D.X.; Zhu, B.; Zhang, P.; Xu, J.; Gan, Q.Q.; Yu, Z.F.; Zhu, J. Self-assembly of highly efficient, broadband plasmonic absorbers for solar steam generation. Sci. Adv. 2016, 2, e1501227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Tan, Y.L.; Wang, J.Y.; Xu, W.C.; Yuan, Y.; Cai, W.S.; Zhu, S.N.; Zhu, J. 3D self-assembly of aluminium nanoparticles for plasmon-enhanced solar desalination. Nat. Photon. 2016, 10, 393–398. [Google Scholar] [CrossRef]
- Zhu, M.W.; Li, Y.J.; Chen, F.J.; Zhu, X.Y.; Dai, J.Q.; Li, Y.F.; Yang, Z.; Yan, X.J.; Song, J.W.; Wang, Y.B.; et al. Plasmonic Wood for High-Efficiency Solar Steam Generation. Adv. Energy Mater. 2017, 8, 1701028. [Google Scholar] [CrossRef]
- Shi, Y.; Li, R.; Jin, Y.; Zhuo, S.; Shi, L.; Chang, J.; Hong, S.; Ng, K.C.; Wang, P. Power and Technology Scaling into the 5 nm Node with Stacked Nanosheets. Joule 2018, 2, 1171–1186. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.; Liu, Y.B.; Krueger, D.; Prasad, M.; Lee, S.E.; Park, Y. Visible-Light Induced Sustainable Water Treatment Using Plasmo-Semiconductor Nanogap Bridge Array, PNA. Small 2021, 17, 2006044. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.Y.; Deng, L.; Wei, N.N.; Weng, Y.K.; Dong, S.; Qi, D.P.; Qiu, J.; Chen, X.D.; Wu, T. High-Performance Photothermal Conversion of Narrow-Bandgap Ti2O3 Nanoparticles. Adv. Mater. 2017, 29, 1603730. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.Y.; Guo, Y.H.; Zhao, F.; Shi, W.; Yu, G.H. Topology-Controlled Hydration of Polymer Network in Hydrogels for Solar-Driven Wastewater Treatment. Adv. Mater. 2020, 32, 2007012. [Google Scholar] [CrossRef]
- Huang, C.H.; Huang, J.X.; Chiao, Y.H.; Chang, C.M.; Hung, W.S.; Lue, S.J.; Wang, C.F.; Hu, C.C.; Lee, K.R.; Pan, H.H.; et al. Tailoring of a Piezo-Photo-Thermal Solar Evaporator for Simultaneous Steam and Power Generation. Adv. Funct. Mater. 2021, 31, 2010422. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Ravi, S.K.; Tan, S.C. Food-derived carbonaceous materials for solar desalination and thermo-electric power generation. Nano Energy 2019, 65, 104006. [Google Scholar] [CrossRef]
- Li, C.Z.; Liu, K.; Liu, H.D.; Yang, B.; Hu, X.J. Capillary driven electrokinetic generator for environmental energy harvesting. Mater. Res. Bull. 2017, 90, 81–86. [Google Scholar] [CrossRef]
- Duan, Y.M.; Weng, M.C.; Zhang, W.; Qian, Y.Q.; Luo, Z.L.; Chen, L.Z. Multi-functional carbon nanotube paper for solar water evaporation combined with electricity generation and storage. Energy Convers. Manag. 2021, 241, 114306. [Google Scholar] [CrossRef]
- Gao, X.; Xu, T.; Shao, C.X.; Han, Y.Y.; Lu, B.; Zhang, Z.P. Electric power generation using paper materials. J. Mater. Chem. A 2019, 7, 20574. [Google Scholar] [CrossRef]
- Li, Z.H.; Chen, C.J.; Xie, H.; Yao, Y.; Zhang, X.; Brozena, A.; Li, J.G.; Ding, Y.; Zhao, X.P.; Hong, M.; et al. Sustainable high-strength macrofibres extracted from natural bamboo. Nat. Sustain. 2021, 5, 235–244. [Google Scholar] [CrossRef]
- An, N.; Tang, C.S.; Xu, S.K.; Gong, X.P.; Shi, B.; Inyang, H.I. Effects of soil characteristics on moisture evaporation. Eng. Geol. 2018, 239, 126–135. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Wu, T.; White, J.C.; Lin, D.H. A new strategy using nanoscale zero-valent iron to simultaneously promote remediation and safe crop production in contaminated soil. Nat. Nanotechnol. 2021, 16, 197–205. [Google Scholar] [CrossRef]
- Dhaliwal, S.S.; Singh, J.; Taneja, P.K.; Mandal, A. Remediation techniques for removal of heavy metals from the soil contaminated through different sources: A review. Environ. Sci. Pollut. Res. 2020, 27, 1319–1333. [Google Scholar] [CrossRef]
- Zivdar, Z.; Heidarzadeh, N.; Asadollahfardi, G. Remediation of diesel contaminated soil by low temperature thermal desorption. Int. J. Environ. Sci. Technol. 2019, 16, 6113–6124. [Google Scholar] [CrossRef]
- Global Soil Regions Map/NRCS Soils. Available online: http://soils.usda.gov/use/worldsoils/mapindex/order.html (accessed on 15 June 2021).
- Khormali, F.; Monger, C. Hot desert soils—Global distribution and unique characteristics. Geoderma Regional 2020, 23, e00330. [Google Scholar] [CrossRef]
- Chen, G.Y.; Sun, J.M.; Peng, Q.; Sun, Q.; Wang, G.; Cai, Y.J.; Gu, X.G.; Shuai, Z.G.; Tang, B.Z. Biradical-Featured Stable Organic-Small-Molecule Photothermal Materials for Highly Efficient Solar-Driven Water Evaporation. Adv. Mater. 2020, 32, 1908537. [Google Scholar] [CrossRef]
- Xiao, P.; He, J.; Ni, F.; Zhang, C.; Liang, Y.; Zhou, W.; Gu, J.C.; Xia, J.Y.; Kuo, S.W.; Chen, T. Exploring interface confined water flow and evaporation enables solar-thermal-electro integration towards clean water and electricity harvest via asymmetric functionalization strategy. Nano Energy 2020, 68, 104385. [Google Scholar] [CrossRef]
- Ying, P.J.; Ai, B.; Hu, W.; Geng, Y.; Li, L.; Sun, K.; Tan, S.C.; Zhang, W.; Li, M. A bio-inspired nanocomposite membrane with improved light-trapping and salt-rejecting performance for solar-driven interfacial evaporation applications. Nano Energy 2021, 89, 106443. [Google Scholar] [CrossRef]
- Ito, Y.; Tanabe, Y.; Han, J.H.; Fujita, T.; Tanigaki, K.; Chen, M.W. Multifunctional Porous Graphene for High-Efficiency Steam Generation by Heat Localization. Adv. Mater. 2015, 27, 4302–4307. [Google Scholar] [CrossRef] [PubMed]
- Meng, T.T.; Jiang, B.; Li, Z.T.; Xu, X.T.; Li, D.G.; Henzie, J.; Nanjundan, A.K.; Yamauchi, Y.; Bando, Y. Programmed design of selectively-functionalized wood aerogel: Affordable and mildew-resistant solar-driven evaporator. Nano Energy 2021, 87, 106146. [Google Scholar] [CrossRef]
- Jia, C.; Li, Y.J.; Yang, Z.; Chen, G.; Yao, Y.G.; Jiang, F.; Kuang, Y.; Pastel, G.; Xie, H.; Yang, B.; et al. Rich Mesostructures Derived from Natural Woods for Solar Steam Generation. Joule 2017, 1, 588–599. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; He, S.M.; Falinski, M.M.; Wang, Y.X.; Li, T.; Zheng, S.X.; Sun, D.Y.; Dai, J.Q.; Bian, Y.H.; Zhu, X.B.; et al. Sustainable off-grid desalination of hypersaline waters using Janus wood evaporators. Energy Environ. Sci. 2021, 14, 5347–5357. [Google Scholar] [CrossRef]
- Shao, C.X.; Ji, B.X.; Xu, T.; Gao, J.; Gao, X.; Xiao, Y.K.; Zhao, Y.; Chen, N.; Jiang, L.; Qu, L.T. Large-Scale Production of Flexible, High-Voltage Hydroelectric Films Based on Solid Oxides. ACS Appl. Mater. Interfaces 2019, 11, 30927–30935. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; World Health Organization: Geneva, Switzerland, 2011.
- Mani, D.; Kumar, C.; Patel, N.K. Integrated micro-biochemical approach for phytoremediation of cadmium and zinc contaminated soils. Ecotoxicol. Environ. Saf. 2015, 111, 86–95. [Google Scholar] [CrossRef]
- Li, Z.; Jia, M.Y.; Christie, P.; Ali, S.; Wu, L.H. Use of a hyperaccumulator and biochar to remediate an acid soil highly contaminated with trace metals and/or oxytetracycline. Chemosphere 2018, 204, 390–397. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.J.; Xu, H.B.; Xia, P.; Wang, H.; Jing, H.P.; Li, J.; Zhao, J.F. High zinc removal from water and soil using struvite supported diatomite obtained by nitrogen and phosphate recovery from wastewater. Environ. Chem. Lett. 2018, 16, 569–573. [Google Scholar] [CrossRef]
- Li, H.K.; Xu, F.; Xie, Y.L.; Wang, C.; Zhang, A.K.; Li, L.L.; Xu, H. Effect of modified coconut shell biochar on availability of heavy metals and biochemical characteristics of soil in multiple heavy metals contaminated soil. Sci. Total Environ. 2018, 645, 702–709. [Google Scholar] [CrossRef]
- Xia, Z.H.; Zhang, S.R.; Cao, Y.R.; Zhong, Q.M.; Wang, G.Y.; Li, T.; Xu, X.X. Remediation of cadmium, lead and zinc in contaminated soil with CETSA and MA/AA. J. Hazard. Mater. 2019, 366, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Myung, E.; Kim, H.; Park, C.; Choi, N.; Park, C. Effect of Soil Washing Solutions on Simultaneous Removal of Heavy Metals and Arsenic from Contaminated Soil. Int. J. Environ. Res. Public Health 2020, 17, 3133. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.J.; Wei, J.F.; Wang, D.; Kong, Z.Y.; Zhang, H.; Wang, H.C. A novel remediation method of cadmium (Cd) contaminated soil: Dynamic equilibrium of Cd2+ rapid release from soil to water and selective adsorption by PP-g-AA fibers-ball at low concentration. J. Hazard. Mater. 2021, 416, 125884. [Google Scholar] [CrossRef] [PubMed]
- Bahemmat, M.; Farahbakhsh, M.; Kianirad, M. Humic substances-enhanced electroremediation of heavy metals Contaminated soil. J. Hazard. Mater. 2016, 312, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Cameselle, C.; Gouveia, S.; Cabo, A. Enhanced Electrokinetic Remediation for the Removal of Heavy Metals from Contaminated Soils. Appl. Sci. 2021, 11, 1799. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wang, Z.; Liang, H.; Li, Y.; Liu, T.; Guo, Q.; Wang, L.; Yang, Y.; Chen, N. Solar-Driven Soil Remediation along with the Generation of Water Vapor and Electricity. Nanomaterials 2022, 12, 1800. https://doi.org/10.3390/nano12111800
Liu X, Wang Z, Liang H, Li Y, Liu T, Guo Q, Wang L, Yang Y, Chen N. Solar-Driven Soil Remediation along with the Generation of Water Vapor and Electricity. Nanomaterials. 2022; 12(11):1800. https://doi.org/10.3390/nano12111800
Chicago/Turabian StyleLiu, Xiaoting, Zhe Wang, Hanxue Liang, Yuanyuan Li, Tianfu Liu, Qiang Guo, Liru Wang, Ya’nan Yang, and Nan Chen. 2022. "Solar-Driven Soil Remediation along with the Generation of Water Vapor and Electricity" Nanomaterials 12, no. 11: 1800. https://doi.org/10.3390/nano12111800





