Defect Density-Dependent pH Response of Graphene Derivatives: Towards the Development of pH-Sensitive Graphene Oxide Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Single-Layer Graphene Transfer Process
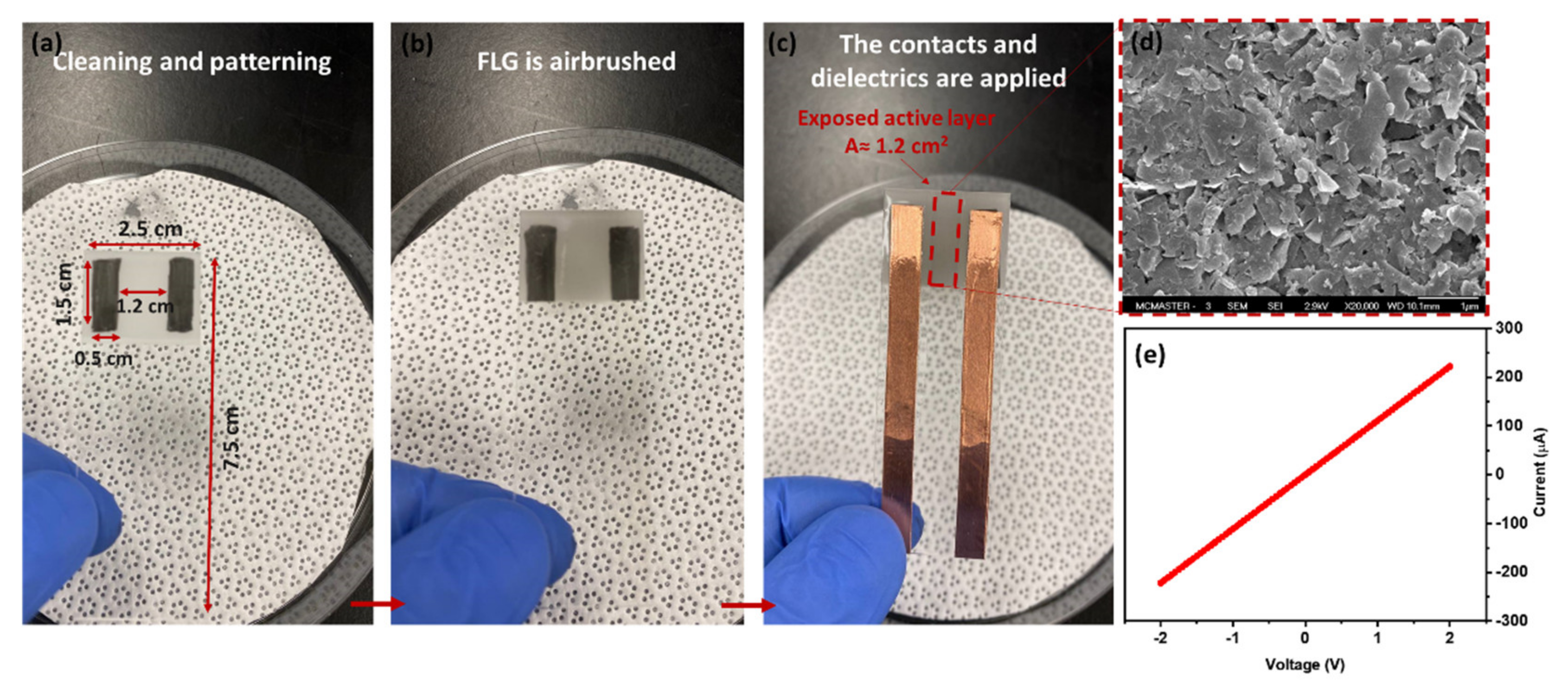
2.2. Synthesis of FLG and Sensor Fabrication
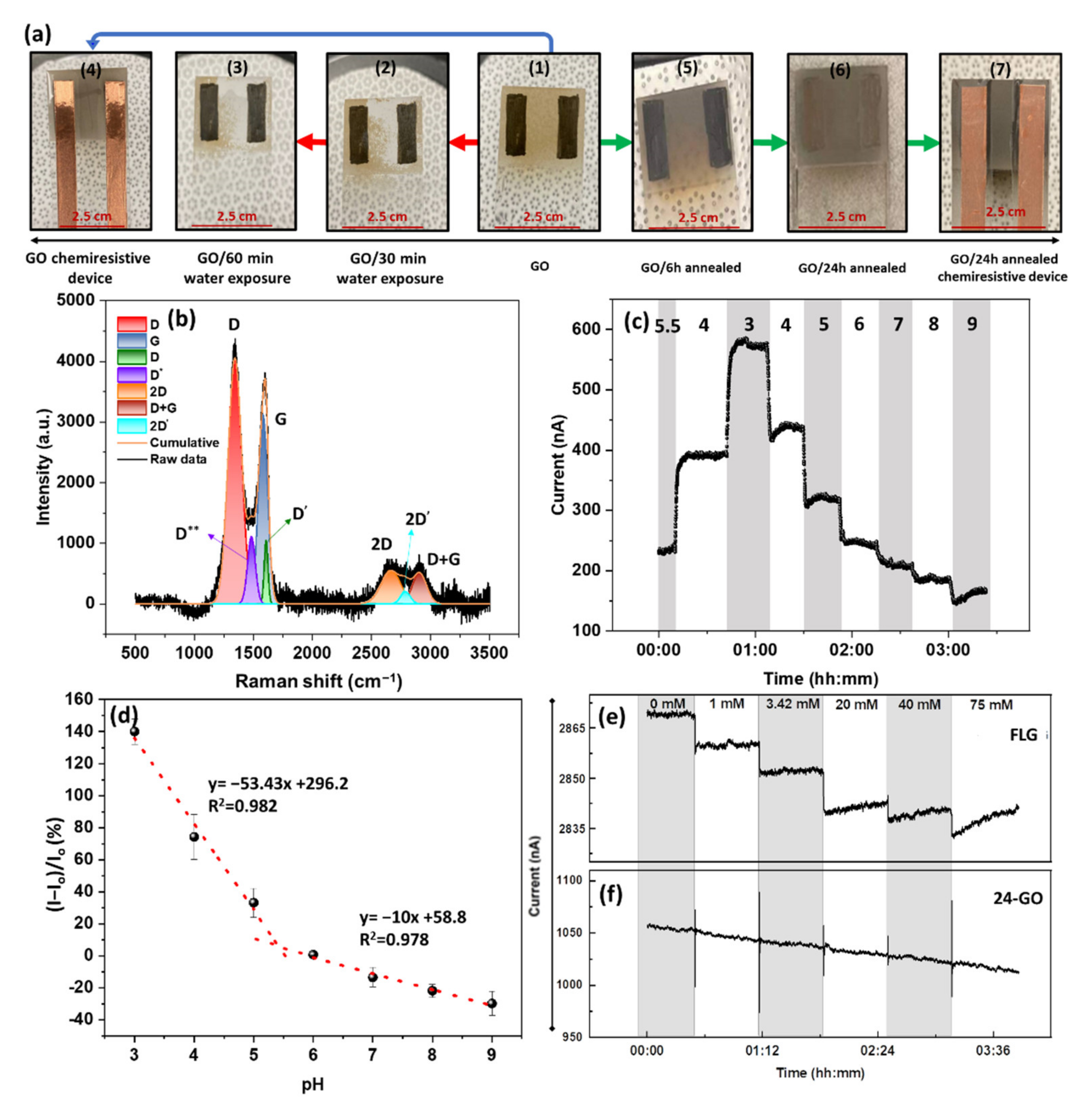
2.3. GO Preparation and SENSOR fabrication
2.4. Characterization
3. Results and Discussions
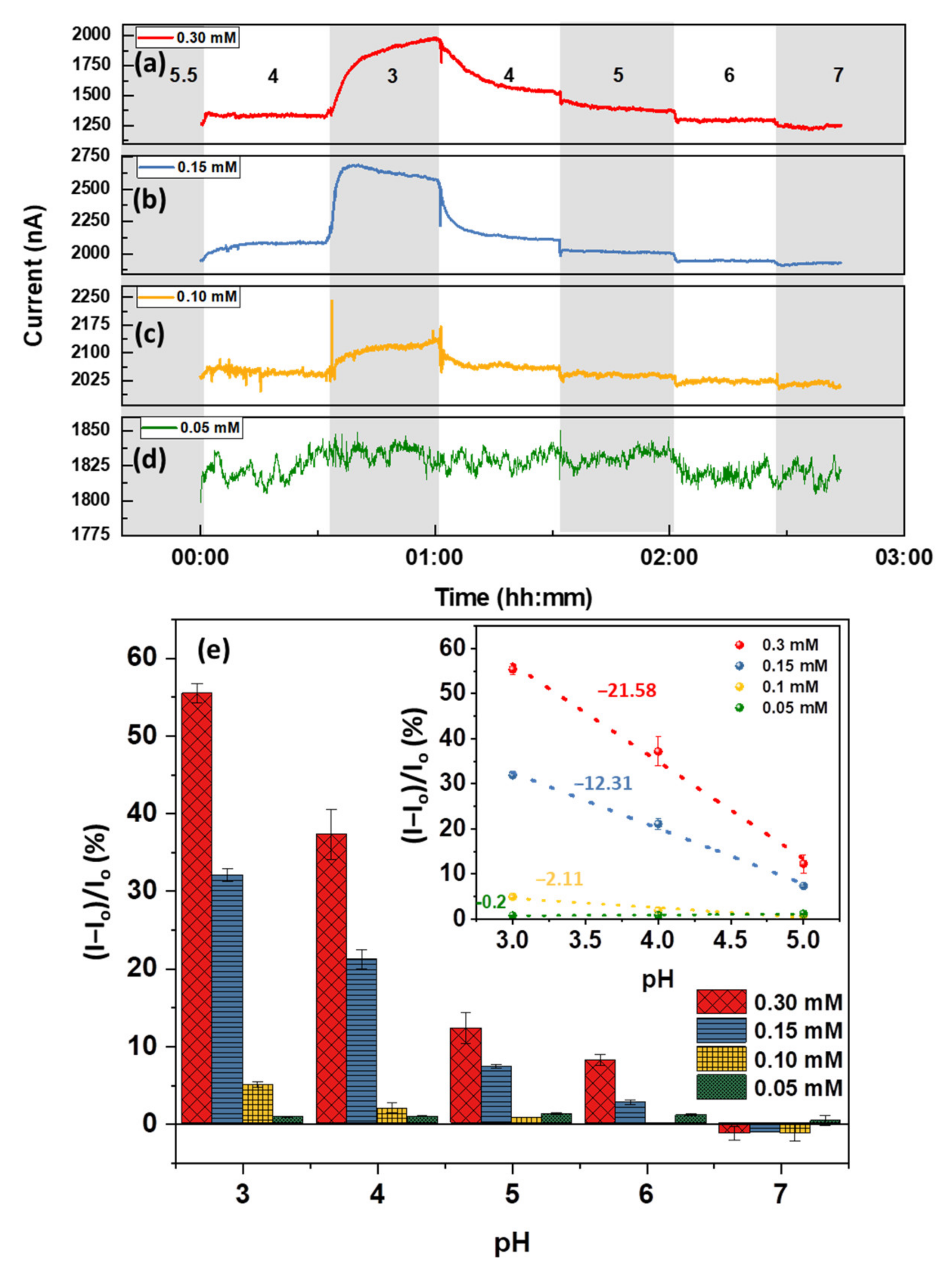
3.1. pH Response of Bare Graphene
3.2. Selective Functionalization of Graphene
3.3. pH Response of GO and Its Application towards the Development of GO-Based pH Sensors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and Regulators of Intracellular PH. Nat. Rev. Mol. Cell Biol. 2009, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Posadas, E.; del Morales, M.M.; Gomez, C.; Acién, F.G.; Muñoz, R. Influence of PH and CO2 Source on the Performance of Microalgae-Based Secondary Domestic Wastewater Treatment in Outdoors Pilot Raceways. Chem. Eng. J. 2015, 265, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Kasi, V.; Sedaghat, S.; M Alcaraz, A.; Kannan Maruthamuthu, M.; Heredia-Rivera, U.; Nejati, S.; Nguyen, J.; Rahimi, R. Low-Cost Flexible Glass-Based PH Sensor via Cold Atmospheric Plasma Deposition. ACS Appl. Mater. Amp Interfaces 2022, 14, 9697–9710. [Google Scholar] [CrossRef] [PubMed]
- Wiora, A.; Wiora, J. Over One-Year Long-Term Laboratory Tests of PH Electrodes in Terms of Industrial Applications Checking Stabilities of Their Parameters and Their Influence on Uncertainties of Measurements. Sensors 2018, 18, 4102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spijkman, M.-J.; Brondijk, J.J.; Geuns, T.C.T.; Smits, E.C.P.; Cramer, T.; Zerbetto, F.; Stoliar, P.; Biscarini, F.; Blom, P.W.M.; de Leeuw, D.M. Dual-Gate Organic Field-Effect Transistors as Potentiometric Sensors in Aqueous Solution. Adv. Funct. Mater. 2010, 20, 898–905. [Google Scholar] [CrossRef] [Green Version]
- Bartic, C.; Palan, B.; Campitelli, A.; Borghs, G. Monitoring PH with Organic-Based Field-Effect Transistors. Sens. Actuators B Chem. 2002, 83, 115–122. [Google Scholar] [CrossRef]
- Ghoneim, M.T.; Nguyen, A.; Dereje, N.; Huang, J.; Moore, G.C.; Murzynowski, P.J.; Dagdeviren, C. Recent Progress in Electrochemical PH-Sensing Materials and Configurations for Biomedical Applications. Chem. Rev. 2019, 119, 5248–5297. [Google Scholar] [CrossRef]
- Chen, Y.; Mun, S.C.; Kim, J. A Wide Range Conductometric PH Sensor Made With Titanium Dioxide/Multiwall Carbon Nanotube/Cellulose Hybrid Nanocomposite. IEEE Sens. J. 2013, 13, 4157–4162. [Google Scholar] [CrossRef]
- Avolio, R.; Grozdanov, A.; Avella, M.; Barton, J.; Cocca, M.; de Falco, F.; Dimitrov, A.T.; Errico, M.E.; Fanjul-Bolado, P.; Gentile, G.; et al. Review of PH Sensing Materials from Macro- to Nano-Scale: Recent Developments and Examples of Seawater Applications. Crit. Rev. Environ. Sci. Technol. 2022, 52, 979–1021. [Google Scholar] [CrossRef]
- Magnusson, E.B.; Halldorsson, S.; Fleming, R.M.T.; Leosson, K. Real-Time Optical PH Measurement in a Standard Microfluidic Cell Culture System. Biomed. Opt. Express 2013, 4, 1749. [Google Scholar] [CrossRef] [Green Version]
- Wencel, D.; Kaworek, A.; Abel, T.; Efremov, V.; Bradford, A.; Carthy, D.; Coady, G.; McMorrow, R.C.N.; McDonagh, C. Optical Sensor for Real-Time PH Monitoring in Human Tissue. Small 2018, 14, 1803627. [Google Scholar] [CrossRef] [PubMed]
- Goldcamp, M.J.; Conklin, A.; Nelson, K.; Marchetti, J.; Brashear, R.; Epure, E. Inexpensive and Disposable PH Electrodes. J. Chem. Educ. 2010, 87, 1262–1264. [Google Scholar] [CrossRef]
- Takeshita, Y.; Martz, T.R.; Johnson, K.S.; Dickson, A.G. Characterization of an Ion Sensitive Field Effect Transistor and Chloride Ion Selective Electrodes for PH Measurements in Seawater. Anal. Chem. 2014, 86, 11189–11195. [Google Scholar] [CrossRef]
- Shao, Y.; Ying, Y.; Ping, J. Recent Advances in Solid-Contact Ion-Selective Electrodes: Functional Materials, Transduction Mechanisms, and Development Trends. Chem. Soc. Rev. 2020, 49, 4405–4465. [Google Scholar] [CrossRef] [PubMed]
- Vonau, W.; Guth, U. PH Monitoring: A Review. J. Solid State Electrochem. 2006, 10, 746–752. [Google Scholar] [CrossRef]
- Kim, D.-M.; Cho, S.J.; Cho, C.-H.; Kim, K.B.; Kim, M.-Y.; Shim, Y.-B. Disposable All-Solid-State PH and Glucose Sensors Based on Conductive Polymer Covered Hierarchical AuZn Oxide. Biosens. Bioelectron. 2016, 79, 165–172. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Castro Neto, A.H. 2D Materials and van Der Waals Heterostructures. Science 2016, 353. [Google Scholar] [CrossRef] [Green Version]
- Angizi, S.; Akbar, M.A.; Darestani-Farahani, M.; Kruse, P. Review—Two-Dimensional Boron Carbon Nitride: A Comprehensive Review. ECS J. Solid State Sci. Technol. 2020, 9, 083004. [Google Scholar] [CrossRef]
- Hatamie, A.; Rahmati, R.; Rezvani, E.; Angizi, S.; Simchi, A. Yttrium Hexacyanoferrate Microflowers on Freestanding Three-Dimensional Graphene Substrates for Ascorbic Acid Detection. ACS Appl. Nano Mater. 2019, 2, 2212–2221. [Google Scholar] [CrossRef]
- Rezvani, E.; Hatamie, A.; Berahman, M.; Simchi, M.; Angizi, S.; Rahmati, R.; Kennedy, J.; Simchi, A. Synthesis, First-Principle Simulation, and Application of Three-Dimensional Ceria Nanoparticles/Graphene Nanocomposite for Non-Enzymatic Hydrogen Peroxide Detection. J. Electrochem. Soc. 2019, 166, H3167–H3174. [Google Scholar] [CrossRef]
- Wehling, T.O.; Novoselov, K.S.; Morozov, S.V.; Vdovin, E.E.; Katsnelson, M.I.; Geim, A.K.; Lichtenstein, A.I. Molecular Doping of Graphene. Nano Lett. 2008, 8, 173–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zubiarrain-Laserna, A.; Angizi, S.; Akbar, M.A.; Divigalpitiya, R.; Selvaganapathy, P.R.; Kruse, P. Detection of Free Chlorine in Water Using Graphene-like Carbon Based Chemiresistive Sensors. RSC Adv. 2022, 12, 2485–2496. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The Electronic Properties of Graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Angizi, S.; Selvaganapathy, P.R.; Kruse, P. Graphene-Silicon Schottky Devices for Operation in Aqueous Environments: Device Performance and Sensing Application. Carbon 2022, 194, 140–153. [Google Scholar] [CrossRef]
- Angizi, S.; Yu, E.Y.C.; Dalmieda, J.; Saha, D.; Selvaganapathy, P.R.; Kruse, P. Defect Engineering of Graphene to Modulate pH Response of Graphene Devices. Langmuir 2021, 37, 12163–12178. [Google Scholar] [CrossRef] [PubMed]
- Leenaerts, O.; Partoens, B.; Peeters, F.M. Water on Graphene: Hydrophobicity and Dipole Moment Using Density Functional Theory. Phys. Rev. B 2009, 79, 235440. [Google Scholar] [CrossRef] [Green Version]
- Taherian, F.; Marcon, V.; van der Vegt, N.F.A.; Leroy, F. What Is the Contact Angle of Water on Graphene? Langmuir 2013, 29, 1457–1465. [Google Scholar] [CrossRef]
- Angizi, S.; Hatamie, A.; Ghanbari, H.; Simchi, A. Mechanochemical Green Synthesis of Exfoliated Edge-Functionalized Boron Nitride Quantum Dots: Application to Vitamin C Sensing through Hybridization with Gold Electrodes. ACS Appl. Mater. Interfaces 2018, 10, 28819–28827. [Google Scholar] [CrossRef]
- Angizi, S.; Shayeganfar, F.; Azar, M.H.; Simchi, A. Surface/Edge Functionalized Boron Nitride Quantum Dots: Spectroscopic Fingerprint of Bandgap Modification by Chemical Functionalization. Ceram. Int. 2020, 46, 978–985. [Google Scholar] [CrossRef]
- Tehrani, Z.; Whelan, S.P.; Mostert, A.B.; Paulin, J.V.; Ali, M.M.; Ahmadi, E.D.; Graeff, C.F.O.; Guy, O.J.; Gethin, D.T. Printable and Flexible Graphene PH Sensors Utilising Thin Film Melanin for Physiological Applications. 2D Mater. 2020, 7, 024008. [Google Scholar] [CrossRef]
- Jung, S.-H.; Seo, Y.-M.; Gu, T.; Jang, W.; Kang, S.-G.; Hyeon, Y.; Hyun, S.-H.; Lee, J.-H.; Whang, D. Super-Nernstian PH Sensor Based on Anomalous Charge Transfer Doping of Defect-Engineered Graphene. Nano Lett. 2021, 21, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Salvo, P.; Melai, B.; Calisi, N.; Paoletti, C.; Bellagambi, F.; Kirchhain, A.; Trivella, M.G.; Fuoco, R.; di Francesco, F. Graphene-Based Devices for Measuring PH. Sens. Actuators B Chem. 2018, 256, 976–991. [Google Scholar] [CrossRef]
- Vivaldi, F.; Bonini, A.; Melai, B.; Poma, N.; Kirchhain, A.; Santalucia, D.; Salvo, P.; Francesco, F. di A Graphene-Based pH Sensor on Paper for Human Plasma and Seawater. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany; 23–27 July 2019; IEEE: New York, NY, USA; pp. 1563–1566.
- Sha, R.; Komori, K.; Badhulika, S. Amperometric PH Sensor Based on Graphene–Polyaniline Composite. IEEE Sens. J. 2017, 17, 5038–5043. [Google Scholar] [CrossRef]
- Konkena, B.; Vasudevan, S. Understanding Aqueous Dispersibility of Graphene Oxide and Reduced Graphene Oxide through p K a Measurements. J. Phys. Chem. Lett. 2012, 3, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Ito, J.; Nakamura, J.; Natori, A. Semiconducting Nature of the Oxygen-Adsorbed Graphene Sheet. J. Appl. Phys. 2008, 103, 113712. [Google Scholar] [CrossRef]
- Chinnathambi, S.; Euverink, G.J.W. Hydrothermally Reduced Graphene Oxide as a Sensing Material for Electrically Transduced PH Sensors. J. Electroanal. Chem. 2021, 895, 115530. [Google Scholar] [CrossRef]
- Kim, T.; Hong, S.; Yang, S. A Solid-State Thin-Film Ag/AgCl Reference Electrode Coated with Graphene Oxide and Its Use in a PH Sensor. Sensors 2015, 15, 6469–6482. [Google Scholar] [CrossRef] [Green Version]
- Neupane, S.; Subedi, V.; Thapa, K.K.; Yadav, R.J.; Nakarmi, K.B.; Gupta, D.K.; Yadav, A.P. An Alternative pH Sensor: Graphene Oxide-Based Electrochemical Sensor. Emergent Mater. 2021, 5, 509–517. [Google Scholar] [CrossRef]
- Sohn, I.-Y.; Kim, D.-J.; Jung, J.-H.; Yoon, O.J.; Nguyen Thanh, T.; Tran Quang, T.; Lee, N.-E. PH Sensing Characteristics and Biosensing Application of Solution-Gated Reduced Graphene Oxide Field-Effect Transistors. Biosens. Bioelectron. 2013, 45, 70–76. [Google Scholar] [CrossRef]
- Dalmieda, J.; Zubiarrain-Laserna, A.; Ganepola, D.; Selvaganapathy, P.R.; Kruse, P. Chemiresistive Detection of Silver Ions in Aqueous Media. Sens. Actuators B Chem. 2021, 328, 129023. [Google Scholar] [CrossRef]
- Sengupta, I.; Sharat Kumar, S.S.S.; Pal, S.K.; Chakraborty, S. Characterization of Structural Transformation of Graphene Oxide to Reduced Graphene Oxide during Thermal Annealing. J. Mater. Res. 2020, 35, 1197–1204. [Google Scholar] [CrossRef]
- Gupta, B.; Kumar, N.; Panda, K.; Kanan, V.; Joshi, S.; Visoly-Fisher, I. Role of Oxygen Functional Groups in Reduced Graphene Oxide for Lubrication. Sci. Rep. 2017, 7, 45030. [Google Scholar] [CrossRef] [PubMed]
- Kwan, Y.C.G.; Ng, G.M.; Huan, C.H.A. Identification of Functional Groups and Determination of Carboxyl Formation Temperature in Graphene Oxide Using the XPS O 1s Spectrum. Thin Solid Film. 2015, 590, 40–48. [Google Scholar] [CrossRef]
- Achtyl, J.L.; Vlassiouk, I.V.; Fulvio, P.F.; Mahurin, S.M.; Dai, S.; Geiger, F.M. Free Energy Relationships in the Electrical Double Layer over Single-Layer Graphene. J. Am. Chem Soc. 2013, 135, 979–981. [Google Scholar] [CrossRef]
- Li, X.; Shi, J.; Pang, J.; Liu, W.; Liu, H.; Wang, X. Graphene Channel Liquid Container Field Effect Transistor as PH Sensor. J. Nanomater. 2014, 2014, 547139. [Google Scholar] [CrossRef] [Green Version]
- He, H.; Kim, K.H.; Danilov, A.; Montemurro, D.; Yu, L.; Park, Y.W.; Lombardi, F.; Bauch, T.; Moth-Poulsen, K.; Iakimov, T.; et al. Uniform Doping of Graphene Close to the Dirac Point by Polymer-Assisted Assembly of Molecular Dopants. Nat. Commun. 2018, 9, 3956. [Google Scholar] [CrossRef] [Green Version]
- Gierz, I.; Riedl, C.; Starke, U.; Ast, C.R.; Kern, K. Atomic Hole Doping of Graphene. Nano Lett. 2008, 8, 4603–4607. [Google Scholar] [CrossRef] [Green Version]
- Kiani, M.J.; Ahmadi, M.T.; Karimi Feiz Abadi, H.; Rahmani, M.; Hashim, A.; Che harun, F.K. Analytical Modelling of Monolayer Graphene-Based Ion-Sensitive FET to PH Changes. Nanoscale Res. Lett. 2013, 8, 173. [Google Scholar] [CrossRef] [Green Version]
- Zahed, M.A.; Barman, S.C.; Das, P.S.; Sharifuzzaman, M.; Yoon, H.S.; Yoon, S.H.; Park, J.Y. Highly Flexible and Conductive Poly (3, 4-Ethylene Dioxythiophene)-Poly (Styrene Sulfonate) Anchored 3-Dimensional Porous Graphene Network-Based Electrochemical Biosensor for Glucose and PH Detection in Human Perspiration. Biosens. Bioelectron. 2020, 160, 112220. [Google Scholar] [CrossRef]
- Medina, H.; Lin, Y.-C.; Obergfell, D.; Chiu, P.-W. Tuning of Charge Densities in Graphene by Molecule Doping. Adv. Funct. Mater. 2011, 21, 2687–2692. [Google Scholar] [CrossRef]
- Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Novoselov, K.S.; Basko, D.M.; Ferrari, A.C. Raman Spectroscopy of Graphene Edges. Nano Lett. 2009, 9, 1433–1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osella, S.; Kiliszek, M.; Harputlu, E.; Unlu, C.G.; Ocakoglu, K.; Kargul, J.; Trzaskowski, B. Controlling the Charge Transfer Flow at the Graphene/Pyrene–Nitrilotriacetic Acid Interface. J. Mater. Chem. C 2018, 6, 5046–5054. [Google Scholar] [CrossRef] [Green Version]
- Zhen, X.V.; Swanson, E.G.; Nelson, J.T.; Zhang, Y.; Su, Q.; Koester, S.J.; Bühlmann, P. Noncovalent Monolayer Modification of Graphene Using Pyrene and Cyclodextrin Receptors for Chemical Sensing. ACS Appl. Nano Mater. 2018, 1, 2718–2726. [Google Scholar] [CrossRef]
- Tang, B.; Guoxin, H.; Gao, H. Raman Spectroscopic Characterization of Graphene. Appl. Spectrosc. Rev. 2010, 45, 369–407. [Google Scholar] [CrossRef]
- Díez-Betriu, X.; Álvarez-García, S.; Botas, C.; Álvarez, P.; Sánchez-Marcos, J.; Prieto, C.; Menéndez, R.; de Andrés, A. Raman Spectroscopy for the Study of Reduction Mechanisms and Optimization of Conductivity in Graphene Oxide Thin Films. J. Mater. Chem. C 2013, 1, 6905. [Google Scholar] [CrossRef]
- Sengupta, I.; Chakraborty, S.; Talukdar, M.; Pal, S.K.; Chakraborty, S. Thermal Reduction of Graphene Oxide: How Temperature Influences Purity. J. Mater. Res. 2018, 33, 4113–4122. [Google Scholar] [CrossRef]
- Farah, S.; Farkas, A.; Madarász, J.; László, K. Comparison of Thermally and Chemically Reduced Graphene Oxides by Thermal Analysis and Raman Spectroscopy. J. Therm. Anal. Calorim. 2020, 142, 331–337. [Google Scholar] [CrossRef]
- Vacchi, I.A.; Raya, J.; Bianco, A.; Ménard-Moyon, C. Controlled Derivatization of Hydroxyl Groups of Graphene Oxide in Mild Conditions. 2d Mater. 2018, 5, 035037. [Google Scholar] [CrossRef]
- Kwon, K.C.; Choi, K.S.; Kim, C.; Kim, S.Y. Role of Metal Cations in Alkali Metal Chloride Doped Graphene. J. Phys. Chem. C 2014, 118, 8187–8193. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s Law Constants (Version 4.0) for Water as Solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Zhen, X.V.; Swanson, E.G.; Nelson, J.T.; Zhang, Y.; Su, Q.; Koester, S.J.; Bühlmann, P. Noncovalent Monolayer Modification of Graphene Using Pyrene and Cyclodextrin Receptors for Chemical Sensing. ACS Appl. Nano Mat. 2018, 1, 2718–2726. [Google Scholar] [CrossRef]
- An, X.; Simmons, T.; Shah, R.; Wolfe, C.; Lewis, K.M.; Washington, M.; Nayak, S.K.; Talapatra, S.; Kar, S. Stable Aqueous Dispersions of Noncovalently Functionalized Graphene from Graphite and Their Multifunctional High-Performance Applications. Nano Lett. 2010, 10, 4295–4301. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]




| SLG | FLG | |||||
|---|---|---|---|---|---|---|
| Area % | at% * | Ratio to C=C at% | Area % | at% * | Ratio to C=C at% | |
| C=O | 0.3 | 0.1119 | 0.003074 | 2.8 | 2.22 | 0.05 |
| O-C=O | 0.2 | 0.0746 | 0.002049 | 3.4 | 2.70 | 0.061 |
| C-OH/O-C-O | 1.3 | 0.4849 | 0.01332 | 11.9 | 9.46 | 0.214 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angizi, S.; Huang, X.; Hong, L.; Akbar, M.A.; Selvaganapathy, P.R.; Kruse, P. Defect Density-Dependent pH Response of Graphene Derivatives: Towards the Development of pH-Sensitive Graphene Oxide Devices. Nanomaterials 2022, 12, 1801. https://doi.org/10.3390/nano12111801
Angizi S, Huang X, Hong L, Akbar MA, Selvaganapathy PR, Kruse P. Defect Density-Dependent pH Response of Graphene Derivatives: Towards the Development of pH-Sensitive Graphene Oxide Devices. Nanomaterials. 2022; 12(11):1801. https://doi.org/10.3390/nano12111801
Chicago/Turabian StyleAngizi, Shayan, Xianxuan Huang, Lea Hong, Md Ali Akbar, P. Ravi Selvaganapathy, and Peter Kruse. 2022. "Defect Density-Dependent pH Response of Graphene Derivatives: Towards the Development of pH-Sensitive Graphene Oxide Devices" Nanomaterials 12, no. 11: 1801. https://doi.org/10.3390/nano12111801







